Abstract
Purpose of Review
Since omalizumab has been approved for urticaria, numerous randomized and real-life observational trials have been published. We reviewed the period January 2017–February 2018.
Recent Findings
Omalizumab is effective for the control of urticaria recalcitrant to antihistamines in different populations globally. The ratio of total serum IgE 4-week/baseline ≥2 can predict response with a high likelihood. In observational real-life trials, doses have been adjusted on an individual basis: in some populations, up to two-thirds of the patients can be controlled with 150 mg/month; however, others are still not controlled with 300 mg/month. In these, 150 mg bimonthly could be tried, before up-dosing to 450 mg/month. On the long run (up to 3 years) omalizumab kept its efficacy. In many patients, dosing intervals could be augmented (6–8 weeks, some even more). After a 12-month treatment, about 20% showed long-term remission without relapse.
Summary
Some biomarkers are being detected. Adjusting omalizumab doses in urticaria patients could enhance efficacy (shortening dosing interval and/or augmenting dose) and save costs (after 12 months: extending dosing interval and/or reducing dose).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Omalizumab (OMA), a humanized monoclonal antibody against the constant region of the immunoglobulin E (IgE) molecule, was launched almost 2 decades ago for the treatment of severe allergic asthma. Since its release, it has been used in several other allergic diseases, but its efficacy has been most astonishing in chronic spontaneous urticaria, also called chronic idiopathic urticaria (CSU/CIU). After small proof-of-concept trials, in 2013, large pivotal phase III trials were conducted: ASTERIA I, ASTERIA II and GLACIAL. These showed that 300 mg omalizumab monthly seemed to be the most effective dose, independent of the patient’s weight or baseline total serum IgE [1, 2].
Since 2013, omalizumab has been approved for the treatment of CSU/CIU in many parts of the world. With its more widespread use, several new aspects are gradually becoming clear and are being published. We report here a review of original papers published from January 2017 onward.
Methods
We conducted a literature search in MEDLINE and Embase from January 2017 to February 2018 by using logical combinations of the following terms: ‘urticaria, chronic’, ‘urticaria, idiopathic’, ‘urticaria, chronic spontaneous’, ‘omalizumab’ and ‘anti-IgE’. We only considered reports of original data, including double-blind placebo-controlled, randomized controlled trials (DBPC-RCT), RCTs, open controlled trials, observational studies, retrospective trials and case reports. We excluded: (1) systematic reviews with or without meta-analyses; (2) review articles; (3) not published in English, Spanish, Portuguese, French or Dutch. Review articles were used to cross-check for identified articles. Data extraction was carried out without a pre-established protocol, as data on different aspects were collected. Data presentation is descriptively divided into several subtitles covering mechanisms and biomarkers, larger trials in non-European, non-US patients, real-life trials, dose and interval adjustment in non-responders, trials on long-term use of omalizumab in urticaria and dose-adjustment preparing omalizumab clearance, omalizumab in special patient groups, omalizumab in chronic inducible urticaria (CindU), adverse effects and omalizumab-urticaria in guidelines.
Biomarkers and Mechanisms of Omalizumab in Chronic Spontaneous/Idiopathic Urticaria: Where Do we Stand?
About half of the patients with chronic spontaneous urticaria (CSU) have auto-antibodies, some against IgE, but by far the majority against FcεRI, the high affinity receptor for IgE on basophils and mast cells. Functional auto-antibodies are presumed to be the cause of a positive basophil activation test (CU-BAT), in which the patient’s serum activates donor basophils to express the surface activation markers, CD63 and CD203c. Also, the less-sensitive basophil histamine release assay (BHRA) is used for this purpose. On the other hand, the FcεRI receptor density on the surface of the patient’s basophils might be a marker of basophil ‘releasibility’.
The mechanisms of action that contribute to the efficacy of omalizumab in CSU/CIU are still unclear. What we know to date is that omalizumab binds to the constant region of the IgE molecule, inhibiting it from binding to its receptor, FcεRI. As a result, total IgE levels in peripheral blood rise, but free IgE is reduced to very low levels. Consequently, there is a downregulation of the FcεRI, probably because of a lack of occupancy by IgE. As such, a reduction of the FcεRI receptor density on mast cells and basophils has been reported with OMA. There is heterogeneity among the results [3]. To fully understand its mechanisms, a functional and qualitative insight of effector cells and immune mediators is needed. We here present the latest findings from 2017 onward (Table 1).
Mechanisms of the Effect of OMA in Urticaria
In a DBPC-RT trial of four monthly injections of 300 mg OMA in CU patients, investigators showed a 66% reduction in FcεRI receptor density on basophils in the active group. This effect continued during the treatment phase and lasted for 2 months after the last dose. Interestingly, no change in the CU-BAT test was observed: patients’ sera continued to activate donor basophils [4].
Using an in vitro system of donor basophils, OMA added to sera from CSU patients did not modify the ability of the sera to induce cell degranulation. Also, sera from patients successfully treated with OMA were still capable of activating mast cells and basophils. OMA does not seem to change factors in the patient’s serum which induce cell degranulation [5].
Biomarkers for Response to OMA
Two DBPC-RTs [4, 6] found that low baseline IgE (<43 UI/mL) was associated with a poor response, while the complete responders had the highest serum baseline IgE values. Patients with baseline IgE (<43 UI/mL) had a 33% chance of non-response at 12 weeks, while only 5% did not respond when IgE was above 43 UI/mL. However, the best predictor of OMA response was the serum [baseline IgE]/[4-week IgE] ratio. The authors invite physicians to use the rule of ‘2 × 4’, which stands for the following: when serum IgE levels fail to rise to twice (2×) the baseline value after the first 4 [4] weeks of treatment, non-response might be expected [6].
