Abstract
Purpose of Review
This review will cover what is known regarding exosomes and allergy, and furthermore discuss novel mechanism of exosome-mediated immune modulation and metabolic regulation via the transfer of mitochondria.
Recent Findings
Exosomes are nano-sized extracellular vesicles (EVs) derived from the endosome that play a direct role in governing physiological and pathological conditions by transferring bioactive cargo such as proteins, enzymes, nucleic acids (miRNA, mRNA, DNA), and metabolites. Recent evidence suggest that exosomes may signal in autocrine but, most importantly, in paracrine and endocrine manner, being taken up by neighboring cells or carried to distant sites. Exosomes also mediate immunogenic responses, such as antigen presentation and inflammation. In asthma and allergy, exosomes facilitate cross-talk between immune and epithelial cells, and drive site-specific inflammation through the generation of pro-inflammatory mediators like leukotrienes. Recent studies suggest that myeloid cell-generated exosomes transfer mitochondria to lymphocytes.
Summary
Exosomes are nano-sized mediators of the immune system which can modulate responses through antigen presentation, and the transfer of pro- and anti-inflammatory mediators. In addition to conventional mechanisms of immune modulation, exosomes may act as a novel courier of functional mitochondria that is capable of modulating the recipient cells bioenergetics, resulting in altered cellular responses. The transfer of mitochondria and modulation of bioenergetics may result in immune activation or dampening depending on the context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allergy is a multifaceted immunologic disease where our innate and adaptive defense mechanisms become activated by what should be a benign signal, resulting in rampant and deregulated immune responses and chronic inflammation [1]. Many different cell types are involved and they each secrete unique soluble mediators of inflammation that drive disease pathology [1, 2]. Some well described cell types include, but are not limited to, CD4+ T cells (Th2, Th17 and hybrid Th2/Th17 subsets) [3, 4], dendritic cells (DCs) [5, 6], macrophages, myeloid-derived regulatory cells (MDRCs) [6,7,8,9,10,11,12], natural killer (NK) cells [13,14,15], and epithelial cells [5, 16, 17]. For an effective immune system, various signaling mechanisms must come to play. The same is true in the case of allergy, where a coordinated, albeit inappropriate, immune signaling cascade results in persistent inflammation that is harmful for the host. Generally, these signaling cascades are mediated by soluble factors, such as cytokines and chemokines, as well as membrane-bound receptors, such as class-II molecules, the CD1 family of receptors, and Fcε receptor [1, 2]. Class-II molecules, such as HLA-DR, are antigen presentation molecules usually found on antigen presenting cells which play an important role in activating CD4+ T cells through the engagement of the T cell receptor (TCR) [18,19,20]. The CD1 family of receptors is a type of scavenger molecule found on macrophages and dendritic cells that can activate T cells [21,22,23]. These scavenger receptors recognize foreign lipids, such as of bacterial and fungal origin [23, 24]. Fcε receptor is an important player in allergy and is found on mast cells and basophils [25]. This receptor binds free IgE and activates degranulation of mast cells and basophils. All three of these molecules play an important role in activating the immune system, and have been found on exosomes.
Extracellular vesicles (EVs), such as exosomes, are essentially couriers of bioactive material, such as nucleotides, proteins, lipids, and metabolites, which have a substantial impact on the phenotype of the recipient cell [26]. In recent years, exosomes, which are secreted by many types of cells, have emerged as key-signaling mediators in various immunologic diseases [27]. The roles of exosomes pertaining to lung pathology are being increasingly described. In particular, exosomes are being appreciated as immunogenic potentiators especially in the context of allergy [28, 29•, 30, 31, 32•]. Many studies have reported a pro-inflammatory role of exosomes in allergy as well as in asthma [29•, 30, 31, 32•]. Exosomes have been described to transfer pro-inflammatory mediators, such as leukotrienes, and process antigens on surface class-II receptors [29•, 30, 32•, 33]. Similarly in allergic skin diseases, exosomes have been shown to transfer antigens that activate immune responses [30, 34]. In addition to host cell-generated exosomes, microbial EVs have also been implicated in immune activation and hypersensitivity [35,36,37].
In addition to transfer of bioactive materials, recent studies have also described the packaging and transfer of mitochondria via EVs and exosomes [38•, 39••, 40••]. The transfer of mitochondria results in alterations in the host cell bioenergetics and may have lasting consequences on cellular function and tissue homeostasis. For example, our laboratory has observed the transfer of exosomes containing mitochondria by myeloid-derived regulatory cells (MDRCs) and subsequent internalization of these exosomes by CD4+ T lymphocytes [40••]. Together, the discovery of novel exosome-mediated mechanisms in modulating cellular and tissue homeostasis, and the host immune system will help us understand the intricate and complex mechanism of allergic disease pathology, which will indubitably aid in fruitful advances in the research and development of improved therapies. This review intends to explore in detail each of the unique mechanisms by which exosomes modulate immune responses in the context of asthma and other allergic diseases.
