Abstract
Purpose of Review
To review innate lymphoid cells (ILCs) and their role in chronic rhinosinusitis (CRS).
Recent Findings
The immune system consists of the innate and adaptive response. Until the recognition of ILCs, chronic inflammatory diseases were characterized by cytokines linked only to T helper cells. However, these immune responses are now described more broadly to include contributions from both the innate and adaptive immunity. In CRS, focus had been on ILC2s in CRS with nasal polyps. These studies also highlight the importance of epithelial cell–derived cytokines in coordinating these responses. In addition to indirect crosstalk via cytokines, ILCs and T helper cells can utilize the OX40/OX40 ligand and major histocompatibility complex class II pathways to directly interact and coordinate responses.
Summary
In addition to T helper cells, ILCs contribute to the inflammatory response associated with CRS. The understanding of these cells along with pathways that activate and perpetuate these cells leads to new potential therapeutic targets for CRS treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Innate lymphoid cells (ILCs) are innate cell counterparts to the adaptive T helper cells. Their recognition in the early 2000s has resulted in a surge of studies on these cells and a renewed interest in the innate immune response. Chronic inflammatory diseases such as chronic rhinosinusitis result from immune dysregulation originally attributed to T helper cells. This review summarizes ILCs and highlights recent understanding of these cells in particular to their roles in the pathophysiology of CRS.
Chronic Rhinosinusitis, Once Defined by T Helper Cytokines
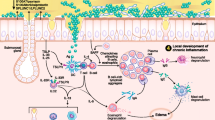
Chronic rhinosinusitis (CRS) describes a heterogenous group of diagnoses characterized by inflammation of the mucosa lining the paranasal sinus cavities resulting in symptoms such as discolored nasal drainage, nasal congestion, and facial pain/pressure persisting for longer than 3 months. Affecting approximately 39 million Americans, the economic direct cost burden is estimated between $10 and $13 billion per year [1]. Within this broad group, CRS is clinically subdivided by the absence or presence of polyps resulting in CRS without (CRSsNP) and with nasal polyps (CRSwNP) (Fig. 1). This clinical distinction provides physicians some broad guidance in considering possible etiologies/risk factors, associated comorbidities, treatment options, and overall long-term management outcomes.
Support for clinical phenotyping of CRS based on the absence and presence of polyps came from finding distinct immunologic profiles associated with CRSwNP as compared to CRSsNP. Initial studies of inflamed sinus mucosa linked CRSwNP with elevated levels of cytokines, interleukin (IL)-4, IL-5, and IL-13. At the time, the contribution of the innate immune response to these cytokines was not appreciated and hence these cytokines were considered T helper 2 (Th2) cytokines. Histologic evaluation of CRSwNP mucosa further characterized it as a Th2 inflammatory disease with elevated eosinophils and mast cells [2]. On the other hand, inflamed sinus mucosa from CRSsNP revealed a Th1 profile with increased presence of neutrophils and higher levels of IFN-γ and TNF-α [3]. In addition to CRS, other chronic inflammatory diseases such as asthma and dermatitis were similarly subcategorized as either Th1 or Th2, emphasizing the perceived importance of the adaptive immune response. The discovery of innate lymphoid cells less than 20 years ago generated new interest in the innate immune response contributing to chronic inflammatory diseases.
Emerging Understanding of Innate Lymphoid Cells and Their Physiological Roles
In the early 2000s, several independent groups identified sources for cytokines typically attributed to T helper cells from non-B and non-T cell lymphocytes. These innate lymphoid cells, lacking antigen-specific receptors, were activated by various stress signals such as exposure to microbes and allergens. As resident tissue cells, ILCs can respond within minutes to potential immunologic threats. Three ILC subgroups exist defined by their profile of secreted cytokines and transcriptional requirements. These subgroups parallel the T helper subgroups such that IFN-γ is the main cytokine of ILC1; Th1, ILC2, and Th2 are defined by secretion of IL-5, IL-13, and IL-4; and ILC3 produce IL-17 and IL-22 (Table 1) [4]. As such, ILCs are capable of immediately responding to environmental stresses and serve as the initial response while T and B cells require days to generate an immunologic response. Given the significant overlap in the expressed cytokines between ILCs and T helper cell counterparts, it remains unclear if ILCs have independent functions or serve as redundancy in the immune system.