The measurement of basophil FcεRI receptor density at baseline is a reliable biomarker between non-responders and responders, as it was significantly lower in the former group. The hypothesis relies on patients’ levels being too low to even down-regulate the inflammatory response, as patients exhibiting significant clinical improvement showed a sharp reduction in the levels of the basophil FcεRI receptor. This measurement is proposed as another predictor of the therapeutic response to OMA in CSU (100% sensitivity and 73.2% specificity, observational cohort within case and controls [7]). In line with these observations, in a DBPC-RTm Metz et al. found a positive correlation between a decrease in FcεRI within lesional and nonlesional skin cells and the clinical efficacy of OMA. This clinical efficacy was associated with histopathological findings that focused on other cells rather than mast cells (peripheral basophils and skin T cells and Langerhans cells) [8].
In a retrospective chart review of 112 patients with CU treated with OMA, Straesser et al. investigated, in pre-omalizumab sample,s serum IgE, anti-FcεRI antibodies and elements of the complete blood count and differential for possible biomarkers for response. Patients with lower serum IgE and patients with positive anti-FcεRI antibodies had a statistically significant poorer response. However, the authors call for care in interpreting these results, as concomitant steroid use might have caused bias [9].
Other Factors that might Affect Response and Predict Relapse after OMA Discontinuation
A proportion of patients with CU are still refractory to treatment with omalizumab. Different co-factors have been hypothesized. Angiotensin converting enzyme (ACE) inhibitors decrease bradykinin degradation and could exacerbate urticaria in a histamine-independent way. Thus, its co-administration with omalizumab could interfere with its effect and efficacy. It is important to consider the correlation between CU and the coagulation cascade. It is not easy to distinguish between cause and consequence, but an increase in D-dimer seemed to be a useful disease biomarker in two cases, where its rise was closely correlated with an urticaria flare; its increase might indicate a rise in the inflammatory state, exacerbating urticaria [10].
In Turkey, after 12 weeks of OMA, 47% of 93 patients had a complete response, and 39% a partial response. Due to local legislative issues, OMA had to be stopped. Baseline IgE over 100 UI/mL was correlated with a faster relapse rate after discontinuation of OMA [11].
Fast and Slow Responders
Among people with CSU, the clinical presentation might be indistinguishable, but the response to OMA can be fast (<1 week) or slower (1 week–3 months) or even very slow (12–24 weeks [12]). Here, an immunological profile difference has been elucidated and novel biomarkers could be considered predictors of response. Positivity of tests that detect auto-antibodies, both in vitro (basophil histamine release assay) and in vivo (autologous serum skin test) correlate with slow responders, hypothetically due to higher levels of auto-antibodies (observational study, without controls [13])
In conclusion, it progressively becomes clearer that patients with CSU can be stratified into phenotypes/immunotypes of responders versus non-responders and fast versus slow responders. Patients seem to have a higher probability to respond to OMA if they have:
-
higher baseline serum IgE level or, more specifically, a rise in baseline/4-week sIgE
-
higher baseline FcεRI density on basophil or mast cells
-
BHRA or ASST positivity: patients do respond, but generally slower.
-
Low D-dimer levels (as marker of inflammation)
-
No intake of concomitant ACE inhibitors (?)
Omalizumab in Urticaria: Large Trials after the Pivotal Studies in US and Europe and Dosing till Response
After the original trials (ASTERIA I and II and GLACIAL) showing the efficacy of OMA in urticaria in European [1] and US [2] patients, respectively, in 2015 Ensina et al. was already reporting favorable results with OMA in CSU in Brazilian patients [16]. Since then, several other large trials have been conducted in patient groups in the Far and Middle East (see Table 2).
Moreover, in the US, Wang et al. showed efficacy of OMA for urticaria in real life [19], and two trials focussed on the effect of OMA on the quality of life in CSU patients.
The phase 3 DBPC-RT POLARIS trial showed that OMA was effective and safe in Japanese and Korean patients [17]. In a subgroup analysis, focussing on 105 Japanese patients with refractory CSU, a clear dose-effect could be seen between the improvement in the 300-mg monthly group, the 150-mg monthly group and the placebo patients in ISS/7 and in the secondary outcome variables (e.g. UAS7, number of hives, DLQI). Only one systemic reaction reported by the investigator as serious was documented, in the 150-mg arm. This was a pharyngeal edema without anaphylaxis [18]. As such, the results are similar to those found in the complete trial [17]. Moreover, just as in the original trials, an ‘early responders’ group could be detected of subjects achieving UAS7 < 6 as early as week 4. However, the precise characterization of these patients was still difficult. A smaller retrospective study in 13 Thai patients with urticaria found a good response with 150 mg monthly in 9/13 patients, most of them with antihistamines as add-on, and the other 4 patients reached control with the 300-mg monthly dose [24].
In an elegantly conducted, randomized trial of 280 Israeli patients with CSU, 50 were started on 150 mg and 230 on 300 mg OMA monthly. Half of the 150-mg dosing group were well controlled, the rest did well after changing to 300 mg. Also, a third of the 300-mg omalizumab group had to up-dose to 450 mg before reaching control. Finally, only 12% were non-responders. After 12 months’ treatment, 6% could be taken off OMA without relapse, and in 32%, the dosing -interval could be augmented to 6–8 weeks [28]. A smaller retrospective report on 15 Asian patients (Thailand) treated with OMA showed 11/15 patients fared well with 150 mg monthly, while the rest needed 300 mg to control the disease. After 12 months’ treatment,3/15 (20%) remained disease-free for at least 6 months, 4/15 could space injection intervals to every 6 months (sic), while the rest still needed frequent omalizumab injections to control the wheals [25]. For more data on long-term outcomes, see below.
A real-life, non-interventional multicentre study in Sweden, Norway and Denmark reported on baseline data of 158 CU patients. Their quality of life was moderately affected (mean DLQI 7.7). 8.2% of all patients were on OMA [33].
