Abstract
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a common health problem in the world. However, its etiology remains unclear. Recent researches have hypothesized that Staphylococcus aureus (SA) exotoxins which act as superantigens might be associated with inflammatory mucosal changes seen in CRSwNP. The objective of this study is to evaluate the relationship between Staphylococcus aureus superantigens and CRSwNP. PubMed, MEDLINE, EMBASE, Cochrane Library and CNKI were searched to collect the case–control studies on the relationship between SA superantigens and CRSwNP from the date of establishment of the databases to May 2013. The extracted data were analyzed by RevMan 5.0. The main outcome measures were SA culture-positive rate, the detection rate of SA superantigens and its specific IgE. Twelve studies including 340 cases and 178 controls were selected. The results showed that SA culture-positive rate in the CRSwNP group was significantly higher than that in the control group (OR 4.85, 95 % CI 1.80–13.05, P = 0.002), the detection rate of SA superantigens and its specific IgE in the CRSwNP group were both significantly higher than that in the control group (OR 12.07, 95 % CI 4.57–31.90, P < 0.00001; OR 17.03, 95 % CI 5.43–53.39, P < 0.00001, respectively) and the CD4+ T cell counts and Lund-Mackay CT scores were statistically higher in the IgE-positive group than in the IgE-negative group (MD 16.26, 95 % CI 4.86–27.67, P = 0.005, MD 2.43, 95 % CI 0.39–4.48, P = 0.02, respectively). However, the eosinophil and CD8+ T cell counts showed no difference between IgE-positive group and -negative group. This meta-analysis indicated that the SA superantigens may be a risk factor for CRSwNP, and the presence of SA superantigen is related to the disease severity of CRSwNP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis is one of the most usual nasal diseases, whose main clinical symptoms consist of persistent nasal congestion, purulent nasal discharge, dysosmia and headaches, etc. There are two types of chronic rhinosinusitis, including chronic rhinosinusitis with nasal polyps (CRSwNP) and without nasal polyps (CRSsNP). CRSwNP is more apt to relapse than CRSsNP, and the active drugs and surgical treatment have poor efficacy on CRSwNP, thus impairing the patients’ health and the quality of life more severely. Its etiology and pathogenesis are still not expounded yet. In recent years, some studies [1, 2] have put forward that the pathogenesis of CRSwNP has a relationship with the infection of Staphylococcus aureus, especially Staphylococcus aureus enterotoxins (SEs) which it secretes that play an important role in CRSwNP. SA is one of the three pathogenic Gram-positive coccus; it always settles in the upper respiratory tract, especially in the nasal cavity. SE as a classic superantigen (SAg) is a set of a related thermal stable structure protein family, whose primary subtypes contain SEA, SAB, SAC, SAD, SAE and toxic shock syndrome toxin-1 (TSST-1) [3]. SA might impact on some factors and then cause chronic inflammatory disease through the superantigen effect of SE [4], such as toxic shock syndrome, psoriasis, atopic dermatitis, mucocutaneous lymph node syndrome (MCLS), and so on. A meta-analysis [5] of some clinical studies has also supported an underlying role of S. aureus superantigens in asthma and allergic rhinitis.
In the past few years, several reports abroad supported bacterial superantigen theory, as Schubert [6] indicated that SE might act as superantigens in chronic rhinosinusitis, particularly in patients with nasal polyps. Bernsein et al. [7] suggested that more than one kind of SEs was detected in the mucus near 55 % of nasal polyps. But there were some studies against this hypothesis. For example, Damm et al. [8] reported the nasal Staphylococcus aureus culture rate in the patients of nasal polyps was not higher than the control group and that Staphylococcus aureus carrying condition was not a risk factor of disease extent and severity of chronic rhinosinusitis. Recently, there are numerous studies that investigated the association between SA superantigens and CRSwNP, but the results remain controversial and have various opinions. To explore the exact role of SA superantigens in the risk of CRSwNP, we performed a meta-analysis by collecting and analyzing the related indicators about them from all eligible studies.