Although a recent study in humans with severe combined immunodeficiency (SCID) suggests that ILCs may be redundant to an intact adaptive immunity for typical immune homeostasis and response, others argue that ILCs have critical unique physiologic roles [5•]. With the ability to secrete IFN-γ, ablating ILC1s prior to infection with mouse cytomegalovirus led to significantly higher viral loads and hence ILC1s seem critical in the immunosurveillance of the local site of infection [6]. In addition to classic T helper cytokines, ILC2s secrete unique molecules such as amphiregulin, which mediates tissue repair, and ILC3s secrete lymphotoxin-α which promotes immunoglobulin A production [7]. Arguing against independent roles of ILCs to T cells, Vely et al. studied a unique rare cohort of SCID patients. With mutations in IL2RG and JAK3, these SCID patients were deficient of both functional T cells and ILCs. With a follow-up of up to 39 years, they followed these patients before and after treatment with hematopoietic stem cell transplantation that reconstituted only the T cell population. They found no increase in infection or inflammatory conditions in these patients as compared to other SCID patients with intact ILCs [5]. Although there were limitations to this observational cohort study, it did provide compelling data suggesting redundancy of ILCs with an intact adaptive immunity.
Innate Lymphoid Cells Linked to Chronic Inflammatory Diseases
Similar to chronic rhinosinusitis, several chronic inflammatory diseases have been linked to immunodysfunction including inflammatory bowel disease (IBD) and asthma. Characterized by cytokines initially attributed to T cells, many chronic inflammatory diseases and treatment options were focused on the adaptive immune response. However, with the appreciation that ILCs are also significant sources of these cytokines and can orchestrate the innate and adaptive immune response, recent studies in the pathophysiology of these diseases have shifted focus towards the innate immune response. Therefore, although ILCs may be redundant during homeostasis, they seem to be significant players in chronic inflammatory disease states.
Like chronic rhinosinusitis, both IBD and asthma describe clinical syndromes that consist of several different subtypes. For IBD, studies on the role of ILCs have primarily focused on Crohn’s disease characterized as a type-1 inflammatory dysfunction. These studies highlight the importance of ILC3 in gut homeostasis and the plasticity of ILCs. Through the production of IL-22 and IL-17, ILC3 helps to induce mucosal wound healing and production of antimicrobial peptides in healthy intestines [8]. In Crohn’s, there seems to be a loss of IL-22 producing ILC3 and an emergence in IFN-γ producing ILC1s, ultimately promoting gut inflammation by the recruitment of lymphocytes and deterioration of the intestinal epithelial cell barrier [9]. This skewing of ILC subtype distribution between healthy and disease states is in part associated to the plasticity of ILCs induced by the inflammatory cytokine milieu. Human ILC3s differentiate to IFN-γ producing ILC1s in the presence of IL-12, a pro-inflammatory cytokine [10]. In fact, plasticity is a shared characteristic among the ILC subtypes. Ulcerative colitis, the other main IBD, is characterized by a predominantly type 2 inflammatory response with elevated IL-13 and IL-5. The role of ILCs in ulcerative colitis remains unclear.
Asthma describes pulmonary inflammation resulting in airway hyperreactivity and is a frequent comorbidity with CRS, especially CRSwNP. Although a large proportion of asthmatics suffer from allergic asthma characterized by elevated IL-13, IL-5, and IL-4, there are other asthma subtypes including neutrophilic, obesity-related, and virus-induced highlighting other cytokine profiles. With the discovery of ILCs, investigators found expansion of IL-5- and IL-13-expressing ILC2s in the OVA allergen-induced asthma mouse model [11]. The significant role of ILC2 in the pathophysiology of allergic asthma is highlighted by inducing asthma using a protease-containing allergen, papain, in mice with no B- or T cells (Rag1−/−). In these mice with only an intact innate immunity, papain induced mucus hypersecretion and pulmonary infiltration of eosinophils, hallmarks of pulmonary inflammation of asthma [12].