Another group of investigators in Turkey has proposed that the omalizumab administration schedule in CSU could be more flexible, depending on the patients’ symptoms. Particularly for patients experiencing worsening of symptoms just before the next 300-mg injection, an approach of omalizumab 150 mg/2 weeks could be tried first, before deciding to increase the dose [27].
In a retrospective observational cohort study, which used data from health plan administrative claims integrated with medical records from CSU patients treated with omalizumab, 75.4% of the patients with 12 months post-index eligibility and 72.4% with 18 months post-index eligibility received an index dose of 300 mg, and approximately 67% remained with this dose throughout the follow-up period. A positive response to omalizumab was reported in 83.7% (12 months post-index) and 77.8% (18 months post-index) and less than 10% showed no response to therapy. Discontinuation of omalizumab was observed in 22% of both groups because of the cost of treatment, insurance disenrollment, side effects, no response to treatment, no time or availability for treatment, or symptom resolution [19].
Finally, two studies concentrated on the quality of life improvement of the patients with CSU on omalizumab. According to the data collected by the CU-Q2oL (chronic urticaria questionnaire on the quality of life) and SF-36 (short form-36) questionnaires of 13 patients, the most bothersome symptom was pruritus (61%). The second and third most bothersome symptoms were the limitation that the disease had on their daily activities and swelling plus nervousness (55.5 and 50%, respectively). In addition, 38% reported their condition caused them depression and shame and 33% reported adverse effects of their medication. Statistical analysis showed a significant increase in the SF-36 score with a marked reduction of UAS7, VAS, and CU-Q2oL scores in the patients who were receiving omalizumab 1 month after starting the drug, and re-evaluations at 6 and 12 months showed a sustained effect [20]. The second study was a post-hoc analysis of data collected in the context of the original pivotal trials, ASTERIA I, II and GLACIAL. In all, 4-monthly injections of 300 mg omalizumab resulted in a statistically significant improvement in the Dermatology Life Quality Index (DLQI). A ≥ 4-point reduction in DLQI is considered a minimal clinically important difference. This was reached by three-quarters of the patients in each one of the three trials [21].
In conclusion, the efficacy of omalizumab in CSU patients has now also been shown in other parts of the world, in real-life settings and in enhancing the quality of life in CSU patients. At least a third of the patients might already respond with 150 mg monthly, while on the other hand, a fifth might need doses higher than 300 mg or a shorter dosing interval (every 2 weeks) to become responders. With these adjustments, in general, over 85% of the CSU patients finally respond.
Long-Term Treatment with Omalizumab and Dose-Adjustment over Time
Unlike the extensive evidence supporting the use of OMA in CU due to its rapid effect on the control of symptoms [12, 30], there are few data evaluating the impact of anti-IgE as a modulator of the long-term immune response. Therefore, some questions arise:
-
1.
Can omalizumab change the course of allergic diseases?
-
2.
Is its prolonged use safe?
-
3.
When to discontinue it?
Multiple studies with urticaria patients and follow-up for 6–12 months have shown that, while the medication is administered, an excellent control of the symptoms according to the scales of quality of life and symptom severity is achieved [22, 29]. These studies also suggest that the drug’s effectiveness does not diminish with time. Some studies suggest that around 20% of the patients tolerated stopping omalizumab after 12 months, but a high proportion may relapse [25, 31], suggesting that, in the majority of the patients, the drug does not eliminate the underlying immune response that causes the disease. At the experimental level, it has been observed that, after stopping omalizumab, a re-population of FcεRI, the high affinity receptor of IgE, in the membrane of mast cells and basophils may occur, even greater than before the use of the drug, which could potentially lead to a relapse [34]. However, this risk has not been demonstrated clinically.
Recent European urticaria guidelines and some authors suggest omalizumab over the use of cyclosporine [35], mainly based on the safety profile of the drug. In a recent study, a group of 35 patients with urticaria not responding to antihistamines in high doses, omalizumab or cyclosporine was administered [22]. The majority of the patients presented good clinical control with these therapies but two patients with cyclosporine had to be shifted to omalizumab due to an increase in blood pressure. At the moment, there are no head-to-head trials in a larger number of patients comparing the clinical response of these two treatments or their safety profile. Although most patients achieve clinical improvement with 300 mg of Omalizumab, some studies suggest that, after 4 months of treatment, the dose may be increased in case of non-response [26]. However, the number needed to treat for this effect has not yet been defined; probably, it lies between 1 in 4 to 1 in 12 patients (ASTERIA 1 and ASTERIA 2). Thus, it cannot yet be recommended as a routine step in clinical management.
Although the guidelines on urticaria are very clear in terms of the stepwise management of symptoms [36], there are still no studies that evaluate when and how to perform omalizumab discontinuation once the patient’s urticaria is controlled. The results of different studies on asthma suggest that, after a year of use, an attempt could be made to reduce by half the monthly omalizumab dose or to stop it [37]. Another alternative is to space the omalizumab administration to every 6–8 weeks or even 2–3 months, as some real-life trials have reported, see above. If the patient does not tolerate the discontinuation, some studies have shown that the reintroduction of omalizumab can achieve clinical control of these patients within 12 weeks [29, 30, 32].
In summary, there are multiple questions that require a response on the use of omalizumab for periods longer than 1 year in patients with urticaria, so more observational and experimental studies are required to establish its long-term safety and efficacy.
Case-Reports of Omalizumab for Urticaria: Pregnancy, HIV-Positive Patients, a.O.
Omalizumab has recently been assigned pregnancy category B risk status by the FDA [38]. In the EXPECT registry, a post-marketing, prospective, observational study in 191 pregnant women with asthma who received one or more doses of omalizumab within 8 weeks prior to conception or at any time during pregnancy and no adverse effects were observed [39].
