Abstract
Objective
Chronic rhinosinusitis (CRS) is a complicated disease with several variants caused by different cellular and molecular mechanisms. The characterization of this heterogeneity supports the definition that the disease consists of many endotypes, such as eosinophilic and neutrophilic CRS, and so on. This study aimed to explore group 2 innate lymphoid cells (ILC2s) in neutrophilic CRS without nasal polyps (CRSsNP) and with nasal polyps (CRSwNP), and evaluate ILC2s across characteristics of the disease.
Methods
Nasal biopsy samples were obtained from normal subjects or subjects with CRSsNP or CRSwNP during surgery. ILC2s were sorted and purified as CD45+Lin−CD127+CD4−CD8−CRTH2+CD161+ cells through flow cytometry, and were compared among three groups of subjects. Then, these samples were cultured in vitro, and inflammatory factors were assessed in tissue cultures. After that, human recombinant (rm) interleukin (IL)-33 or IL-17 were administered into the cultures, and we again examined relevant inflammatory substances.
Results
ILC2s were upregulated in neutrophilic CRSsNP and CRSwNP patients, and there were no statistical differences between them. Eosinophil cation protein (ECP), myeloperoxidase (MPO), IL-25, IL-33, IL-5, IL-13, interferon (IFN)-γ and IL-17 were increased in the cultures, however, only concentrations of MPO, IFN-γ and IL-17 were enhanced in CRSwNP tissues compared to CRSsNP ones. After administration of rmIL-33, ECP, IL-5 and IL-13 were all increased in tissues from CRSsNP and CRSwNP patients, however, there were no significant differences between them. Finally, we evaluated concentrations of several above inflammatory factors after the treatment of rmIL-17, and found that MPO and IFN-γ were enhanced in these two phenotypes of patients, and were elevated significantly in CRSwNP tissue cultures.
Conclusion
These findings show that ILC2s might be inactivated in neutrophilic CRSsNP and CRSwNP based on this pilot study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of chronic rhinosinusitis (CRS) ranges from approximately 1–9% of the general population [1]. Studies show that the prevalence of CRS is to be 10.9% in Europe and 12.5% in USA [2]. In mainland China, the disease affects approximately 8% population [3]. CRS is defined as inflammation of the nose and the paranasal sinuses characterized by two or more symptoms, one of which should be either nasal blockage or nasal discharge. Other symptoms may be facial pain/pressure, reduction or loss of smell, or both [1].
CRS is a complex disease including several disease variants with different underlying mechanisms. It is generally accepted that there are clinically relevant CRS phenotypes defined by observable characteristics, such as CRS without nasal polyps (CRSsNP) or with NP (CRSwNP). However, clinical phenotypes do not present full insight into the underlying cellular and molecular pathophysiologic mechanisms of CRS. The characterization of this heterogeneity supports the definition that the disease consists of many endotypes, such as eosinophilic and neutrophilic CRS, and other biological endotypes, which are defined by different underlying pathophysiologies that may be identified by corresponding molecular biomarkers [4].
Group 2 innate lymphoid cells (ILC2s) have been identified as a major source of interleukin (IL)-5 and IL-13, in response to IL-25 and IL-33 expression [5]. ILC2s have been identified in Th2-type inflammatory diseases, and shown to mediate helminth expulsion in the intestine [6], airway hyperreactivity [7,8,9], obstruction in asthma [10] and influenza infection [11]. Whilst ILC2s have now been widely investigated in murine models, human studies remain limited. Several researches indicated that these cell types are defined by expressions of biomarker CRTH2 and CD161 [12], and the transcription factor GATA-binding protein 3 (GATA3) [13], however, their functional significance in Th2-mediated disease in humans is not clearly understood. An elegant study indicated the elevation of ILC2s in patients with CRSwNP, and concluded that ILC2s may drive NP formation in CRS, and may be linked with high tissue and blood eosinophilia and have a potential role in the activation and survival of eosinophils during the Th2 immune response [14]. Unfortunately, that study only investigated eosinophilic CRS, and barely involved the neutrophilic endotype of CRS, which characterized the main endotype of Chinese patients [15]. In mainland China, only a minority of NP tissues are Th2 biased, whereas the majority express IFN-γ, Th17, or other neutrophil-related cytokines [16].
Therefore, we performed this current study. Here, we aimed to explore ILC2s in neutrophilic CRSsNP and CRSwNP patients. Then, we cultured tissue samples in vitro, and assessed inflammatory factors in cultures. After that, we administered human recombinant (rm) IL-33 or rmIL-17 into the cultures, and again examined relevant inflammatory substances.