Methods
Selection of studies
Literature searches were performed on PubMed, MEDLINE, EMBASE, Cochrane Library and CNKI from the date of establishment of the databases to May 2013 using the following search strategies: ‘Staphylococcus aureus enterotoxins’ or ‘superantigen’ and ‘chronic rhinosinusitis’ or ‘nasal polyps’. All searches were restricted to human studies. In addition, we performed a manual search of the references of all identified articles to find other studies. There was no language restriction. The articles identified by the search were then screened for inclusion or exclusion. The inclusion criteria were: (1) case–control studies, (2) studies about the relationship between SA superantigens and CRSwNP, (3) the patients in the CRSwNP group were diagnosed clearly based on the guideline [9], and (4) studies with sufficient available data to further analyses. Articles were excluded if they were duplicate publications, reviews, comments, or there was lack of sufficient original data, or failure to ask for missing data.
Data extraction
Two assessors read the included articles and performed data extraction independently, and then created databases and cross-checked data. They reached a consensus on all items by discussion. If they were unable to reach an agreement, an expert was invited to the discussion. The following information was extracted from each included study: first author, published year, country, ethnicity of study population, demographic characteristics of samples (age, gender, and numbers of samples), the outcomes in each article and the detection technology. If the included literatures had missing information, we would contact with authors directly.
Statistical analysis
The data analysis was performed using RevMan 5.0. The relationship between SA superantigens and CRSwNP was assessed by odds ratio (OR) or mean difference (MD) and 95 % confidence interval (95 % CI). A Chi square test was used to test the presence of heterogeneity between the included studies. If P > 0.1, I 2 < 50 %, these several similar studies were considered with homogeneity, and the fixed effects model was chosen for the meta-analysis or the random effects model was chosen instead. If P < 0.1, and the source of heterogeneity could not be estimated, meta-analysis was not been performed and descriptive analysis would be chosen instead. The significance of pooled ORs was detected by the Z test (P < 0.05 was considered significant).
Results
Study characteristics
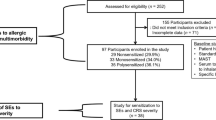
Using the search strategy described, we found 71 relevant articles. 59 studies were excluded for not meeting the inclusion criteria: 37 studies were not relevant to CRSwNP and superantigens; five studies did not have sufficient data for further analysis; 15 studies were review articles; and two studies were comment articles. Therefore, 12 studies [10–21] consisting of 340 cases and 178 controls were included in this meta-analysis of the association between Staphylococcus aureus superantigens and the risk of CRSwNP (Fig. 1). 115 CRSwNP patients in the total group had comorbidities such as asthma, allergic rhinitis, aspirin sensitivity, etc. The characteristics of the included studies are summarized in Table 1.
Quantitative data synthesis
The association between Staphylococcus aureus superantigen and the risk of CRSwNP was evaluated through the detection rate of SA culture in the nasal cavity, SE and the specific IgE against SE in the serum. Among the 12 included studies, four studies [10, 12, 17, 19] evaluated the association between SA culture-positive rate and the risk of CRSwNP. As shown in Fig. 2a, we found that SA culture-positive rate in the CRSwNP group was significantly higher than that in the control group (OR 4.85, 95 % CI 1.80–13.05, P = 0.002). Five studies [12, 15, 19–21] reported the relationship between SE and CRSwNP. Significant difference in the detection rate of SE between the two groups was found (OR 12.07, 95 % CI 4.57–31.90, P < 0.00001) (Fig. 2b), and four studies [11, 14, 16, 18] assessed the association between the specific IgE against SE and CRSwNP. Meta-analysis indicated that the detection rate of SE-specific IgE in the CRSwNP group was significantly higher than that in the control group (OR 17.03, 95 % CI 5.43–53.39, P < 0.00001) (Fig. 2c). Besides, we discovered that the SA culture-positive rate and the detection rate of SE in patients with CRSwNP, who did not have comorbidities of asthma, allergic rhinitis, and aspirin sensitivity, were still significantly higher than that in the control group (OR 3.93, 95 % CI 1.13–13.68, P = 0.03, OR 10.73, 95 % CI 3.66–31.46, P < 0.0001, respectively) (Fig. 2d, e). However, we found that the detection rate of SE in the CRSsNP group was not significantly higher than that in the control group (OR 2.06, 95 % CI 0.21–20.72, P = 0.54) (Fig. 3).