Recently, Gonzalez-Medina et al. have described two 37-year-old women with exacerbation of CSU during pregnancy. Both women received a dose of 300 mg of omalizumab and no teratogen effects were seen with a normal development of the pregnancy. Efficacy of the drug was similar to that observed in the general population [40]. In addition, Ensina et al. reported two other women with CSU, of 29 and 32 years of age, respectively. The first patient received omalizumab at a dose of 150 mg throughout the course of two pregnancies with a good response, while the latter patient was treated with 300 mg. No complications were observed during pregnancy and delivery or in the subsequent development of the children. In the latter case, the mother breastfed her son without complications up to the publication of this report [41].
Although the use of omalizumab during pregnancy has not been approved, currently it may be considered as a safe and efficient treatment in patients who are refractory to standard therapy, weighing the benefits against possible risks.
Recently, the efficacy of omalizumab (300 mg) has been reported to reduce the frequency and severity of angioedema in patients with antihistamine-resistant CSU [42]. In the X-ACT study (a phase III, DBPC-RCT), patients between 18 and 75 years of age with CSU and at least 4 episodes of angioedema over the past 6 months were enrolled to receive omalizumab 300 mg versus placebo. After completion of the treatment, statistically significant improvements were observed in the angioedema-related quality of life (AE-QoL) score and the DLQI in patients with moderate to severe CSU, showing a marked and early improvement of the symptoms of angioedema and of the psychological well being of the patients who received the drug [43]. Analyzing data from the ASTERIA I [44], the ASTERIA II [1], and the Glacial [2] studies, Maurer et al. found that angioedema is more often observed in patients with CSU refractory to standard therapy and describe a marked reduction of days/weeks with angioedema associated with a relevant improvement in QoL in patients receiving OMA 300 mg versus those receiving placebo [45].
As for OMA in elderly patients, a group of Italian investigators documented the effect of 40 weeks OMA, comparing efficacy and safety in patients with CSU of group I (15–64 years, n = 290) against group II (≥65 years, n = 32). There was no difference in efficacy between the two groups and there were no specific safety concerns among the elderly patients [46]. In a recent retrospective study, Syrigos et al. [47] analyzed 20 patients with a mean age of 54.5 years and found that 85% had a complete (UAS7 = 0) response to treatment. Half of the patients had an early response after the first dose, 10% an intermediate response after the second dose, and 45% had a late response after the fourth or fifth doses. Disease duration was significantly less in patients with a late response compared to those with an early response (P = 0.026).
Furthermore, a male patient with HIV undergoing highly active antiretroviral therapy with good response who concomitantly had refractory CSU and was treated with omalizumab has been reported. The drug shown to be efficacious and safe and no changes in viral load or TCD4 cell counts were observed [48]. A similar response was observed in a female patient with relapsing-remitting multiple sclerosis and refractory CSU who was successfully treated with omalizumab. The drug was also efficacious and safe administered concurrently with azathioprine and/or interferon beta 1 therapy [49].
Omalizumab in Inducible Urticaria
As to the efficacy of omalizumab for the treatment of inducible urticaria, a clinical response has been described in the treatment of antihistamine-resistant solar urticaria in a series of eight cases treated with omalizumab at doses of 300 and 450 mg [50]. Among other reports of patients with a favorable response to omalizumab, a patient with severe cholinergic urticaria was described [51]. Further studies would be necessary to determine the efficacy, treatment length, maintenance dose, etc. in this type of urticaria.
Kocatürk et al. conducted a prospective controlled trial using the guideline-indicated treatment algorithm for a group of CSU versus CindU patients. The response rate of CindU patients was low on standard dose antihistamines, improved with up-dosing and reached almost 80% adding OMA in the anti-histamine-resistant patients [52]. A small study of OMA in dermographism [53] and a systematic review of published evidence on OMA in CindU reinforced these findings [54].
Adverse Effects of Omalizumab
Omalizumab in urticaria has shown a good safety profile [26, 35, 55], although some adverse events (AEs) keep on being reported, most of them in line with previous AE reports. A few studies have followed up evaluating its use for periods longer than 1 year. Although there are not yet any ultra-long studies in urticaria, studies in asthma show a low frequency of adverse effects, even after 9 years of use [37], and with even higher doses than those used for the control of urticaria. However, a recent study, sponsored by Novartis at the request of the FDA in patients with moderate–severe asthma, suggests that, in asthmatic patients with cardiovascular risk factors, it may be appropriate to perform lipid profile tests and an assessment of the cardiovascular risk before starting omalizumab [56]. No data on this topic are available for patients with urticaria.
In urticaria patients, a possible adverse effect was reported in four female subjects who presented transient hair loss [57, 58]. Although this may be a rare adverse effect, the association of hair loss and omalizumab is currently uncertain, and different causes may explain this possible association. In a prospective observational trial in 13 Portuguese patients, one presented mild headache, and one presented severe angioedema with worsening of urticaria 6 h after the administration [31]. Finally, in one of the long-term trials, there was again one case of late-onset anaphylaxis. A 46-ear-old female developed headache, malaise, worsening of hives, itchy throat, stomach cramps, wheezing and escalating cough 9 h after the first dose of omalizumab. She self-injected epinephrine and went to the emergency department from where she was released several hours later with no sequelae [32].
Long Term Trials with OMA and Malignancy (Mainly Asthma Trials)
The relationship between malignancy and long-term treatment with OMA has been studied mainly in patients with asthma due to the suspicion of a higher incidence of this type of disease in patients receiving the monoclonal antibody [59].
In 2014, the prospective observational cohort study EXCELS assessed the clinical effectiveness and long-term safety profile of OMA in patients with moderate–severe asthma. The primary outcome measures included all confirmed, study-emergent primary malignancies. The result showed that the crude malignancy rates were similar in the OMA-treated and in the OMA-untreated groups, ratio of 0.84 (95% CI, 0.62–1.13) and 0.98 (95% CI, 0.71–1.36), respectively, for all malignancies. The study concluded that OMA therapy is not associated with a risk of malignant diseases [60].