Methods
Study population
The normal group (normal mucosa) consisted of samples collected from the inferior turbinates of 8 subjects (3 men and 5 women), aged between 32 and 55 years (mean age 45.45 years), undergoing nasal septoplasty-inferior turbinoplasty because of the clinical symptom of nasal blockage. Ethmoid sinus mucosa removed during the course of endoscopic sinus surgery was collected from 11 patients (5 men and 6 women), aged between 27 and 61 years (mean age 46.13 years), who had CRSsNP and were referred to the Department of Otorhinolaryngology-Head and Neck Surgery, Huashan Hospital of Fudan University, Shanghai, China. Polyp tissues were obtained by functional endoscopic sinus surgery from 11 patients (7 men and 4 women), aged between 33 and 64 years (mean age 46.36 years), who had CRSwNP and were referred to the Department of Otorhinolaryngology-Head and Neck Surgery, Huashan Hospital of Fudan University, Shanghai, China. The diagnosis of CRSsNP or CRSwNP was confirmed according to European Position Paper on Rhinosinusitis and Nasal Polyps 2012 [1]. Patients were classified as CRSwNP or CRSsNP based on the presence of NP on pre-surgery endoscopy, and categorized as eosinophilic or neutrophilic CRS in accordance with their histopathological parameters. All patients had not taken any medication for at least 1 month before operation. The atopic status of them was assessed according to skin reactivity to house dust mite (HDM) and other 12 common airborne allergens on skin prick test (SPT). The CRSsNP or CRSwNP patients who only had the positive result on SPT to HDM were included. In this study, all the normal subjects had a negative result, and 3 patients with CRSsNP and 4 patients with CRSwNP had a positive result. The reaction to the SPT was considered positive if the wheal area caused by HDM was larger than 7 mm2 (diameter > 3 mm). The exclusion criteria of asthma were based on history, physical examination and lung function test such as a reduction in forced expiratory volume in 1 s and/or peak expiratory flow during the attacks. Other exclusion criteria included history of allergic fungal sinusitis, cystic fibrosis, aspirin intolerance, immunodeficiency, Churg-Strauss syndrome, coagulation disorder and pregnancy, and current use of topical steroid, oral steroid, antihistamines or antibiotics. This investigation was approved by the Ethical Committee of Huashan Hospital of Fudan University (no. 2018-057), and signed informed consent was obtained from all patients.
Sample preparation
Samples from inferior turbinates, ethmoid sinus and NP were obtained and cut into three portions, respectively: one was for confocal immunofluorescence microscopic analysis, one was analyzed by flow cytometry and one was cultured for treatments in vitro.
Immunocytochemistry and confocal microscopy
Sections were incubated overnight at room temperature with primary antibodies against eosinophil cation protein (ECP) (MyBioSource, Inc., San Diego, CA, USA) or myeloperoxidase (MPO) (MyBioSource, Inc., San Diego, CA, USA). After rinsing with phosphate-buffered saline (PBS) (3 × 5 min), samples were incubated 1 h at room temperature with allophycocyanin (APC) or fluorescein isothiocyanate (FITC)-conjugated secondary antibodies in the darkness. Coverslips were then washed twice with PBS-T before nucleus staining with 10 mg/mL 4′, 6-Diamidino-2-phenylindole dihydrochloride (DAPI) for 5 min at room temperature. Finally, the coverslips were washed three times by PBS-T and twice by PBS before mounting with Vectashield (Vector Laboratories,Burlingame, CA, USA). As negative controls, cell samples were incubated either with the secondary antibody alone or with the primary antibody preabsorbed with a specific blocking peptide (1:5 w/w). Images were obtained with a Leica TCS SP5 Confocal Laser Scanning Microscope by the Leica confocal software (LCS) 2.61 (LeicaMicrosystems, Wetzlar, Germany). Images were analyzed by LCS Lite (Leica) software. To enable comparison, all images were acquired using the same parameters of laser power and photomultiplier sensitivity. Images shown are representative of at least three separate experiments in each condition, and were processed with identical values for contrast and brightness. Numbers of infiltrating eosinophils and neutrophils were determined microscopically in a blinded manner at a high power field (HPF) of 400 × magnification. Neutrophilic CRS including CRSsNP and CRSwNP was classified as < 10 eosinophils/HPF histopathologically.