Forest plots of studies comparing the SA culture-positive rate (a), the detection rate of SE (b) and SE-specific IgE (c) in patients with CRSwNP and controls; the forest plots of studies comparing the SA culture-positive rate (d) and the detection rate of SE (e) in patients with controls and CRSwNP, who did not have comorbidities of asthma, allergic rhinitis and aspirin sensitivity
In a further analysis, the mechanism of SAg triggering CRSwNP remained unclear. Analysis only indicated the CD4+ T cell counts in the SE-specific IgE-positive group was significant higher than that in the SE-specific IgE-negative group (MD 16.26, 95 % CI 4.86–27.67, P = 0.005) (Fig. 4a), whereas the eosinophil and CD8+ T cell counts showed no differences between the two groups above (MD 36.75, 95 % CI −8.09 to 81.60, P = 0.11; MD 97.99, 95 % CI −110.02 to 306.00, P = 0.36, respectively) (Fig. 4b, c).
In addition, two studies [13, 16] also reported the relation between exposure to SAg and CRSwNP severity. In these studies, the disease severity of CRSwNP was assessed by Lund–Mackay CT scores. Lund–Mackay CT scores were statistically higher in the SE-specific IgE-positive group than in the SE-specific IgE-negative group (MD 2.43, 95 % CI 0.39–4.48, P = 0.02) (Fig. 4d).
Discussion
Nowadays, there are a variety of studies on bacterial superantigen hypothesis, but they have different opinions. Our meta-analysis confirmed it in a more robust way by combining data from 12 included studies and the results indicated that the nasal SA culture-positive rate, the detection rate of SE and its specific IgE in patients with CRSwNP is significantly higher than that in the control group. The infection of SA, especially the presence of its endotoxins, may lead to the increase of the risk of CRSwNP. It is consistent with the findings reported by Bachert et al. [1], Schubert [6] and Bernsein et al. [7]. Since SA superantigens also have a relationship with asthma and allergic rhinitis [5], we performed another analysis to exclude the effect of other chronic inflammatory diseases. The SA culture-positive rate and the detection rate of SE in CRSwNP patients without asthma, allergic rhinitis and aspirin sensitivity are still significantly higher than controls. Staphylococcus aureus superantigens play a role in chronic rhinosinusitis with nasal polyps. Interestingly, we found that the detection rate of SE in patients with CRSsNP was not significantly higher than that in the control group. The role of Staphylococcus aureus superantigens in CRSsNP remains uncertain.
To explore the pathogenic mechanism of Staphylococcus aureus superantigens, some scholars pointed out that SA superantigens led to the occurrence of CRSwNP by promoting the infiltration of eosinophils and lymphocytes [6]. The main feature of superantigens is to bypass the process of antigen-presenting cells (APCs), but directly link with the major histocompatibility II complex (MHC II) by binding outside the peptide-binding grove on the surface of APCs through a complete protein molecule [22]. It makes SA alter the immune response to escape from host defense and enables colonization effectively. SEs act as superantigens and activate a large amount of T cells containing the specific Vβ region non-specifically [23] resulting in a skewing of the TCR Vβ repertoire, thereby synthesizing inflammatory cytokines. Superantigens can not only activate lymphocytes directly, but also stimulate eosinophils, mast cells and other inflammatory cells to assemble [24]. Perez-Novo et al. [25] put forward the hypothesis that the immune response caused by SA superantigens affected the eicosane hormone regulatory pathways and raised its synthesis. Since eicosane hormones, which include leukotrienes, prostaglandins and lipoxin, are important inflammatory mediators, they led to the increase of eosinophils infiltration and then produced several relevant cytokines and inflammatory mediators, thus causing chronic inflammation of the nasal sinus mucosa by the cascade