Di Bona et al. published a real-life trial to evaluate the long-term safety of omalizumab in patients with uncontrolled asthma. This retrospective study, conducted in two centers in southern Italy between March 2007 and March 2016, included 91 patients. Only one case of malignancy was reported. A tumor of the digestive tract was diagnosed 1 year after the beginning of OMA treatment. Due to its characteristics, the authors considered that the treatment was unlikely to be related to the tumor [37]. These data are consistent with the results of the EXCELS study.
Omalizumab Versus Cyclosporine as Third-Line Therapy in Recalcitrant CSU
Sanchez et al. recruited patients with CSU older than 12 years and treated them according to the international guideline. They were randomized to receive one of five H1-antihistamine options frequently used in Colombia. After the first month, patients with DLQI ≥5 were considered unresponsive, and higher doses of non-sedating H1-antihistamines were given for another 30 days. One month later, those without a clinical response were randomized to OMA 300 mg/month or cyclosporine 3–5 mg/kg/day for 4 months (see Table 2).
From the 150 patients recruited, 35 were unresponsive to H1-antihistamines in high doses and were randomized to cyclosporine [18] or OMA [17]. After 2 weeks, one patient in the cyclosporine group developed systemic hypertension and had to change to OMA. Headache and pain at the injection site were observed in four and nine patients treated with OMA respectively. Transitory hypertension in two patients and abdominal pain in six were the main side effects seen in patients treated with cyclosporine. After 4 months of treatment, 12/18 patients treated with OMA and 11/17 treated with cyclosporine were under complete control. In this study, a minority of the patients required third-line treatment, and both omalizumab and cyclosporine showed similar efficacy. However, as many patients could not adequately register the UAS7 score, the tool used by the authors to assess response to treatment was the DLQI, despite its moderate correlation with disease activity [22].
Local and Global Guiding Documents on the Use of Omalizumab in Urticaria
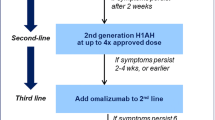
The current version of the EAACI/GA2LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria (International Guideline) recommends adding OMA in the treatment of patients unresponsive to high doses of H1-antihistamines as a third-line treatment. This position differs from the previous version of the same guideline, where montelukast and cyclosporine-A were also considered third-line drugs. Efficacy of cyclosporine-A has been shown in many trials, but, due to its safety profile, it is currently considered a fourth-line therapy. On the other hand, montelukast has been excluded from the algorithm due to the lack of evidence regarding its efficacy [36].
According to its local population characteristics and access to diagnosis and treatment, regional guidelines are also relevant and may differ from the international one. Members of the Skin Allergy Research Society of India discussed the available data and have proposed an evidence-based therapeutic approach in an Indian perspective. They recommend the use of OMA or cyclosporine over other drugs as the third line of treatment in patients who are unresponsive or incompletely responsive to up-dosing of modern second-generation antihistamines. However, they emphasize that, due to its cost and the requirement of a hospital setting for administration, omalizumab’s use may be limited [61].
The clinical impact of guidelines’ recommendations for the management of CSU was evaluated by Sanchez et al. (see discussion in previous paragraph on OMA versus cyclosporine and Table 2) [22].
As there is no consensus definition on who is a “complete”, “partial” or “non-responder” for patients under treatment with OMA, the use of a disease measurement tool is required. Ferrer et al., to harmonize treatment management and compare data in clinical trials and real-life studies, proposed the use of the UAS7 and the urticaria control test (UCT) to assess response to treatment [62]. The authors suggest that the UAS7 should be assessed weekly during OMA treatment, especially during the first 12 weeks, to identify fast and slow responders, as well as partial responders. A UAS7 ≤ 6 is proposed to define treatment response; achieving this score during the first 12 weeks characterizes fast responders. Moreover, the authors recommend continuing recording UAS7 until month 6 to detect slow responders. The absence or little improvement in the UAS7 after 6 doses would suggest a non-response. Ferrer et al. further discuss that a disadvantage of the UAS7 is that it does not include a component related to angioedema, which might be present in more than 50% of patients with urticaria. The Angioedema Activity Score is a validated tool to measure angioedema activity, but the recently promoted UCT score also comprises angioedema, besides wheals and pruritus. Unlike the UAS7 which needs recording 7 days a week, the UCT is a point-assessment during the medical visit, which could be useful for those patients who not adequately register the UAS7. A UCT of 0 indicates “no disease control,” a score of ≤11 indicates poor disease control, and a score of ≥12 indicates controlled disease. The use of these tools can be supplemented by a patient-orientated tool that measures quality of life, such as the DLQI or the CU-Q2oL [62].
The standardization of response to treatment will allow clinicians and researchers to determine predictors of a good response, as well as optimal dose and dosing intervals for patients under treatment with OMA. Based on a review of selected relevant literature, Asero et al. discuss these and other unresolved issues related to the use of OMA in CSU patients in Italy. As CSU has a variable and unpredictable disease course, there may be no optimal treatment duration for OMA. In clinical trials, treatment duration ranged from 12 to 24 weeks, but long-term treatment has been described in real-life studies. According to the Italian Medicine Agency (AIFA), OMA should be administered for 3 months and for a further months in cases with clinical response, followed by an 8-week treatment interruption to evaluate symptom recurrence. A second cycle of five doses is recommended in cases of relapse (defined as a UAS score > 3 or UAS7 > 16 after 30 days of antihistamines at the maximum approved dose). Despite not having a personal specific recommendation regarding the optimal duration of treatment, the authors suggest that the AIFA recommendations should be followed in Italy. In their opinion, patients with a partial response (UAS7 > 50% and < 90%) after the third dose, should receive extended treatment for up to 6 months before discontinuing the drug. The international guidelines recommend OMA as an add-on therapy for patients with antihistamine-resistant CSU. However, there is no consensus regarding the maintenance or modification of the antihistamines regimen when an adequate control is achieved with OMA. Asero et al. advise that, in those patients who respond to add-on OMA, a gradual reduction in antihistamines doses or even discontinuation could be performed, and antihistamines could be resumed as needed. They also suggest maintaining the current antihistamine agent and dosage until the first follow-up visit after starting omalizumab, with reduction or discontinuation based on UAS7 reduction [63].