Tissue cell preparation
Tissues were minced using sterile scissors to approximately less than 2-mm pieces and digested with 200 µL of 200 U/mL collagenase type III (Sigma–Aldrich, St. Louis, MO, USA) and 200 µL of 200 µg/mL DNase type IV (Sigma–Aldrich, St. Louis, MO, USA) in 1.6-mL tissue culture medium, and then incubated at 37 °C, shaking at 110 rpm for 1 h, with manual shaking every 20 min to dissociate any tissue clumping. Digested tissues were passed through a 70 µm Falcon cell strainer. Red blood cells were lysed in Tris-buffered ammonium chloride solution (0.83% NH4Cl and 20 mM Tris/Cl).
Flow cytometry analysis of ILC2s
Tissue cell suspensions from normal, CRSsNP or CRSwNP subjects were centrifuged for 10 min at 200×g at 4 °C, and cells were harvested for flow cytometry analysis, as described elsewhere [14]. These cells were washed with 200 µL flow cytometry medium (FCM, 1% BSA and 0.1% sodium azide in PBS) and incubated with 50 µL rabbit serum for 5 min at room temperature to block the Fcγ receptor. These cells then were washed three times with FCM, and stained with 50 µL of monoclonal antibodies against the following surface molecules at 4 °C for 30 min at 1 × 108 cells/mL: Alexa Fluor (AL)-647-conjugated anti-CRTH2; AL-700-conjugated anti-CD4; APC-Cy7-conjugated anti-CD45; FITC-conjugated lineage cocktail (consisting of CD3, CD14, CD16, CD19, CD20, CD56); FITC-conjugated anti-Epcam; Brilliant Violet (BV)-421-conjugated anti-CD127; Horizon-V500-conjugated anti-CD8; phycoerythrin (PE)-conjugated anti-CD161; and PE-Cy7-conjugated anti-CD25. All antibodies purchased from BioLegend, San Diego, CA, USA or MyBioSource, Inc., San Diego, CA, USA. They were washed again and incubated with 50 µL secondary antibody (biotin-conjugated rabbit anti-mouse IgG; Dako Denmark A/S, Glostrup, Denmark) followed by 50 µL R-phycoerythrin-conjugated streptavidin (RPE-streptavidin; Dako Denmark A/S, Glostrup, Denmark). After staining, the cells were resuspended in staining buffer containing DAPI for the exclusion of dead cells. Controls for single color analysis were carried out by incubating cells with a primary antibody of the same isotype, but of irrelevant specificity at matched concentrations followed by secondary reagents, as described above. The samples were collected on an LSR II flow cytometer equipped with FACSDIVA software (BD Biosciences, Mountain View, CA) and analyzed with FlowJo version 10 (Tree Star, Inc., Ashland, OR). Set-up of the instrument was carried out visually, and selection of the isotype control antibody was in accordance with the primary antibodies’ host species, isotype, and conjugation format. ILC2s were identified as CD45+Lin−CD127+CD4−CD8−CRTH2+CD161+ through the gating strategy.
Organ culture of tissues
Tissues from normal, CRSsNP or CRSwNP subjects were cultured using an air–liquid interface method [17]. Blades were used to cut the previously described tissues into 2- to 3-mm3 pieces under sterile conditions. Tissue fragments were washed 3 times with PBS containing an antimycotic (5 µg/mL fungizone) and an antibiotic (300 µg/mL penicillin G) and then were rinsed with 98% Dulbecco’s minimum essential medium (DMEM) supplemented with 10% calf serum and gentamicin (20 µg/mL). To determine the effects of rmIL-33 or rmIL-17 treatment, the above tissues were next saturated for 1 h in culture medium (DMEM + 10% calf serum + 10 µg/mL gentamicin) in the presence of 100 ng/mL of rmIL-33 (MyBioSource, Inc., San Diego, CA, USA) or 100 ng/mL of rmIL-17 (MyBioSource, Inc., San Diego, CA, USA). Then the tissues were placed on hydrated 1 × 1-cm gelatin sponge with the mucosa facing upward and the submucosa downward.