inflammatory effect. In this meta-analysis, patients in the CRSwNP group were divided into two groups according to the detection of SE-specific IgE. However, we only found significant difference in CD4+ T cell counts between SE-specific IgE-positive group and -negative group. But the differences of eosinophil and CD8+ T cell counts between the two groups were not statistically significant. On the contrary, Gevaert et al. [17] discovered an opposite result: eosinophil counts in the SE-positive group was higher than in the -negative group. The inconformity of these conclusions may be associated with different active states of the detected eosinophils. Therefore, the detection only of active eosinophils, while ignoring degranulated and inactive cells, may result in a false-negative result. Nevertheless, the research by Gevaert et al. [17] lacks sufficient data to calculate merge OR and 95 % CI in the meta-analysis. However, the significant difference in CD4+ counts in the two groups is not enough to explain the pathogenic mechanism of SA superantigens, because this meta-analysis of CD4+ is based on only two studies of small sample sizes and CD4+ T cell is just a kind of lymphocyte. Besides, Wang et al. [21] found that the TCRVβ spectrum skewing in patients with CRSwNP was closely related to SA superantigens. Bernstein et al. [26] also had a similar conclusion. But the two articles could not provide sufficient data to perform a meta-analysis. Thus, more high-quality researches are needed to provide an evidence-based proof that the up-regulation of TCRVβ is one of the mechanisms of SA superantigens causing CRSwNP. Moreover, Clark et al. [13] and Tripathi et al. [16] found the relationship between the exposure to SA superantigens and the disease severity of CRSwNP. In this meta-analysis, Lund-Mackay CT scores were statistically higher in the SE-specific IgE-positive group than the -negative group. The presence of SA superantigens could be used as an index of CRSwNP severity.
In summary, this meta-analysis demonstrated that the Staphylococcus aureus superantigen may be a risk factor for the persistence and severity of chronic rhinosinusitis with nasal polyps, and the presence of SA superantigens is related to the disease severity of CRSwNP. So the therapy targeting SA superantigens is expected to be a new method for the treatment of CRSwNP. Nevertheless, since the included literatures are all case–control studies, their proof intensity is not strong enough and only the association could be provided. Therefore, there is still a need for large sample, high-quality, scientific and normative randomized controlled trials of the multi-center, so as to further confirm the relationship between Staphylococcus aureus superantigens and chronic rhinosinusitis with nasal polyps and its mechanism.
References
Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P (2001) Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol 107:607–614
Wang JH, Kwon HJ, Jang YJ (2010) Staphylococcus aureus increases cytokine and matrix metalloproteinase expression in nasal mucosae of patients with chronic rhinosinusitis and nasal polyps. Am J Rhinol Allergy 24:422–427
Balaban N, Rasooly A (2000) Staphylococcal enterotoxins. Int J Food Microbiol 61:1–10
White J, Herman A, Pullen AM, Kubo R, Kappler JW, Marrack P (1989) The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell 56:27–35
Pastacaldi C, Lewis P, Howarth P (2011) Staphylococci and staphylococcal superantigens in asthma and rhinitis: a systematic review and meta-analysis. Allergy 66:549–555
Schubert MS (2001) A superantigen hypothesis for the pathogenesis of chronic hypertrophic rhinosinusitis, allergic fungal sinusitis, and related disorders. Ann Allergy Asthma Immunol 87:181–188
Bernstein JM, Ballow M, Schlievert PM, Rich G, Allen C, Dryja D (2003) A superantigen hypothesis for the pathogenesis of chronic hyperplastic sinusitis with massive nasal polyposis. Am J Rhinol 17:321–326