Investigating the role of ILC2s in asthma has revealed significant understanding of ILC2 biology. ILC2s express a number of receptors to epithelial cell-derived cytokines including IL-33, IL-25, and thymic stromal lymphopoietin (TSLP). As such, ILC2s are poised to respond rapidly to environmental triggers stimulating epithelial cells. Although ILC2s can induce components of asthma in the absence of the adaptive immunity, ILC2s and T cells express cytokines and receptors that support crosstalk between the two cell types and hence amplification of pulmonary inflammation [13]. Therefore, mouse models support the role of ILC2s in the initiation of allergic asthma. However, T cells are preferentially triggered in allergen sensitized mice and IL-5 production from memory T cells is critical in the eosinophilic pulmonary inflammation activated by the IL-33-ST2 axis [14]. Therefore, the role of ILC2 seems less prominent than the adaptive immune response in the maintenance of allergic asthma [13].
Other ILC subtypes have been linked to non-type 2 asthma. Increasing obesity rates in humans are highlighting obesity as a risk factor for asthma. In obese asthmatic patients, ILC3s are found in the bronchoalveolar lavage fluid [13]. In mice made obese by being fed a high-fat diet for 3 months, airway hyperreactivity along with elevated levels of IL-17A producing ILC3 is present [13].
ILCs Critical Source of Cytokines in CRSwNP
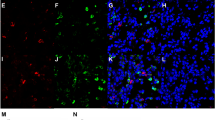
Many studies into ILCs in asthma and IBD are based on mice models with limited studies using human tissue. On the other hand, the management of CRS often involves surgery that removes diseased tissue. Utilizing this access to tissue, we isolated and characterized Lin−CD127+ ILC subtypes. ILC2s, defined by presence of CRTH2+, make up the largest ILC population within inflamed sinus mucosa, whether from patients with or without nasal polyps, while ILC3s, defined by expression of NKp44+, are the smallest population (Fig. 2) representing less than 0.2% of lineage negative cells. Consistent with its type 2 immune characterization, in CRSwNP, ILC2s represent the largest proportion of innate lymphoid cells within inflamed sinus mucosa. However, percentages of ILC1s and ILC2s are near equally present [15, 16]. Finally, allergic fungal rhinosinusitis, a subtype of CRS with nasal polyps defined by sensitization to fungi and fungal components identified within sinus mucosa, was notable for an elevated percentage of ILC3s. However, given the new appreciation of the plasticity of ILCs, additional characterization studies are warranted. CRS represents a unique opportunity to isolate and study human ILCs.
ILC1-3s are elevated in CRSwNP. a ILC1 gating strategy in inflamed mucosa. Lineage negative/CD127+ events were analyzed for PE-CXCR3+ (b), PE-CRTH2+ (c), and PE-NKp44+ cells (d). Representative histogram (black) and FMO control (shaded) from CRS subtypes. Percentage of ILC1–3+ cells from CRSsNP (n = 8), CRSwNP (n = 13), and AFRS (n = 10). Data presented as mean + SD
Human ILCs were initially isolated from fetal tissue and inflamed sinus tissue from CRS patients. In 2011, Mjosberg et al. were the first to describe a Lin−CD127+CRTH2+ innate lymphoid cell in sinus mucosal tissue which we now know are ILC2s as a source of IL-13 when stimulated with either IL-25 or IL-33 in conjunction with IL-2 [17]. Building on this finding, we showed that ILC2 levels are elevated in polyp tissue, and found that although mast cells and T helper 2 cells can potentially secrete IL-13 in response to IL-33, ILC2s were the primary source of IL-13 in response to IL-33 stimulation [18]. Other groups have confirmed an increased presence of ILC2 in polyp tissue and demonstrated that these cells were also a source for IL-5, another type 2 cytokine [19•]. Finally, elevated levels of ILC2 are associated with higher tissue and peripheral eosinophil levels in CRSwNP, supporting a possible role of ILC2 in the activation and viability of eosinophils [20]. Characterization of surface receptors on ILC2s shows expression of receptors for IL-25, IL-33, and TSLP [21]. Taken together, ILC2s in nasal polyps can respond to IL-33, IL-25, and TSLP released rapidly from stimulated respiratory epithelial cells quicker than T helper 2 counterparts that require antigen stimulation.