There are still open questions regarding the use of OMA in clinical practice. Regional and personal experiences must be taken into account to answer these questions. The availability and access to the different drugs must be considered when opting for one or another treatment in patients with refractory CSU. Tools to assess disease control and activity should be used, but one tool may not fit all populations or types of practice (private, hospital, university, etc.). Regional and global experts meetings are fundamental to discuss and come up with answers to those not yet solved issues.
Conclusion
Since January 2017, a wealth of articles have been published on OMA in CSU, enhancing our knowledge on possible mechanisms of the drug in CSU, biomarkers for response, clinical responses in patients other than American and European, long-term outcomes and OMA use in special groups. Interestingly, the effect of OMA in CSU seems to lie in the non-soluble (cellular?) fraction, as post-omalizumab serum is still able to elicit a positive BHRA. For now, an accessible biomarker might be the ratio of the total serum IgE at baseline/after 4 weeks treatment. A 2-fold rise in total serum IgE indicates response is highly probable. As for the new clinical data, OMA is effective for CSU in patients in Latin America and the Far and Middle East. Concerning dose adjustments, investigators have shown that almost a third of their patients already respond with 150 mg monthly and of the non-responders to 300 mg monthly some might fare well with 150 mg every 2 weeks, while others need 450–600 mg every month. Also, OMA maintains its efficacy, even after interruption and re-treatment. Finally, after a year of treatment, a small fraction of patients can be taken off the drug, while others might stay controlled with intervals of 6–8 weeks or even 2–3 months.
There are now some promising efficacy and safety reports on OMA in pregnant patients with CSU, elderly patients and patients with HIV infection and CSU, patients with CSU and angioedema and CindU patients. Cases of anaphylaxis due to OMA keep on being reported, but they are extremely rare. With growing evidence and experience in the use of OMA in CSU, this treatment is now also being included in several local urticaria guidelines.
The reviewers are positive that even more interesting data will be published on OMA in CSU for ears to come which will continue to enhance our knowledge on the drug and the disease.
References
Maurer M, Rosen K, Hsieh HJ, Saini S, Grattan C, Gimenez-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368(10):924–35.
Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132(1):101–9.
Kaplan AP, Gimenez-Arnau AM, Saini SS. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy. 2017;72(4):519–33.
Jorg L, Pecaric-Petkovic T, Reichenbach S, Coslovsky M, Stalder O, Pichler W, et al. Double-blind placebo-controlled trial of the effect of omalizumab on basophils in chronic urticaria patients. Clin Exp Allergy. 2018;48(2):196–204.
Serrano-Candelas E, Martinez-Aranguren R, Vega O, Gastaminza G, Bartra J, Audicana MT, et al. Omalizumab efficacy in cases of chronic spontaneous urticaria is not explained by the inhibition of sera activity in effector cells. Sci Rep. 2017;7(1):8985.
Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in CSU patients is linked to and predicted by IgE levels and their change. Allergy. 2017.
Deza G, Bertolin-Colilla M, Pujol RM, Curto-Barredo L, Soto D, Garcia M, et al. Basophil FcepsilonRI expression in chronic spontaneous Urticaria: a potential immunological predictor of response to Omalizumab therapy. Acta Derm Venereol. 2017;97(6):698–704.
Metz M, Staubach P, Bauer A, Brehler R, Gericke J, Kangas M, et al. Clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with a reduction of FcepsilonRI-positive cells in the skin. Theranostics. 2017;7(5):1266–76.
Straesser M, Palacios T, Kyin T, Borish L, Lawrence MG. Biomarkers which may predict response to omalizumab in chronic urticaria: serum IGE and CD203C. Ann Allergy Asthma Immunol. 2017;119(5):S39.
Asero R. Serial D-dimer plasma levels in a patient with chronic spontaneous urticaria developing resistance to omalizumab. Clin Exp Dermatol. 2017;42(6):667–9.
Ertas R, Ozyurt K, Ozlu E, Ulas Y, Avci A, Atasoy M, et al. Increased IgE levels are linked to faster relapse in patients with omalizumab-discontinued chronic spontaneous urticaria. J Allergy Clin Immunol. 2017.
Casale TB, Win PH, Bernstein JA, Rosen K, Holden M, Iqbal A, et al. Omalizumab response in patients with chronic idiopathic urticaria: insights from the XTEND-CIU study. J Am Acad Dermatol. 2017;
Gericke J, Metz M, Ohanyan T, Weller K, Altrichter S, Skov PS, et al. Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J Allergy Clin Immunol. 2017;139(3):1059–61. e1
Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy. 2018;73(3):705–12.
Straesser MD, Oliver E, Palacios T, Kyin T, Patrie J, Borish L, et al. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J Allergy Clin Immunol Pract 2017.
Ensina LF, de Lacerda AE, Machado LM, Camelo-Nunes I, Sole D. Long-term omalizumab therapy for refractory chronic spontaneous urticaria: a real-life experience. Ann Allergy Asthma Immunol. 2015;115(6):536.
Hide M, Park HS, Igarashi A, Ye YM, Kim TB, Yagami A, et al. Efficacy and safety of omalizumab in Japanese and Korean patients with refractory chronic spontaneous urticaria. J Dermatol Sci. 2017;87(1):70–8.