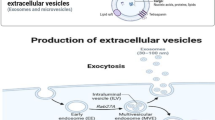
Exosome Biogenesis
Exosomes were first described in the calcification of collagen in the extracellular matrix [41]. Since then, various “blebbings” from cells have been described as extracellular vesicles. The classification and nomenclature to describe EVs have been based on mode of biogenesis and biochemical properties [42, 43]. Currently, exosomes are described as vesicles derived from the endosome and released to the extracellular space [44,45,46,47, 48••]. Tetraspanins are highly enriched in exosomes and are used as reliable markers of exosomes [49]. Tetraspanins are transmembrane proteins which interact with one another and with other transmembrane proteins, such as integrins and receptors, acting as a scaffold to organize surface proteins and support cellular signaling [50]. When studying exosomes, reliable markers used in the field include CD63, CD81, CD9, tumor susceptibility 101 (TSG101), and ALG-2 interacting protein X (ALIX). Specifically, endosomal markers or markers that are part of the endosomal sorting complexes required for transport (ESCRT) complex (such as TSG101, CD81, and ALIX) are preferred as they indicate an endosomal origin of the extracellular vesicle, which is part of the definition of an exosome [49, 51]. The biogenesis of exosomes starts with the outward invagination of the endosome, resulting in the formation of vesicles within the endosomal body, referred to as a multi-vesicular body (MVB) [44]. Although the biogenesis of exosomes results from the invagination of the endosome, the process is described as an “outward invagination” to clarify that the lipid bilayer topology is maintained throughout the biogenesis and secretion process. The MVB can either merge with a lysosome, resulting in the degradation of its cargo, or it can fuse with the cytoplasmic membrane, causing the release of exosomes into the extracellular space. Exosomes are released from various different cell types, and can be isolated from several sources of biological fluids, such as bronchoalveolar lavage (BAL) fluid, synovial fluid, serum, urine, breast milk, and semen [27, 33, 52,53,54,55]. Although the biological functions of these vesicles are still being characterized, and their association with disease being elucidated, exosomes have been thought to be part of a complex intercellular and systemic messaging system, that also play a role in cellular homeostasis via the autophagy pathways [26, 56]. Exosomes impart their effects on recipient cells through receptor interactions or by transfer of bioactive cargo [29•, 30, 57,58,59,60]. Studies have shown that in addition to antigen-specific activation, immune cells can use adhesion molecules to “capture” exosomes [61, 62]. We explore the various mechanisms by which exosomes can modulate the immune system in the following sections.
Exosomes and Inflammation
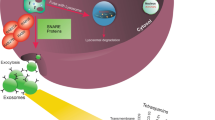
Exosomes have been described as efficient cell-to-cell messengers that can cross biological barriers and modulate the immune system [26, 27, 63,64,65]. Inflammation can be triggered by many mechanisms, such as antigen presentation, cytokines, chemokines, leukotrienes, and other lipid mediators of inflammation. Exosomes have been characterized with several membrane-associated immunogenic markers found on the surface, such as class-I and class-II major histocompatibility complex (MHC) molecules, co-stimulatory molecules (CD86, CD80, and CD54), and even functional enzymes that produce lipid mediators of inflammation [30, 31, 55, 66,67,68].
Antigen loaded exosomes have been demonstrated to induce strong antigen-specific immune responses. Specifically, dendritic cells pulsed with antigens produce exosomes that can activate CD8+ T cells in an antigen-specific manner likely through antigen presentation by MHCI-peptide complexes [69••]. Antigen-loaded exosomes, as well as peptide class-II complexes associated with exosomes, have been found to be attached to the surface of follicular dendritic cells (FDCs). This exosome-mediated transfer of antigens is suggested as a mechanism by which exosomes can promote antigen-specific activation of T and B cells in primary and secondary lymphoid nodes [70]. Furthermore, adhesion of exosomes to the surface of FDCs is through the oligomerization and binding of tetraspanins between the exosomes and FDCs. We speculate that the adhesion of exosomes may be facilitated by adhesion molecules such as CD54 [71]. CD54 (ICAM-1) has been reported by others and our lab to be expressed on exosomes [29•, 33, 72]. Segura et al. have shown that CD8+ dendritic cells use LFA-1 (the ligand for CD54) to capture MHC-peptide complexes from exosomes [61]. Furthermore, Hao et al. have reported that the internalization of exosomes in immune cells may be mediated by CD54/LFA-1 interactions on dendritic cells [73]. Nolte-’t Hoen et al. have also shown that LFA-1 is important for the recruitment of exosomes to T cells and their subsequent activation [62]. Bone marrow-derived mesenchymal stromal cells internalized PC12 pheochromocytoma cell-derived exosomes through clathrin-dependent endocytosis, resulting in delivery of miR-21 [74]. Additionally, endothelial cells have been shown to internalize exosomes via a dynamin-dependent matter through endocytosis [75]. Together, these observations suggest different modes of internalization that may be cell-type specific. Furthermore that the effects imparted by the exosomes are multi-modal (receptor-ligand interaction, or through transfer of cargo).