Epithelial Cell–Derived Cytokines Regulate ILCs
The increased appreciation of ILCs in the pathophysiology of CRS also highlights the importance of epithelial cell–derived cytokines IL-33, IL-25 and TSLP in CRS. Beyond serving as a physical barrier, the respiratory epithelial cells through expression of cytokines and chemokines can regulate immune responses to various environmental triggers such as viruses, bacteria, fungi, and allergens. Initially linked by their promotion of type 2 cytokines from Th2 cells and localization of IL-33, IL-25, and TSLP receptors on ILCs, several immune effector cells and epithelial cells in addition to T helper cells broadened the role of these cytokines to the orchestration of both the innate and adaptive immune responses.
IL-33 was first described in 2005 as the ligand to the orphan ST2 receptor [22]. ST2 is a transmembrane receptor and member of the IL-1 receptor family expressed on several immune cells including mast cells, Th2 cells, eosinophils, and ILCs [23]. A number of different tissues and cells including skin, lung, dendritic cells, and respiratory epithelial cells express IL-33 [22]. Interest in the role of ST2 in the pathophysiology of type 2 chronic inflammatory respiratory diseases developed after genome-wide association studies strongly associated IL-33 and its receptor ST2 with atopic asthma [22, 24]. In CRSwNP, ILC2s are the primary source of IL-13 in response to IL-33 [18]. In turn, the IL-13 promotes goblet cell hyperplasia and mucus production. IL-33 also activates IL-5 production from ILC2s to similar levels as IL-13 [19•]. As compared to IL-25 and TSLP, IL-33 is the most potent epithelial cell cytokine to activate ILC2s to express type 2 cytokines [25•]. In addition, IL-33 expression from respiratory epithelial cells is activated by several triggers including protease-active allergens and molecules associated with tissue damage inducing and supporting the type 2 immune response characteristic of CRSwNP [18, 26, 27].
TSLP and IL-25 are two other epithelial cell–derived cytokines whose expression is upregulated by proteases and have receptors on ILC2s [28]. TSLP and IL-25 expression are upregulated in nasal polyp tissue. The TSLP protein in nasal polyp tissue was initially elusive. TSLP protein is susceptible to cleavage and not detected by typical commercial antibodies. This TSLP fragment is the active form of the cytokine, and its activity is elevated in nasal polyp tissue relative to healthy control tissue [29]. Using isolated ILC2s from human peripheral blood, in vitro studies showed that TSLP was the primary epithelial cytokine that increased viability of ILC2s [25•]. Current studies suggest that IL-25 has the least effect on ILC2s relative to IL-33 or TSLP. Clearly, epithelial cell–derived cytokines are central to the initiation and coordination of the type 2 immune response characteristic of CRSwNP.
Crosstalk Between ILCs and T Helper Cells
The significant overlap in expressed cytokines from the innate and adaptive immunity leads to questions of coordination and division of labor between these two immune responses. As tissue resident cells, ILCs can rapidly respond to environmental triggers. Dendritic cells then process these allergens or microbes, which then activate T cells, but this pathway may take days to mount a reaction. The type 2 immune response highlights the various pathways that coordinate the innate and adaptive immunity as well as means for direct crosstalk.
As discussed above, epithelial cell–derived cytokines are critical in activating ILC2s. These same cytokines, IL-33, IL-25, and TSLP, are also important in completion of the Th2 activation. Naïve CD4+ T cells are primed in lymph nodes by processed antigens and this priming is independent of ILC2. However, once these primed T cells migrate to the inflamed tissues, the milieu of epithelial cell–derived cytokines signals the final process of differentiation of these T cells [30]. In this model, ILC2 does not directly interact with T cells to coordinate the type 2 immune response, but rather the epithelial cell–derived cytokines function to coordinate the innate and adaptive type 2 response. In a different model activated by an epithelial cell–derived cytokine, IL-33 incites not only IL-13 but also tissue-restricted expression of OX40 ligand (OX40L) on ILC2s. The OX40L on ILC2s allows direct interaction with OX40 expressed on dendritic cells to support expansion and differentiation of Th2 cells [31], and an adaptive Type 2 immune response triggered by IL-33 is dependent on OX40L expression on ILC2s [32•].