Hide M, Igarashi A, Yagami A, Chinuki Y, Inomata N, Fukunaga A, et al. Efficacy and safety of omalizumab for the treatment of refractory chronic spontaneous urticaria in Japanese patients: subgroup analysis of the phase 3 POLARIS study. Allergol Int. 2017.
Wang L, Ke X, Kavati A, Wertz D, Huang Q, Willey VJ, et al. Real-world treatment patterns and outcomes of omalizumab use in patients with chronic idiopathic urticaria. Curr Med Res Opin. 2018;34(1):35–9.
Larrea-Baca I, Gurpegui-Resano M. Improvement in the quality of life of patients with chronic spontaneous urticaria treated with omalizumab in real life. Enferm Clin. 2017;27(6):361–8.
Finlay AY, Kaplan AP, Beck LA, Antonova EN, Balp MM, Zazzali J, et al. Omalizumab substantially improves dermatology-related quality of life in patients with chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2017;31(10):1715–21.
Sanchez J, Zakzuk J, Cardona R. Evaluation of a guidelines-based approach to the treatment of chronic spontaneous Urticaria. J Allergy Clin Immunol Pract. 2018;6(1):177–82. e1
Ensina LF, Valle SO, Juliani AP, Galeane M. Vieira dos Santos R, Arruda LK, et al. Omalizumab in Chronic Spontaneous Urticaria: A Brazilian Real-Life Experience. Int Arch Allergy Immunol. 2016;169(2):121–4.
Kulthanan K, Tuchinda P, Chularojanamontri L, Likitwattananurak C, Ungaksornpairote C. Omalizumab therapy for treatment of recalcitrant chronic spontaneous urticaria in an Asian population. J Dermatol Treat. 2017;28(2):160–5.
Kulthanan K, Tuchinda P, Likitwattananurak C, Weerasubpong P, Chularojanamontri L. Does omalizumab modify a course of recalcitrant chronic spontaneous urticaria?: a retrospective study in Asian patients. J Dermatol. 2018;45(1):17–23.
Curto-Barredo L, Spertino J, Figueras-Nart I, Exposito-Serrano V, Guilabert A, Mele-Ninot G, et al. Omalizumab updosing allows disease activity control in refractory patients with chronic spontaneous urticaria. Br J Dermatol. 2018;
Turk M, Kocaturk E, Cure K, Yilmaz I. Two-week intervals during omalizumab treatment may provide better symptom control in selected patients with chronic urticaria. J Allergy Clin Immunol Pract. 2018;
Vadasz Z, Tal Y, Rotem M, Shichter-Confino V, Mahlab-Guri K, Graif Y, et al. Omalizumab for severe chronic spontaneous urticaria: real-life experiences of 280 patients. J Allergy Clin Immunol Pract. 2017;5(6):1743–5.
Turk M, Yilmaz I, Bahcecioglu SN. Treatment and retreatment with omalizumab in chronic spontaneous urticaria: real life experience with twenty-five patients. Allergol Int. 2018;67(1):85–9.
Nettis E, Di Leo E, Foti C, Cegolon L, Vacca A. Efficacy and rapid activity of omalizumab retreatments in chronic spontaneous urticaria. J Am Acad Dermatol. 2017.
Pinto Gouveia M, Gameiro A, Pinho A, Goncalo M. Long-term management of chronic spontaneous urticaria with omalizumab. Clin Exp Dermatol. 2017;42(7):735–42.
Maurer M, Kaplan A, Rosen K, Holden M, Iqbal A, Trzaskoma BL, et al. The XTEND-CIU study: long-term use of omalizumab in chronic idiopathic urticaria. J Allergy Clin Immunol. 2018;141(3):1138–9.e7.
Thomsen SF, Pritzier EC, Anderson CD, Vaugelade-Baust N, Dodge R, Dahlborn AK, et al. Chronic urticaria in the real-life clinical practice setting in Sweden, Norway and Denmark: baseline results from the non-interventional multicentre AWARE study. J Eur Acad Dermatol Venereol. 2017;31(6):1048–55.
Macglashan DW Jr, Saini SS. Omalizumab increases the intrinsic sensitivity of human basophils to IgE-mediated stimulation. J Allergy Clin Immunol. 2013;132(4):906–11. e1-4
Koski R, Kennedy KK. Treatment with omalizumab or cyclosporine for resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2017;119(5):397–401.
Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. The 2017 revision and update. Allergy. 2018.
Di Bona D, Fiorino I, Taurino M, Frisenda F, Minenna E, Pasculli C, et al. Long-term "real-life" safety of omalizumab in patients with severe uncontrolled asthma: a nine-year study. Respir Med. 2017;130:55–60.
FDA U. (Omalizumab): safety information 2015. 2015 [Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103976s5224lbl.pdf.
Namazy J, Cabana MD, Scheuerle AE, Thorp JM Jr, Chen H, Carrigan G, et al. The Xolair pregnancy registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol. 2015;135(2):407–12.
Gonzalez-Medina M, Curto-Barredo L, Labrador-Horrillo M, Gimenez-Arnau A. Omalizumab use during pregnancy for chronic spontaneous urticaria (CSU): report of two cases. J Eur Acad Dermatol Venereol. 2017;31(5):e245–e6.
Ensina LF, Cusato-Ensina AP, Camelo-Nunes IC, Sole D. Omalizumab as third-line therapy for urticaria during pregnancy. J Investig Allergol Clin Immunol. 2017;27(5):326–7.
Staubach P, Metz M, Chapman-Rothe N, Sieder C, Brautigam M, Canvin J, et al. Effect of omalizumab on angioedema in H1 -antihistamine-resistant chronic spontaneous urticaria patients: results from X-ACT, a randomized controlled trial. Allergy. 2016;71(8):1135–44.