Exosomes have been found to transfer or even help generate pro-inflammatory lipid mediators. For example, exosomes from human macrophages and dendritic cells contain enzymes for the biosynthesis of leukotrienes and promote migration of granulocytes [68]. Furthermore, pulmonary epithelial cell-derived exosomes metabolize myeloid cell-derived leukotriene C4 to leukotriene D4 [76]. In addition to leukotrienes, ceramides and sphingolipids have been found in exosomes and potentially implicated in inflammation [67, 77]. Pro-inflammatory cytokines, such as TNF-α and IFN-γ haven been shown to drive release of ceramides into exosomes, which become mediators of cell death signaling [78]. Additionally, hepatocytes have been shown to also release pro-inflammatory ceramide-enriched extracellular vesicles under stress [79].
Exosomes can package miRNA which have various different functional implications to the target cells which internalize these vesicles, and is often dependent on the context of disease. In one study, serum exosomes from rats treated with zinc oxide nanoparticles were identified with 16 different pro-inflammatory miRNAs [80]. Additionally, miR-155 and miR-146a, two pro-inflammatory miRNAs, were found enriched in exosomes purified from dendritic cells following treatment with endotoxin [60]. From a clinical perspective, a pro-inflammatory miRNA signature was found from serum exosomes isolated from septic patients admitted to the ICU [81]. In asthma, the exosomes from human bronchoalveolar lavage fluid have been found to contain miRNAs with pro-inflammatory signatures [31]. Several miRNA involved in immune modulation, including miR-27 and miR-24, (important for Th2 responses [82]), miR-21 (important for metabolic regulation of pathogenic Th17 [83]), and Let-7c (M2 polarization) were identified in exosomes from asthmatics [84]. This indicates that exosomal transfer of miRNA can modulate gene programing and promote inflammation in an antigen-independent manner.
Novel Mechanisms of Exosome-Mediators
As discussed earlier, exosomes are couriers of various biological cargo with functional effects [26]. In recent years, exosomes and other extracellular vesicles (EVs) have been shown to alter cellular metabolism by transfer of metabolites to recipient cells or by altering regulation of metabolic enzyme pathways [39••, 40••, 85,86,87,88,89,90]. Metabolism has been appreciated beyond fulfilling cellular energy requirements, and is connected to various cellular processing such as epigenetic control [91, 92] and gene regulation [93, 94]. The cellular changes induced by metabolism then may impact at an organismal level, such as in immune response [95, 96], tissue repair [97, 98], and disease pathology [99,100,101]. In particular, the transfer of mitochondria from one cell to another has garnered much attention as a novel mechanism of cellular energetic repair [39••, 85,86,87,88,89,90].
The transfer of mitochondria from one cell to another has been previously described through a structural mechanism called tunneling nanotubes (TNTs) (Fig. 1) [102,103,104,105,106]. TNTs are membrane nanotube protrusions that extend from the plasma membrane and bridge the cytoplasm of two cells over a distance [103, 105]. Jackson et al. have shown that TNTs are important for the transfer of functional mitochondria from mesenchymal stem cells (MSCs) to macrophages to promote antimicrobial functions in in vitro and in vivo models of acute respiratory distress syndrome (ARDS) [107]. Their study demonstrates that the transfer of mitochondria from MSCs to macrophages increases their bioenergetics and phagocytic activity. However, inhibition of TNT formation by cytochalasin B did not completely block intercellular transfer of mitochondria, suggesting an alternative cell-contact independent mechanism.
Transfer of mitochondria from MSCs to macrophages can occur in EVs secreted from MSCs [39••]. Morrison et al. demonstrate that the MSC-derived EVs contain mitochondria, and when transferred to macrophages, promote M2 polarization and enhance oxidative phosphorylation. The authors further demonstrate that functional mitochondria are being transferred by MSCs to macrophages by showing that EVs from rhodamine-treated MSCs, which generate dysfunctional mitochondria, have no effect on macrophages. These results are supported by other studies that illustrate MSC-derived EVs can recapitulate the beneficial effects of cell-based MSC therapies [87,88,89,90].