Damm M, Quante G, Jurk T, Sauer JA (2004) Nasal colonization with Staphylococcus aureus is not associated with the severity of symptoms or the extent of the disease in chronic rhinosinusitis. Otolaryngol Head Neck Surg 131:200–206
Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, Lanza DC, Marple BF, Osguthorpe JD, Stankiewicz JA, Anon J, Denneny J, Emanuel I, Levine H (2003) Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg 129:S1–S32
Wang M, Zhang D, Zhang H, Wang Z, Chen X, Jian J (2006) A primary study about the correlation between superantigens produced by Staphylococcus aureus and chronic rhinosinusitis with nasal polyps. Lin Chuang Er Bi Yan Hou Ke Za Zhi 20:914–916
Foreman A, Holtappels G, Psaltis AJ, Jervis-Bardy J, Field J, Wormald PJ, Bachert C (2011) Adaptive immune responses in Staphylococcus aureus biofilm-associated chronic rhinosinusitis. Allergy 66:1449–1456
Kim ST, Chung SW, Jung JH, Ha JS, Kang IG (2011) Association of T cells and eosinophils with Staphylococcus aureus exotoxin A and toxic shock syndrome toxin 1 in nasal polyps. Am J Rhinol Allergy 25:19–24
Clark DW, Wenaas A, Citardi MJ, Luong A, Fakhri S (2011) Chronic rhinosinusitis with nasal polyps: elevated serum immunoglobulin E is associated with Staphylococcus aureus on culture. Int Forum Allergy Rhinol 1:445–450
Conley DB, Tripathi A, Ditto AM, Reid K, Grammer LC, Kern RC (2004) Chronic sinusitis with nasal polyps: staphylococcal exotoxin immunoglobulin E and cellular inflammation. Am J Rhinol 18:273–278
Guven M, Karabay O, Akidil O, Yilmaz MS, Yildirim M (2013) Detection of staphylococcal exotoxins in antrochoanal polyps and chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg 148:302–307
Tripathi A, Conley DB, Grammer LC, Ditto AM, Lowery MM, Seiberling KA, Yarnold PA, Zeifer B, Kern RC (2004) Immunoglobulin E to staphylococcal and streptococcal toxins in patients with chronic sinusitis/nasal polyposis. Laryngoscope 114:1822–1826
Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C (2005) Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy 60:71–79
Zhang N, Holtappels G, Claeys C, Huang G, van Cauwenberge P, Bachert C (2006) Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol 20:445–450
El-Fiky LM, Khamis N, Mostafa BD, Adly AM (2009) Staphylococcal infection and toxin production in chronic rhinosinusitis. Am J Rhinol Allergy 23:264–267
Seiberling KA, Conley DB, Tripathi A, Grammer LC, Shuh L, Haines GR, Schleimer R, Kern RC (2005) Superantigens and chronic rhinosinusitis: detection of staphylococcal exotoxins in nasal polyps. Laryngoscope 115:1580–1585
Wang M, Shi P, Chen B, Zhang H, Jian J, Chen X, Wang Z, Zhang D (2008) The role of superantigens in chronic rhinosinusitis with nasal polyps. ORL J Otorhinolaryngol Relat Spec 70:97–103
Fleischer B (1994) Superantigens. Apmis 102:3–12
Choi YW, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J (1989) Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc Natl Acad Sci USA 86:8941–8945
Langley R, Patel D, Jackson N, Clow F, Fraser JD (2010) Staphylococcal superantigen super-domains in immune evasion. Crit Rev Immunol 30:149–165
Perez-Novo CA, Watelet JB, Claeys C, Van Cauwenberge P, Bachert C (2005) Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol 115:1189–1196
Bernstein JM, Allen C, Rich G, Dryja D, Bina P, Reiser R, Ballow M, Wilding GE (2011) Further observations on the role of Staphylococcus aureus exotoxins and IgE in the pathogenesis of nasal polyposis. Laryngoscope 121:647–655
Conflict of interest
We declare that we have no conflict of interest. The funding source of the research is National Natural Science Funds of China (No.81371070).
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Ou, J. Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ou, J., Wang, J., Xu, Y. et al. Staphylococcus aureus superantigens are associated with chronic rhinosinusitis with nasal polyps: a meta-analysis. Eur Arch Otorhinolaryngol 271, 2729–2736 (2014). https://doi.org/10.1007/s00405-014-2955-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-014-2955-0