Enzyme-linked immunosorbent assay (ELISA) analysis
IL-5 in the cultures was measured using a 96-well microplate (Corning Inc., New York, NY, USA) coated with 1 µg/mL IL-5 antibody (MyBioSource, Inc., San Diego, CA, USA), and incubated overnight at 4 °C. After washing, the microplate was incubated in 3% bovine serum albumin (Calbiochem, La Jolla, Calif., USA) at 37 °C for 1 h. Samples at 1:10 dilution were then added, followed by incubation at 37 °C for 1 h. After washing, IL-5 antibody that had been biotinylated using a biotinylation kit (American Qualex International Inc., San Clemente, Calif., USA) was then added at 1 µg/mL, and allowed to incubate at 25 °C for 1 h. After washing, 1.5 µg/mL of streptavidin peroxidase was added followed by incubation at 25 °C for 1 h. After washing, tetramethylbenzidine (TMB) substrate [12.5 mL citric phosphate buffer, 200 µL of TMB stock solution (6 mg/mL in dimethyl sulfoxide), 100 µL 1% H2O2] was added to produce a color reaction. The reaction was terminated by the addition of 6 N H2SO4. The optical density was determined at 450 nm using a microplate reader (MTP-32; Corona Electric, Ibaraki, Japan). Concentrations of ECP, MPO, IL-25, IL-33, IL-13, IFN-γ and IL-17 in the cultures were evaluated using the corresponding ELISA kits (all purchased from MyBioSource, Inc., San Diego, CA, USA).
Statistical analysis
Statistical analysis was performed using a commercially available statistical software, Prism 6.0 (GraphPad Software Inc, San Diego, California). Kruskal–Wallis test was performed for comparisons between patient groups. A nonparametric Mann–Whitney test was applied as the initial Kruskal–Wallis test was significant. P < 0.05 was considered statistically significant.
Results
Patient characteristics
Thirty patients were included in the study. Patient characteristics are summarized in Table 1. There was no significant difference in age, gender, allergy, asthma and other status between the three study groups.
Infiltrating eosinophils and neutrophils increase in CRSsNP and CRSwNP
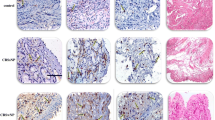
To evaluate the endotype of CRS patients in the present study, we performed confocal immunofluorescence microscopic analysis. The results indicated that counts of eosinophils and neutrophils in CRSsNP and CRSwNP increased compared to normal mucosa (Fig. 1). In addition, there were no statistical differences between CRSsNP and CRSwNP patients (Fig. 1M). However, infiltrating neutrophils in CRSwNP were enhanced in comparison with CRSsNP (Fig. 1N). The findings show the characteristic of neutrophilic endotype in CRSwNP patients in mainland China, as described by other publications [16].
Confocal immunofluorescence microscopic analysis of infiltrating eosinophils and neutrophils in normal subjects (n = 8), chronic rhinosinusitis without nasal polyps (CRSsNP) (n = 11) and chronic rhinosinusitis with nasal polyps (CRSwNP) patients (n = 11). a Eosinophil cation protein (ECP) in eosinophils (allophycocyanin) from normal subjects. b Myeloperoxidase (MPO) in neutrophils (fluorescein isothiocyanate) from normal subjects. c ECP and MPO in eosinophils and neutrophils (merge) from normal subjects. d ECP and MPO in eosinophils and neutrophils [4′, 6-Diamidino-2-phenylindole dihydrochloride (DAPI)] from normal subjects. e ECP in eosinophils (allophycocyanin) from CRSsNP patients. f MPO in neutrophils (fluorescein isothiocyanate) from CRSsNP patients. g ECP and MPO in eosinophils and neutrophils (merge) from CRSsNP patients. (H) ECP and MPO in eosinophils and neutrophils (DAPI) from CRSsNP patients. i ECP in eosinophils (allophycocyanin) from CRSwNP patients. j MPO in neutrophils (fluorescein isothiocyanate) from CRSwNP patients. k ECP and MPO in eosinophils and neutrophils (merge) from CRSwNP patients. l ECP and MPO in eosinophils and neutrophils (DAPI) from CRSwNP patients. m Numbers of eosinophils. n Numbers of neutrophils. Scale bars: 10 µm. Original magnification: A, B, C, D, E, F, G, H, I, J, K, L × 400. Normal normal subjects. CRSsNP CRSsNP patients. CRSwNP CRSwNP patients **p < 0.01 vs CRSsNP
ILC2s arise in CRSsNP and CRSwNP
ILC2s represent a recently discovered cell population which has been implicated in driving Th2 inflammation in CRS. However, their function in this disease has yet to be investigated. In the current study, we identified ILC2s as CD45+Lin−CD127+CD4−CD8−CRTH2+CD161+ through flow cytometry analysis based on previous procedures [14]. We found ILC2s arised in CRSsNP and CRSwNP patients when compared to normal subjects (Fig. 2). However, there were no significant differences between tissue samples from CRSsNP and CRSwNP (Fig. 2d, e). The results show that airway epithelium may regulate innate Th2-type reactions in response to the stimulation of antigen through releases of IL-25 and IL-33 on the basis of the endotype of CRS [12, 17].