The ability of cells to release EVs containing mitochondria has been previously described [38•, 86]. Our lab has also reported that exosomes from bronchoalveolar lavage (BAL) fluid of asthmatics and exosomes derived from myeloid-derived regulatory cells from the airways of asthmatics contain mitochondria, which can be internalized by CD4+ T lymphocytes [40••]. We observe that functional mitochondria that are capable of producing ROS are internalized by CD4+ T cells and merge with the host mitochondrial network. Our studies align with published reports that implicate the importance of mitochondria transfer by EVs and their role in altering cellular function in response to injury and inflammation.
The transfer of healthy mitochondria to cells with damaged mitochondria is an important mechanism for cellular repair. Human mesenchymal stem cells (hMSCs) were shown to package healthy mitochondria inside membrane-bound vesicles that were secreted and subsequently acquired by epithelial cells that were co-cultured in vitro [85]. The study shows that when cultured with A549 ρ° (ρ° phenotype lack mitochondrial DNA) that have defective mitochondria, the transfer of mitochondria by hMSC-derived EVs rescued metabolic activity and aerobic respiration in the A549 ρ° cells.
Functional mitochondrial complex proteins have been reported in exosomes, and viable for the generation of ATP [108•]. Panfoli et al. report that hMSCs from > 37-week old, newborns generated exosomes that contained functional complex proteins that were capable of generating ATP while, hMSC from 28 to 30-week old, newborns generated exosomes that were unable to produce ATP despite having mitochondria complex proteins [108•]. They implicate this difference as potential vulnerability factors between newborn and preterm, such as reduced ability to cope with anoxic environments and repair damaged tissue in preterm.
In addition to the transfer of healthy mitochondria, cells may use EVs to package damaged mitochondria as a danger signal to others as a result of disease pathology, and to maintain mitochondrial quality control [109, 110]. Studies have demonstrated that mitochondria can generate vesicles of various types that are shuttled to the lysosome [111] or peroxisome for degradation [112]. This pathway shares the same pathway as exosome generation—through the late endosome and multivesicular body [111]—and thus would not be alarming if these mitochondrially derived vesicles (MDVs) were secreted. Cells that have damaged mitochondria are undergoing cellular stress that may overwhelm or even shutdown mitophagy and autophagy pathways. The unique coincidence that these pathways are shared with exosome generation may suggest an alternative survival mechanism for cells to shed damaged cellular components extracellular while attempting to regain homeostasis. To support this theory, Davis et al. have shown that damaged mitochondria can be transported to adjacent cells to aid in degradation, which they have coined the term transmitophagy [113].
Conclusions
In allergy, exosomes have been shown to activate T cells in an antigen-specific manner without the need of an APC [30]. The activation is most likely through the engagement of MHCII-peptide complexes on the surface of exosomes with the TCR of CD4+ T cells. The modulation of the immune system by exosomes is not limited to surface receptor interactions. Transfer of RNA by exosomes, such as those produced by mast cells, can alter the transcriptomic landscape of the recipient cell, potentially promoting upregulation of pro-inflammatory genes [28, 59, 60]. Together, exosomes can activate T cells and other immune subsets in a multi-modal manner, such as receptor interaction or fusion and cargo transfer, without the aid of traditional APCs.
Furthermore, we gather that the transfer of mitochondria between cells is not an uncommon occurrence and happens in both healthy and diseases states. Furthermore, new evidence suggests that mitochondria can be packaged into extracellular vesicles, such as exosomes, and transported to recipient cells. Importantly, this transfer has the ability to induce functional changes to the recipient cell, implicating the potential for such transfer to be important in cellular homeostasis and disease pathogenesis. Although the exact pathway by which exosome package mitochondria is still unknown, we speculate that two possible mechanisms exist: 1. mitochondrial fission; and 2. mitophagy. Proteins such as Drp1 induce fission of mitochondria that may promote their packaging into exosomes or other types of EVs. Similarly, the mitophagy pathway may shuttle mitochondria through pathways that are shared with exosome biogenesis. These pathways need to be studied in the context of exosome biogenesis and mitochondrial packaging to better understand how cellular organelles, such as the mitochondria, can be packaged and delivered.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–92. https://doi.org/10.1038/nri2254.
Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118(11):3546–56. https://doi.org/10.1172/JCI36130.
Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol. 2008;26:205–32. https://doi.org/10.1146/annurev.immunol.26.021607.090312.
Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134(5):1175–1186.e7. https://doi.org/10.1016/j.jaci.2014.05.038.
Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8(3):193–204. https://doi.org/10.1038/nri2275.
Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18(5):673–83. https://doi.org/10.1038/nm.2731.