In more direct crosstalk between ILCs and T cells, major histocompatibility complex class II (MHCII) expression on some ILC2s provide a direct bridge between ILC2s and T cells. During an antihelminthic immune response, the depletion of ILC2s resulted in an impaired Th2 response. The MHCII-expressing ILC2s interact with antigen-specific T cells to promote IL-2 production from T cells, which promote ILC2 proliferation, and concurrently, the interaction is necessary to support T cell proliferation [33]. In addition, MHCII-expressing ILC2s can interact with naïve CD4+ cells to induce differentiation towards Th2 versus Th1 cells [34].
Taken together, there is clear coordination of the innate and adaptive immune response and, in some type 2 immune responses, the crosstalk between ILCs and T cells is critical.
Conclusion
In a relatively short time since they were first recognized, much has been learned about ILCs. Although there is significant overlap with their T helper cell counterparts, ILCs express other molecules that highlight some unique roles. Current data suggest that the adaptive immunity is sufficient to maintain immune homeostasis. However, ILCs are actively involved in several chronic inflammatory diseases including CRS. As such, they present a possible future therapeutic target for these diseases. In addition, the coordination of a type 2 immune responses by epithelial cell–derived cytokines represents other future therapeutic targets. As ILCs clearly play a role in the pathophysiology of CRS, future research areas to explore include the plasticity of ILCs and its implication along with the interaction of the innate and adaptive immune response.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Rudmik L. Economics of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2017;17(4):20.
Kamil A, Ghaffar O, Lavigne F, Taha R, Renzi PM, Hamid Q. Comparison of inflammatory cell profile and Th2 cytokine expression in the ethmoid sinuses, maxillary sinuses, and turbinates of atopic subjects with chronic sinusitis. Otolaryngol Head Neck Surg. 1998;118(6):804–9.
Daines SM, Orlandi RR. Inflammatory cytokines in allergy and rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18(3):187–90.
Mjösberg J, Spits H. Human innate lymphoid cells. J Allergy Clin Immunol. 2016;138(5):1265–76.
• Vély F, Barlogis V, Vallentin B, Neven B, Piperoglou C, Ebbo M, et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol. 2016;17(11):1291–9 This prospective observational study follows a small cohort of SCID patients before and after hematopoietic stem cell transplantation, which reconstituted the T cells without much effect on the ILC population. With follow-up from 7 to 39 years, there were no increase in susceptibility to diseases as compared to control cohort with intact T cell and ILC populations. This suggests that for immune homeostasis, ILCs may be redundant to T cells.
Weizman OE, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C, et al. ILC1 confer early host protection at initial sites of viral infection. Cell. 2017;171(4):795–808.
Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–74.
Peters CP, Mjösberg JM, Bernink JH, Spits H. Innate lymphoid cells in inflammatory bowel diseases. Immunol Lett. 2016;172:124–31.
Forkel M, Mjosberg J. Dysregulation of group 3 innate lymphoid cells in the pathogenesis of inflammatory bowel disease. Curr Allergy Asthma Rep. 2016;16(10):73.
Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14(3):221–9.
Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198. e1–4.
Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63.
Kim HY, Umetsu DT, Dekruyff RH. Innate lymphoid cells in asthma: will they take your breath away? Eur J Immunol. 2016;46(4):795–806.
Endo Y, Hirahara K, Iinuma T, Shinoda K, Tumes DJ, Asou HK, et al. The interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity. 2015;42:294–308.
Dietz CP, Luong A. Innate lymphoid cells: the innate counterpart to T helper cells. Adv Otorhinolaryngol. 2016;79:58–68.