ILC2s-derived from tissue samples of normal subjects (n = 8), chronic rhinosinusitis without nasal polyps (CRSsNP) (n = 11) and chronic rhinosinusitis with nasal polyps (CRSwNP) patients (n = 11). a ILC2s were identified as CD45+Lin−CD127+CD4−CD8−CRTH2+CD161+ in normal subjects. b ILC2s were identified as CD45+Lin−CD127+CD4−CD8−CRTH2+CD161+ in CRSsNP patients. c ILC2s were identified as CD45+Lin−CD127+CD4−CD8−CRTH2+CD161+ in CRSwNP patients. d Numbers of ILC2s. e Percentage of ILC2s. Normal normal subjects, CRSsNP CRSsNP patients, CRSwNP CRSwNP patients **p < 0.01 vs CRSsNP
Th1-type inflammation is caused in neutrophilic CRSsNP and CRSwNP
To evaluate cellular functions of ILC2s in neutrophilic CRSsNP and CRSwNP, inflammatory factors were assessed in the cultures using ELISA. ECP, MPO, IL-25, IL-33, IL-5, IL-13, IFN-γ and IL-17 were all increased in tissue cultures from CRS patients compared to normal mucosa (Fig. 3). Additionally, there were no significant differences in contents of ECP, IL-25, IL-33, IL-5 and IL-13 between two types of CRS (Fig. 3a, c–f). However, productions of MPO, IFN-γ and IL-17 were elevated in CRSwNP tissue cultures (Fig. 3b, g, h). This result suggest that Th1-type inflammation is initiated in neutrophilic CRSsNP and CRSwNP [18]. More importantly, the data also indicate that ILC2s might be in an inactive state in the neutrophilic endotype of CRSsNP and CRSwNP, and might promote Th2 inflammation in eosinophilic CRS including CRSsNP and CRSwNP phenotypes [14].
Inflammatory mediators in tissue cultures from normal subjects (n = 8), chronic rhinosinusitis without nasal polyps (CRSsNP) (n = 11) and chronic rhinosinusitis with nasal polyps (CRSwNP) patients (n = 11). a Eosinophil cation protein (ECP) in the cultures. b Myeloperoxidase (MPO) in the cultures. c Interleukin (IL)-25 in the cultures. d IL-33 in the cultures. e IL-5 in the cultures. f IL-3 in the cultures. g interferon (IFN)-γ in the cultures. h IL-17 in the cultures. Normal normal subjects, CRSsNP CRSsNP patients, CRSwNP CRSwNP patients **p < 0.01 vs CRSsNP; ****p < 0.0001 vs CRSsNP
ILC2s are activated after rmIL-33 administration in vitro
Normally, ILC2s were inactivated in neutrophilic inflammation of CRS [18]. To verify whether these cell types could be activated in Th1-biased inflammatory condition, we examined ECP, MPO, Th2-type and Th1-type cytokines after rmIL-33 administration in vitro. The study illustrated that ECP, IL-5 and IL-13 were upregulated in response to rmIL-33 in CRSsNP (Fig. 4f, h, i) and CRSwNP (Fig. 4k, m, n) cultures. However, MPO and IFN-γ were not changed statistically in the two endotypes of CRS (Fig. 4g, j, l, o). Furthermore, all above inflammatory mediators were not increased in normal mucosa of inferior turbinates (Fig. 4a–e). Notably, there were no statistical differences between CRSsNP and CRSwNP groups in ECP, IL-5 and IL-13 productions (Fig. 4p–s). Therefore, we conclude that ILC2s can be activated by rmIL-33 administration in vitro, and we can hypothesize that nasal epithelium might regulate the type 1 or 2-biased inflammations through releases of inflammatory substances based on its activation by external stimuli.