Wang Y, Jin TH, Farhana A, Freeman J, Estell K, Zmijewski JW, et al. Exposure to cigarette smoke impacts myeloid-derived regulatory cell function and exacerbates airway hyper-responsiveness. Lab Investig. 2014;94(12):1312–25. https://doi.org/10.1038/labinvest.2014.126.
Deshane J, Zmijewski JW, Luther R, Gaggar A, Deshane R, Lai JF, et al. Free radical-producing myeloid-derived regulatory cells: potent activators and suppressors of lung inflammation and airway hyperresponsiveness. Mucosal Immunol. 2011;4(5):503–18. https://doi.org/10.1038/mi.2011.16.
Deshane JS, Redden DT, Zeng M, Spell ML, Zmijewski JW, Anderson JT, et al. Subsets of airway myeloid-derived regulatory cells distinguish mild asthma from chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015;135(2):413–424.e15. https://doi.org/10.1016/j.jaci.2014.08.040.
Arora M, Poe SL, Ray A, Ray P. LPS-induced CD11b+Gr1(int)F4/80+ regulatory myeloid cells suppress allergen-induced airway inflammation. Int Immunopharmacol. 2011;11(7):827–32. https://doi.org/10.1016/j.intimp.2011.01.034.
Ray P, Arora M, Poe SL, Ray A. Lung myeloid-derived suppressor cells and regulation of inflammation. Immunol Res. 2011;50(2–3):153–8. https://doi.org/10.1007/s12026-011-8230-1.
Arora M, Poe SL, Oriss TB, Krishnamoorthy N, Yarlagadda M, Wenzel SE, et al. TLR4/MyD88-induced CD11b+Gr-1 int F4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol. 2010;3(6):578–93. https://doi.org/10.1038/mi.2010.41.
Souzdaltseva TV, Makarova TV, Vechkanova NN. NK cells and IgE level in peripheral blood in aspirin-induced and allergic bronchial asthma. Russ J Immunol. 2000;5(3):315–9.
Lunding L, Wegmann M. NK cells in asthma exacerbation. Oncotarget. 2015;6(24):19932–3. https://doi.org/10.18632/oncotarget.4841.
Korsgren M. NK cells and asthma. Curr Pharm Des. 2002;8(20):1871–6.
Boushey HA, Fahy JV. Basic mechanisms of asthma. Environ Health Perspect. 1995;103(Suppl 6):229–33.
Nakagami Y, Favoreto S Jr, Zhen G, Park SW, Nguyenvu LT, Kuperman DA, et al. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J Immunol. 2008;181(3):2203–10.
Gay D, Maddon P, Sekaly R, Talle MA, Godfrey M, Long E, et al. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nature. 1987;328(6131):626–9. https://doi.org/10.1038/328626a0.
Cammarota G, Scheirle A, Takacs B, Doran DM, Knorr R, Bannwarth W, et al. Identification of a CD4 binding site on the beta 2 domain of HLA-DR molecules. Nature. 1992;356(6372):799–801. https://doi.org/10.1038/356799a0.
Konig R, Shen X, Germain RN. Involvement of both major histocompatibility complex class II alpha and beta chains in CD4 function indicates a role for ordered oligomerization in T cell activation. J Exp Med. 1995;182(3):779–87.
Moody DB, Young DC, Cheng TY, Rosat JP, Roura-Mir C, O'Connor PB, et al. T cell activation by lipopeptide antigens. Science. 2004;303(5657):527–31. https://doi.org/10.1126/science.1089353.
Mizumoto N, Takashima A. CD1a and langerin: acting as more than Langerhans cell markers. J Clin Invest. 2004;113(5):658–60. https://doi.org/10.1172/JCI21140.
Zajonc DM, Crispin MD, Bowden TA, Young DC, Cheng TY, Hu J, et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity. 2005;22(2):209–19. https://doi.org/10.1016/j.immuni.2004.12.009.
Hardman CS, Chen YL, Salimi M, Jarrett R, Johnson D, Jarvinen VJ, et al. CD1a presentation of endogenous antigens by group 2 innate lymphoid cells. Sci Immunol. 2017;2(18). https://doi.org/10.1126/sciimmunol.aan5918.
Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–80. https://doi.org/10.1016/j.jaci.2009.11.017.
Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–74.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. https://doi.org/10.1083/jcb.201211138.
Sastre B, Canas JA, Rodrigo-Munoz JM, Del Pozo V. Novel modulators of asthma and allergy: exosomes and MicroRNAs. Front Immunol. 2017;8:826. https://doi.org/10.3389/fimmu.2017.00826.