Staubach P, Metz M, Chapman-Rothe N, Sieder C, Brautigam M, Maurer M, et al. Omalizumab rapidly improves angioedema-related quality of life in adult patients with chronic spontaneous urticaria: X-ACT study data. Allergy. 2018;73(3):576–84.
Saini SS, Bindslev-Jensen C, Maurer M, Grob JJ, Bulbul Baskan E, Bradley MS, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135(1):67–75.
Maurer M, Sofen H, Ortiz B, Kianifard F, Gabriel S, Bernstein JA. Positive impact of omalizumab on angioedema and quality of life in patients with refractory chronic idiopathic/spontaneous urticaria: analyses according to the presence or absence of angioedema. J Eur Acad Dermatol Venereol. 2017;31(6):1056–63.
Nettis E, Cegolon L, Di Leo E, Canonica WG, Detoraki A, Italian OSG. Omalizumab in elderly patients with chronic spontaneous urticaria: an Italian real-life experience. Ann Allergy Asthma Immunol. 2018;120(3):318–23.
Syrigos N, Grapsa D, Zande M, Tziotou M, Syrigou E. Treatment response to omalizumab in patients with refractory chronic spontaneous urticaria. Int J Dermatol. 2018;57(4):417–22.
Iemoli E, Niero F, Borgonovo L, Cossu MV, Piconi S. Successful Omalizumab treatment in HIV positive patient with chronic spontaneous urticaria: a case report. Eur Ann Allergy Clin Immunol. 2017;49(2):88–91.
Syrigos N, Grapsa D, Syrigou E. Omalizumab for refractory chronic spontaneous urticaria during concurrent immunomodulatory therapy for multiple sclerosis. Eur Ann Allergy Clin Immunol. 2017;49(6):286–7.
Morgado-Carrasco D, Fusta-Novell X, Podlipnik S, Combalia A, Aguilera P. Clinical and photobiological response in eight patients with solar urticaria under treatment with omalizumab, and review of the literature. Photodermatol Photoimmunol Photomed. 2017.
Koumaki D, Seaton ED. Successful treatment of refractory cholinergic urticaria with omalizumab. Int J Dermatol. 2018;57(1):114.
Kocaturk E, Can PK, Akbas PE, Copur M, Degirmentepe EN, Kiziltac K, et al. Management of chronic inducible urticaria according to the guidelines: a prospective controlled study. J Dermatol Sci. 2017;87(1):60–9.
Maurer M, Schutz A, Weller K, Schoepke N, Peveling-Oberhag A, Staubach P, et al. Omalizumab is effective in symptomatic dermographism-results of a randomized placebo-controlled trial. J Allergy Clin Immunol. 2017;140(3):870–3.e5.
Maurer M, Metz M, Brehler R, Hillen U, Jakob T, Mahler V, et al. Omalizumab treatment in patients with chronic inducible urticaria: a systematic review of published evidence. J Allergy Clin Immunol. 2018;141(2):638–49.
Nettis E, Cegolon L, Macchia L, Zaza I, Calogiuri G, Di Leo E. Efficacy of omalizumab treatment with concomitant antihistamines as needed for moderate, refractory chronic spontaneous urticaria. Acta Derm Venereol. 2018.
Iribarren C, Rahmaoui A, Long AA, Szefler SJ, Bradley MS, Carrigan G, et al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: results from EXCELS, a prospective cohort study in moderate to severe asthma. J Allergy Clin Immunol. 2017;139(5):1489–95.e5.
Konstantinou GN, Chioti AG, Daniilidis M. Self-reported hair loss in patients with chronic spontaneous urticaria treated with omalizumab: an under-reported, transient side effect? Eur Ann Allergy Clin Immunol. 2016;48(5):205–7.
Noshela Ghazanfar M, Thomsen SF. Transient hair loss in patients with chronic spontaneous urticaria treated with omalizumab. Eur Ann Allergy Clin Immunol. 2017;49(6):284–5.
Busse W, Buhl R, Fernandez Vidaurre C, Blogg M, Zhu J, Eisner MD, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983–9.e6.
Long A, Rahmaoui A, Rothman KJ, Guinan E, Eisner M, Bradley MS, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560–7.e4.
Godse K, De A, Zawar V, Shah B, Girdhar M, Rajagopalan M, et al. Consensus statement for the diagnosis and treatment of urticaria: a 2017 update. Indian J Dermatol. 2018;63(1):2–15.
Ferrer M, Boccon-Gibod I, Goncalo M, Inaloz HS, Knulst A, Lapeere H, et al. Expert opinion: defining response to omalizumab in patients with chronic spontaneous urticaria. Eur J Dermatol. 2017;27(5):455–63.
Asero R, Canonica GW, Cristaudo A, Fierro MT, Girolomoni G, Marzano AV, et al. Critical appraisal of the unmet needs in the treatment of chronic spontaneous urticaria with omalizumab: an Italian perspective. Curr Opin Allergy Clin Immunol. 2017;17(6):453–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Larenas-Linnemann reports personal fees from GSK, Astrazeneca, MEDA, Boehringer Ingelheim, Novartis, Grunenthal, UCB, Amstrong, Siegfried, DBV Technologies, MSD, Pfizer, grants from Sanofi, Astrazeneca, Novartis, UCB, GSK, TEVA, Chiesi, Boehringer Ingelheim, outside the submitted work. The other authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Allergic Skin Diseases
Rights and permissions
About this article
Cite this article
Larenas-Linnemann, D.E.S., Parisi, C.A.S., Ritchie, C. et al. Update on Omalizumab for Urticaria: What’s New in the Literature from Mechanisms to Clinic. Curr Allergy Asthma Rep 18, 33 (2018). https://doi.org/10.1007/s11882-018-0787-5
Published:
DOI: https://doi.org/10.1007/s11882-018-0787-5