Inflammatory mediators after human recombinant interleukin (IL)-33 treatment in tissue cultures from normal subjects (n = 8), chronic rhinosinusitis without nasal polyps (CRSsNP) (n = 11) and chronic rhinosinusitis with nasal polyps (CRSwNP) patients (n = 11). a–e Eosinophil cation protein (ECP), myeloperoxidase (MPO), IL-5, IL-13 and interferon (IFN)-γ in the cultures from normal subjects, respectively. f–j ECP, MPO, IL-5, IL-13 and IFN-γ in the cultures from CRSsNP patients, respectively. k–o ECP, MPO, IL-5, IL-13 and IFN-γ in the cultures from CRSwNP patients, respectively. p–t ECP, MPO, IL-5, IL-13 and IFN-γ in the cultures, respectively. Normal normal subjects, IL-33-treated IL-33-treated tissue samples, CRSsNP CRSsNP patients, CRSwNP CRSwNP patients **p < 0.01 vs CRSsNP; ****p < 0.0001 vs IL-33-treated or CRSsNP
Th1-type inflammation is exacerbated in neutrophilic CRSsNP and CRSwNP after rmIL-17 administration in vitro
Chronic sinus diseases can be differentiated by the measurement of inflammatory mediators [19]. Neutrophilic inflammation of CRS is characterized by overexpressions of MPO, IFN-γ and IL-17 [20]. To estimate relationships between Th1-type and Th2-type inflammation, we carried out the investigation to confirm the response of ILC2s to rmIL-17. After rmIL-17 administration in the cultures, ECP, IL-5 and IL-13 were not changed significantly whether in CRSsNP (Fig. 5f, h, i) or in CRSwNP (Fig. 5k, m, n). However MPO and IFN-γ were elevated greatly in the two endotypes of CRS (Fig. 5g, j, l, o). Furthermore, all above inflammatory mediators were not enhanced in normal mucosa tissues (Fig. 5a–e). Not surprisingly, productions of MPO and IFN-γ were upregulated remarkably in NP tissues after IL-17 treatment (Fig. 5q, t). The increase of neutrophil infiltrate in CRSwNP vs CRSsNP contributed to the further aggregation of Th1-type inflammation. The present study indicates that Th1-type inflammation is exacerbated in neutrophilic CRSsNP and CRSwNP after rmIL-17 administration in vitro.
Inflammatory mediators after human recombinant interleukin (IL)-17 treatment in tissue cultures from normal subjects (n = 8), chronic rhinosinusitis without nasal polyps (CRSsNP) (n = 11) and chronic rhinosinusitis with nasal polyps (CRSwNP) patients (n = 11). a–e Eosinophil cation protein (ECP), myeloperoxidase (MPO), IL-5, IL-13 and interferon (IFN)-γ in the cultures from normal subjects, respectively. f–j ECP, MPO, IL-5, IL-13 and IFN-γ in the cultures from CRSsNP patients, respectively. k–o ECP, MPO, IL-5, IL-13 and IFN-γ in the cultures from CRSwNP patients, respectively. p–t ECP, MPO, IL-5, IL-13 and IFN-γ in the cultures, respectively. Normal normal subjects, IL-17-treated IL-17-treated tissue samples, CRSsNP CRSsNP patients, CRSwNP CRSwNP patients ****p < 0.0001 vs IL-17-treated or CRSsNP
Discussion
According to a proposal for uniform nomenclature, ILCs populations are classified as three subset groups on the basis of their phenotypical and functional characteristics [21]. Group 1 (ILC1s) comprises ILCs that produce IFN-γ [22]. Group 2 comprises ILCs that produce type-2 cytokines including IL‑5 and IL‑13 and are dependent on GATA3 and retinoic acid receptor related orphan receptor-α (RORα) for their development and function [23]. Group 3 (ILC3s) includes all ILC subtypes that produce IL‑17 and/or IL‑22 and depend on the transcription factor RORγt for their development and function [24]. ILC2s are widely concerned about their pathogenicity in CRS recently, however, to our best knowledge, few reports involve functions and cellular activities of ILC1s and ILC3s in airway inflammation.
In current opinions, the underlying pathophysiologic mechanisms of CRS mainly lie in an immune dysfunction of the sinus mucosa, as a disordered interface between the host and the environment, with contributions from environmental damage and antigens [25]. The eosinophilic endotype of CRSsNP and CRSwNP have been correlated to a skewed Th2 immune response through eosinophilic inflammation primarily mediated by IL-5 and IL-13 cytokines [26]. ILC2s promote Th2 inflammation, even in the absence of Th2 cells, which highlights a potential role within the etiological mechanisms of CRS [27,28,29]. An interesting study investigated the role of ILC2s in eosinophilic CRS, but did not refer to the neutrophilic endotype of CRS [14]. Therefore, we conducted a research to examine the function of ILC2s in the pathogenesis of neutrophilic CRS.