• Torregrosa Paredes P, Esser J, Admyre C, Nord M, Rahman QK, Lukic A, et al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy. 2012;67(7):911–9. https://doi.org/10.1111/j.1398-9995.2012.02835.x Novel pro-inflammatory role of exosomes in allergic asthma.
Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, et al. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120(6):1418–24. https://doi.org/10.1016/j.jaci.2007.06.040.
Levanen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL, et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013;131(3):894–903. https://doi.org/10.1016/j.jaci.2012.11.039.
• Kulshreshtha A, Ahmad T, Agrawal A, Ghosh B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol. 2013;131(4):1194–203, 203 e1–14. https://doi.org/10.1016/j.jaci.2012.12.1565 Novel crosstalk of pro-inflammatory exosomes in allergic airway inflammation between cell types.
Admyre C, Grunewald J, Thyberg J, Gripenback S, Tornling G, Eklund A, et al. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J. 2003;22(4):578–83.
Vallhov H, Gutzeit C, Hultenby K, Valenta R, Gronlund H, Scheynius A. Dendritic cell-derived exosomes carry the major cat allergen Fel d 1 and induce an allergic immune response. Allergy. 2015;70(12):1651–5. https://doi.org/10.1111/all.12701.
Nazimek K, Bryniarski K, Askenase PW. Functions of exosomes and microbial extracellular vesicles in allergy and contact and delayed-type hypersensitivity. Int Arch Allergy Immunol. 2016;171(1):1–26. https://doi.org/10.1159/000449249.
Hong SW, Kim MR, Lee EY, Kim JH, Kim YS, Jeon SG, et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy. 2011;66(3):351–9. https://doi.org/10.1111/j.1398-9995.2010.02483.x.
Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, et al. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses--novel mechanisms for host-microbe interactions in atopic eczema. PLoS One. 2011;6(7):e21480. https://doi.org/10.1371/journal.pone.0021480.
• Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. https://doi.org/10.1038/ncomms9472 Excellent review of potential roles for exosomes in mitophagy and mitochondiral health.
•• Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, DF MA, O'Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–86. https://doi.org/10.1164/rccm.201701-0170OC Novel finding of mitochondrial transfer and crosstalk between cell types via extracellular vesicles.
•• Hough KP, Trevor JL, Strenkowski JG, Wang Y, Chacko BK, Tousif S, et al. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol. 2018;18:54–64. https://doi.org/10.1016/j.redox.2018.06.009 Novel finding of mitochondrial transfer by BAL and MDRC-derived exosomes to CD4 + T cells.
Ali SY, Sajdera SW, Anderson HC. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci U S A. 1970;67(3):1513–20.
Simpson RJ, Mathivanan S. Extracellular microvesicles: the need for internationally recognised nomenclature and stringent purification criteria. J Proteomics Bioinformatics. 2012;05. https://doi.org/10.4172/jpb.10000e10.
Witwer KW, Soekmadji C, Hill AF, Wauben MH, Buzas EI, Di Vizio D, et al. Updating the MISEV minimal requirements for extracellular vesicle studies: building bridges to reproducibility. J Extracell Vesicles. 2017;6(1):1396823. https://doi.org/10.1080/20013078.2017.1396823.
Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5(4):317–23. https://doi.org/10.1038/nrm1360.
Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006;Chapter 3:Unit 3 22. doi:https://doi.org/10.1002/0471143030.cb0322s30, 30, 3.22.1, 3.22.29.
Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79. https://doi.org/10.1038/nri855.
Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–93. https://doi.org/10.1038/nri2567.
•• Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. https://doi.org/10.1038/s41556-018-0250-9 Excellent review on exosome tropism and cellular signaling specificities.
Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. https://doi.org/10.3389/fimmu.2014.00442.
Charrin S, Jouannet S, Boucheix C, Rubinstein E. Tetraspanins at a glance. J Cell Sci. 2014;127(Pt 17):3641–8. https://doi.org/10.1242/jcs.154906.
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–65. https://doi.org/10.1242/jcs.128868.
Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–87. https://doi.org/10.1093/intimm/dxh267.
Saez F, Frenette G, Sullivan R. Epididymosomes and prostasomes: their roles in posttesticular maturation of the sperm cells. J Androl. 2003;24(2):149–54.
Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101(36):13368–73. https://doi.org/10.1073/pnas.0403453101.
Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–78.
Baixauli F, Lopez-Otin C, Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol. 2014;5:403. https://doi.org/10.3389/fimmu.2014.00403.
Saha B, Momen-Heravi F, Kodys K, Szabo G. MicroRNA cargo of extracellular vesicles from alcohol-exposed monocytes signals naive monocytes to differentiate into M2 macrophages. J Biol Chem. 2016;291(1):149–59. https://doi.org/10.1074/jbc.M115.694133.
Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36(7):1772–81. https://doi.org/10.1002/eji.200535615.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. https://doi.org/10.1038/ncb1596.
Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. https://doi.org/10.1038/ncomms8321.
Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179(3):1489–96.
Nolte-’t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–81. https://doi.org/10.1182/blood-2008-08-174094.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5. https://doi.org/10.1038/nbt.1807.
Sun D, Zhuang X, Zhang S, Deng ZB, Grizzle W, Miller D, et al. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65(3):342–7. https://doi.org/10.1016/j.addr.2012.07.002.
El Andaloussi S, Lakhal S, Mager I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65(3):391–7. https://doi.org/10.1016/j.addr.2012.08.008.
Chou SH, Lan J, Esposito E, Ning M, Balaj L, Ji X, et al. Extracellular mitochondria in cerebrospinal fluid and neurological recovery after subarachnoid hemorrhage. Stroke. 2017;48(8):2231–7. https://doi.org/10.1161/STROKEAHA.117.017758.
Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41. https://doi.org/10.1016/j.plipres.2017.03.001.
Esser J, Gehrmann U, D’Alexandri FL, Hidalgo-Estevez AM, Wheelock CE, Scheynius A, et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J Allergy Clin Immunol. 2010;126(5):1032–40, 40 e1–4. https://doi.org/10.1016/j.jaci.2010.06.039.
•• Wahlund CJE, Gucluler G, Hiltbrunner S, Veerman RE, Naslund TI, Gabrielsson S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep. 2017;7(1):17095. https://doi.org/10.1038/s41598-017-16609-6 Great report on how exosomes are more immunogenic than microvesicles and how exosomes play an immunogenic role.
Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165(3):1259–65.
McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of uptake. J Circ Biomark. 2015;4:7. https://doi.org/10.5772/61186.
Hough KP, Wilson LS, Trevor JL, Strenkowski JG, Maina N, Kim Y-I, et al. Unique lipid signatures of extracellular vesicles from the Airways of Asthmatics. Sci Rep. 2018;8(1):10340. https://doi.org/10.1038/s41598-018-28655-9.
Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007;120(1):90–102. https://doi.org/10.1111/j.1365-2567.2006.02483.x.
Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289(32):22258–67. https://doi.org/10.1074/jbc.M114.588046.
Chiba M, Kubota S, Sato K, Monzen S. Exosomes released from pancreatic cancer cells enhance angiogenic activities via dynamin-dependent endocytosis in endothelial cells in vitro. Sci Rep. 2018;8(1):11972. https://doi.org/10.1038/s41598-018-30446-1.
Lukic A, Ji J, Idborg H, Samuelsson B, Palmberg L, Gabrielsson S, et al. Pulmonary epithelial cancer cells and their exosomes metabolize myeloid cell-derived leukotriene C4 to leukotriene D4. J Lipid Res. 2016;57(9):1659–69. https://doi.org/10.1194/jlr.M066910.
Torok NJ. Extracellular vesicles and ceramide: new mediators for macrophage chemotaxis? J Lipid Res. 2016;57(2):157–8. https://doi.org/10.1194/jlr.C066191.
Podbielska M, Szulc ZM, Kurowska E, Hogan EL, Bielawski J, Bielawska A, et al. Cytokine-induced release of ceramide-enriched exosomes as a mediator of cell death signaling in an oligodendroglioma cell line. J Lipid Res. 2016;57(11):2028–39. https://doi.org/10.1194/jlr.M070664.
Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res. 2016;57(2):233–45. https://doi.org/10.1194/jlr.M063412.
Qiao Y, Liang X, Yan Y, Lu Y, Zhang D, Yao W, et al. Identification of exosomal miRNAs in rats with pulmonary neutrophilic inflammation induced by zinc oxide nanoparticles. Front Physiol. 2018;9:217. https://doi.org/10.3389/fphys.2018.00217.
Real JM, Ferreira LRP, Esteves GH, Koyama FC, Dias MVS, Bezerra-Neto JE, et al. Exosomes from patients with septic shock convey miRNAs related to inflammation and cell cycle regulation: new signaling pathways in sepsis? Crit Care. 2018;22(1):68. https://doi.org/10.1186/s13054-018-2003-3.
Pua HH, Steiner DF, Patel S, Gonzalez JR, Ortiz-Carpena JF, Kageyama R, et al. MicroRNAs 24 and 27 suppress allergic inflammation and target a network of regulators of T helper 2 cell-associated cytokine production. Immunity. 2016;44(4):821–32. https://doi.org/10.1016/j.immuni.2016.01.003.