Dietz CJ, Yadav A, Fakhri S, Citardi MJ, Luong A. Innate lymphoid cell distributions in chronic rhinosinusitis subtypes. Communication presented at the Cell-VIB-Symposia: the multifaceted roles of type 2 immunity held in Bruges, Belgium. 2014
Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–62.
Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188(4):432–9.
• Tojima I, Kouzaki H, Shimizu S, Ogawa T, Arikata M, Kita H, et al. Group 2 innate lymphoid cells are increased in nasal polyps in patients with eosinophilic chronic rhinosinusitis. Clin Immunol. 2016;170:1–8 Using flow cytometry analysis, the authors analyzed the prevalence of ILC2s within inflamed sinus mucosa and peripheral from healthy controls, eosinophilic, and non-eosinophilic CRSwNP and CRSsNP patients. They confirmed an elevated prevalence of ILC2s in CRSwNP but showed a correlation of elevated ILC2s and eosinophilic CRSwNP. This correlation suggests a role of ILC2s in support and recruitment of eosinophils to sinus mucosa.
Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015;45(2):394–403.
Roediger B, Weninger W. Group 2 innate lymphoid cells in the regulation of immune responses. Adv Immunol. 2015;125:111–54.
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90.
Chen WY, Tsai TH, Yang JL, Li LC. Therapeutic strategies for targeting IL-33/ST2 signalling for the treatment of inflammatory diseases. Cell Physiol Biochem. 2018;49(1):349–58.
Torgerson DG, Ampleford EJ, Chiu GY, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43:887.
• Camelo A, Rosignoli G, Ohne Y, Stewart RA, Overed-Sayer C, Sleeman MA, et al. IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv. 2017;1(10):577–89 In this study, human ILC2s were isolated and cultured in vitro to dissect the individual and combined effects of IL-33, IL-25, and TSLP on ILC2s. In addition, these studies observed the plasticity of ILC2s.
Kouzaki H, Matsumoto K, Kikuoka H, Kato T, Tojima I, Shimizu S, et al. Endogenous protease inhibitors in airway epithelial cells contribute to eosinophilic chronic rhinosinusitis. Am J Respir Crit Care Med. 2017;195(6):737–47.
Paris G, Pozharskaya T, Asempa T, Lane AP. Damage-associated molecular patterns stimulate interleukin-33 expression in nasal polyp epithelial cells. Int Forum Allergy Rhinol. 2014;4(1):15–21.
Poposki JA, Klingler AI, Stevens WW, Peters AT, Hulse KE, Grammer LC, et al. Proprotein convertases generate a highly functional heterodimeric form of thymic stromal lymphopoietin in humans. J Allergy Clin Immunol. 2017;139(5):1559–67.
Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132(3):593–600.
Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang HE, Pollack JL, et al. A tissue checkpoint regulates type 2 immunity. Nat Immunol. 2016;17(12):1381–7.
Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG, et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17(1):57–64.
• Halim TYF, Rana BMJ, Walker JA, Kerscher B, Knolle MD, Jolin HE, et al. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity. 2018;48(6):1195–207 This study demonstrated that IL-33 incited tissue-restricted expression of OX40L on ILC2s, which resulted in expansion of T helper and Treg cells. In a mouse model of parasitic infection using Nippostrongylus brasiliensis, abolishing OX40L ILC2s resulted in an inability to mount an effective Th2 and Treg cell response. This demonstrated the importance of the crosstalk between ILC2s and adaptive immune response.
Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41(2):283–95.
Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192(5):2442–8.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
Patients undergoing medically indicated functional endoscopic sinus surgery (FESS) were consented to having sinonasal tissue, which was removed as a standard of their surgery, collected and analyzed for studies shown in Fig. 2. The Institutional Review Board at the University of Texas Health Science Center at Houston approved the study protocol.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Rhinosinusitis
Rights and permissions
About this article
Cite this article
Luong, A.U., Sun, H. & Yao, W.C. Contributions of Innate Lymphoid Cells in Chronic Rhinosinusitis. Curr Allergy Asthma Rep 19, 28 (2019). https://doi.org/10.1007/s11882-019-0861-7
Published:
DOI: https://doi.org/10.1007/s11882-019-0861-7