Firstly, we confirmed the endotype of CRS including CRSsNP and CRSwNP in the study as a neutrophilic inflammation condition through immunofluorescence microscopic analysis. Then, the data presented in this research showed significantly elevated ILC2 frequencies and percentages in patients with CRSsNP and CRSwNP compared with normal controls, just as described in previous studies [12]. The results affirmed the activation of nasal epithelium in response to environmental damage [30]. As a result, the nasal epithelial cells released IL-25 and IL-33 to activate ILC2s located in the nasal mucosa, which promoted allergic inflammation in the nose and sinus mucosa. IL-33-responsive ILC2s are an important source of IL-13 in CRSwNP [28], and this cytokine regulates the development and function of ILC2s in Th2-mediated inflammatory conditions such as eosinophilic CRSsNP and CRSwNP [31, 32]. The outcomes from our study demonstrated enhancements of IL-25 and IL-33 in the tissue cultures. However, there were no significant differences in contents of IL-25 and IL-33 between two endotypes of CRS. As a consequence, productions of type-2 cytokines IL-5 and IL-13 were also not changed statistically in CRSwNP when compared to CRSsNP. These results implied that airway epithelium might regulate innate Th2-type reactions on the basis of the CRS endotypes. The underlying mechanisms are needed to be investigated further.
To approve whether ILC2s could be activated in Th1 inflammatory condition, we administered rmIL-33 into the cultures. The study displayed that ECP, IL-5 and IL-13 were upregulated in response to rmIL-33 in CRSsNP and CRSwNP. However, MPO and IFN-γ were not increased statistically. Significantly, there were no statistical differences between CRSsNP and CRSwNP groups. Consequently, we deduced that ILC2s can be activated by rmIL-33 in vitro, and nasal epithelium might modulate the type 1- or 2-biased inflammations through releases of inflammatory factors based on its activation by external antigens.
The data also showed that productions of MPO, IFN-γ and IL-17 were elevated in CRSsNP and CRSwNP tissue cultures compared to normal tissues, and these inflammatory mediators were enhanced in CRSwNP vs CRSsNP. This result suggested that Th1-type inflammation was initiated not only in neutrophilic CRSsNP but also in neutrophilic CRSwNP [18], and this type inflammation was more severe in CRSwNP patients because of increased neutrophilia in NP.
IFN-γ (Th1 cytokine) and IL-17 (Th17 cytokine) were revealed to play a significant role in regulating inflammation, modulating airway structural cells and stimulating innate immunity to mediate neutrophil recruitment in asthma and other airway diseases [33]. IFN-γ and IL-17 showed a significant disruption of the epithelial barrier, increased paracellular permeability, which indicated that these two cytokines might contribute to the development of CRS by promoting a leaky mucosal barrier [34, 35].
IL-17A was reported to induce both eosinophilic and neutrophilic inflammation, however, other studies demonstrated that IL-17A-induced inflammation did not influence the development of NP in murine model [36]. Therefore, we further assessed the role of IL-17 in CRSsNP and CRSwNP. After the treatment of rmIL-33, MPO and IFN-γ in cultures were not enhanced statistically in the two endotypes of CRS, which illustrated that type-2 cytokine could not accelerate nasal mucosa inflammation in CRS in the study. That is to say, epithelial cells-derived IL-33 did not contribute to neutrophilic type inflammation in the development of CRS. IL-17 were shown to increase elastase and MPO activity associated with neutrophil recruitment in airways in vivo [37]. Thus we applied IL-17 into the tissue cultures to evaluate the role of Th17 cytokine on Th1 type inflammation in the present study. The results revealed that ECP, IL-5 and IL-13 were not modified significantly whether in CRSsNP or CRSwNP. Nevertheless MPO and IFN-γ were elevated greatly in these endotypes of CRS. Furthermore, expressions of MPO and IFN-γ were upregulated prominently in NP tissues after IL-17 stimulation. The increasing neutrophil infiltrate in CRSwNP vs CRSsNP might lead to the further aggregation of Th1-type inflammation. Hence, this study suggested that IL-17 could exacerbate the neutrophilic inflammation through boosting neutrophils activities.
Conclusion
In conclusion, ILC2s represent a significant new area of research for the understanding of the pathogenesis of eosinophilic type CRS, but did not influence neutrophilic endotype CRSsNP and CRSwNP. It should be emphasized that this is a preliminary analysis in only few patients, and that the relevant significance is derived from an exploratory study. A confirmatory analysis should be further performed based on a larger patient population.