Murugaiyan G, da Cunha AP, Ajay AK, Joller N, Garo LP, Kumaradevan S, et al. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Invest. 2015;125(3):1069–80. https://doi.org/10.1172/JCI74347.
Essandoh K, Li Y, Huo J, Fan GC. MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. 2016;46(2):122–31. https://doi.org/10.1097/SHK.0000000000000604.
Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103(5):1283–8. https://doi.org/10.1073/pnas.0510511103.
Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–65. https://doi.org/10.1038/nm.2736.
Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. 2017;6(3):1018–28. https://doi.org/10.1002/sctm.16-0363.
Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192(3):324–36. https://doi.org/10.1164/rccm.201410-1765OC.
Gennai S, Monsel A, Hao Q, Park J, Matthay MA, Lee JW. Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Transplant. 2015;15(9):2404–12. https://doi.org/10.1111/ajt.13271.
Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32(1):116–25. https://doi.org/10.1002/stem.1504.
Fan J, Krautkramer KA, Feldman JL, Denu JM. Metabolic regulation of histone post-translational modifications. ACS Chem Biol. 2015;10(1):95–108. https://doi.org/10.1021/cb500846u.
Lu C, Thompson CB. Metabolic regulation of epigenetics. Cell Metab. 2012;16(1):9–17. https://doi.org/10.1016/j.cmet.2012.06.001.
van der Knaap JA, Verrijzer CP. Undercover: gene control by metabolites and metabolic enzymes. Genes Dev. 2016;30(21):2345–69. https://doi.org/10.1101/gad.289140.116.
Schvartzman JM, Thompson CB, Finley LWS. Metabolic regulation of chromatin modifications and gene expression. J Cell Biol. 2018;217(7):2247–59. https://doi.org/10.1083/jcb.201803061.
Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208(7):1367–76. https://doi.org/10.1084/jem.20110278.
Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–7. https://doi.org/10.1038/nature08097.
Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–30. https://doi.org/10.1126/science.aam7928.
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–85. https://doi.org/10.1002/path.4133.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. https://doi.org/10.1038/nature05485.
Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–74. https://doi.org/10.1038/nm.2627.
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–66. https://doi.org/10.1038/nm.3444.
Dupont M, Souriant S, Lugo-Villarino G, Maridonneau-Parini I, Verollet C. Tunneling nanotubes: intimate communication between myeloid cells. Front Immunol. 2018;9:43. https://doi.org/10.3389/fimmu.2018.00043.
Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303(5660):1007–10. https://doi.org/10.1126/science.1093133.
Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22(7):1181–91. https://doi.org/10.1038/cdd.2014.211.
Vignais ML, Caicedo A, Brondello JM, Jorgensen C. Cell connections by tunneling nanotubes: effects of mitochondrial trafficking on target cell metabolism, homeostasis, and response to therapy. Stem Cells Int. 2017;2017:6917941. https://doi.org/10.1155/2017/6917941.
Lu J, Zheng X, Li F, Yu Y, Chen Z, Liu Z, et al. Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells. Oncotarget. 2017;8(9):15539–52. https://doi.org/10.18632/oncotarget.14695.
Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34(8):2210–23. https://doi.org/10.1002/stem.2372.
• Panfoli I, Ravera S, Podesta M, Cossu C, Santucci L, Bartolucci M, et al. Exosomes from human mesenchymal stem cells conduct aerobic metabolism in term and preterm newborn infants. FASEB J. 2016;30(4):1416–24. https://doi.org/10.1096/fj.15-279679 Identification of functional mitochondrial complexes and direct measurement of exosome respiration by oximetry.
Kiriyama Y, Nochi H. Intra- and intercellular quality control mechanisms of mitochondria. Cells. 2017;7(1). https://doi.org/10.3390/cells7010001.
Sugiura A, McLelland GL, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 2014;33(19):2142–56. https://doi.org/10.15252/embj.201488104.
Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol. 2012;22(2):135–41. https://doi.org/10.1016/j.cub.2011.11.057.
Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, et al. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol. 2008;18(2):102–8. https://doi.org/10.1016/j.cub.2007.12.038.
Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, et al. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A. 2014;111(26):9633–8. https://doi.org/10.1073/pnas.1404651111.
Funding
The study was funded by the following funding sources: R01HL128502, P01HL114470, FAMRI YCSA 2010, and Parker B Francis Foundation.
Author information
Authors and Affiliations
Contributions
KPH and JSD outlined the review and together drafted and completed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Basic and Applied Science
Rights and permissions
About this article
Cite this article
Hough, K.P., Deshane, J.S. Exosomes in Allergic Airway Diseases. Curr Allergy Asthma Rep 19, 26 (2019). https://doi.org/10.1007/s11882-019-0857-3
Published:
DOI: https://doi.org/10.1007/s11882-019-0857-3