References
Fokkens WJ, Lund VJ, Mullol J et al (2012) European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl 23:3 (p preceding table of contents, 1–298)
Hamilos DL (2011) Chronic rhinosinusitis: Epidemiology and medical management. J Allergy Clin Immunol 128(4):693–707
Shi JB, Fu QL, Zhang H et al (2015) Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy 70(5):533–539
Akdis CA, Bachert C, Cingi C et al (2013) Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 131(6):1479–1490
Moro K, Yamada T, Tanabe M et al (2010) Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463(7280):540–544
Neill DR, Wong SH, Bellosi A et al (2010) Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464(7293):1367–1370
Barlow JL, Bellosi A, Hardman CS et al (2012) Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol 129(1):191–198.e1
Halim TY, Krauss RH, Sun AC, Takei F (2012) Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 36(3):451–463
Kim HY, Chang YJ, Subramanian S et al (2012) Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol 129(1):216–227
Klein Wolterink RG, Kleinjan A, van Nimwegen M et al (2012) Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol 42(5):1106–1116
Monticelli LA, Sonnenberg GF, Abt MC et al (2011) Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12(11):1045–1054
Mjösberg JM, Trifari S, Crellin NK et al (2011) Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 12(11):1055–1062
Mjosberg J, Bernink J, Golebski K et al (2012) The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cell. Immunity 37(4):649–659
Ho J, Bailey M, Zaunders J et al (2015) Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy 45(2):394–403
Zhang N, Liu S, Lin P et al (2010) Remodeling and inflammation in Chinese versus white patients with chronic rhinosinusitis. J Allergy Clin Immunol 125(2):507
Wang X, Zhang N, Bo M et al (2016) Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol 138(5):1344–1353
Fahy JV, Locksley RM (2011) The airway epithelium as a regulator of Th2 responses in asthma. Am J Respir Crit Care Med 184(4):390–392
Derycke L, Eyerich S, Van Crombruggen K et al (2014) Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS ONE 9(6):e97581
Van Zele T, Claeys S, Gevaert P et al (2006) Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61(11):1280–1289
Derycke L, Zhang N, Holtappels G, Dutré T, Bachert C (2012) IL-17A as a regulator of neutrophil survival in nasal polyp disease of patients with and without cystic fibrosis. J Cyst Fibros 11(3):193–200
Spits H, Artis D, Colonna M et al (2013) Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol 13(2):145–149
Gordon SM, Chaix J, Rupp LJ et al (2012) The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 36(1):55–67
Hoyler T, Klose CS, Souabni A et al (2012) The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37:634–648
Takatori H, Kanno Y, Watford WT et al (2009) Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 206(1):35–41
Tan BK, Schleimer RP, Kern RC (2010) Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg 18(1):21–26
Huvenne W, van Bruaene N, Zhang N et al (2009) Chronic rhinosinusitis with and without nasal polyps: what is the difference? Curr Allergy Asthma Rep 9(3):213–220
Miljkovic D, Bassiouni A, Cooksley C et al (2014) Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy 69(9):1154–1161
Shaw JL, Fakhri S, Citardi MJ et al (2013) IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med 188(4):432–439
Walford HH, Lund SJ, Baum RE et al (2014) Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol 155(1):126–135
Ho J, Bailey M, Zaunders J et al (2014) Cellular comparison of sinus mucosa vs polyp tissue from a single sinus cavity in chronic rhinosinusitis. Int Forum Allergy Rhinol 5(1):14–27
Barret NA, Austen KF (2009) Innate cells and T helper 2 cell immunity in airway inflammation. Immunity 31(3):425–437
Spits H, Di Santo JP (2011) The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol 12(1):21–27
Baba S, Kagoya R, Kondo K, Suzukawa M, Ohta K, Yamasoba T (2015) T-cell phenotypes in chronic rhinosinusitis with nasal polyps in Japanese patients. Allergy Asthma Clin Immunol 11:33
Soyka MB, Wawrzyniak P, Eiwegger T et al (2012) Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol 130(5):1087–1096.e10
Ramezanpour M, Moraitis S, Smith JL, Wormald PJ, Vreugde S (2016) Th17 cytokines disrupt the airway mucosal barrier in chronic rhinosinusitis. Mediat Inflamm 2016:9798206
Hong SL, Zhang YL, Kim SW et al (2015) Interleukin-17A-induced inflammation does not influence the development of nasal polyps in murine model. Int Forum Allergy Rhinol 5(5):363–370
Hoshino H, Laan M, Sjöstrand M, Lötvall J, Skoogh BE, Linden A (2000) Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J Allergy Clin Immunol 105(1 Pt 1):143–149
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81371076), and the Shanghai Suburb Tertiary Hospital Clinical Capacity Building Project (Grant No. SHDC12015905).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Lin, L., Wei, J., Chen, Z. et al. Activations of group 2 innate lymphoid cells depend on endotypes of chronic rhinosinusitis. Eur Arch Otorhinolaryngol 275, 3007–3016 (2018). https://doi.org/10.1007/s00405-018-5180-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-5180-4