Abstract
Purpose of Review
The purpose of this study was to review real-life studies on effectiveness and safety of omalizumab in chronic urticaria (CU).
Recent Findings
CU is an itching skin disease characterized by wheals, angioedema, or both (present >6 weeks). Omalizumab is a humanized anti-IgE monoclonal antibody approved for treatment of CU and is becoming one of the main treatment options for antihistamine-resistant CU; however, real-life studies on long-term effectiveness and safety are lacking.
Summary
We present an overview of the real-life literature totaling 505 patients with an age range of 7–82 years, on the effectiveness and safety of omalizumab used for CU since 2013. A complete response to omalizumab was seen among 64% of the patients, whereas 25% obtained partial response. On average, 15% had no or very limited response. Fifteen patients from five studies reported side effects. Overall, omalizumab was effective and well-tolerated for patients with antihistamine-resistant CU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic urticaria is a severely itching skin disease characterized by wheals, angioedema, or both for more than 6 weeks. Chronic urticaria is further subdivided into chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CINDU). CINDU appears after physical stimuli such as heat, cold, or sun exposure [1].
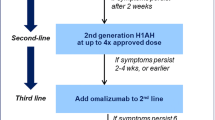
The recommended treatment for chronic urticaria by international guidelines is non-sedating antihistamines once daily as first-line therapy; if no effect is observed, it is recommended to increase the dose of antihistamine up to fourfold. Third-line options include add-on therapy of omalizumab, ciclosporine, or montelukast [2•].
Omalizumab is a humanized monoclonal antibody, which was approved for treatment of CSU in 2014 and for severe allergic asthma in 2004. Omalizumab binds to immunoglobulin E (IgE) and thereby inhibits its binding to the high affinity receptor, FcεRI on the surface of mast cells and basophils [3]. Thus, omalizumab reduces the level of free IgE and downregulates IgE receptors on these cells [4]. Clinical studies have shown that omalizumab significantly reduces the activity of chronic urticaria, with 52–66% of patients obtaining complete or almost complete symptom control when treated with the recommended dose of omalizumab, 300 mg s.c. every 4 weeks [5••, 6, 7]. Further, omalizumab reduced the need for additional medication, improves quality of life, and the number of angioedema-free days [1, 5••, 6,7,8].
Omalizumab is increasingly becoming one of the main treatment options for antihistamine-resistant chronic urticaria; however, real-life studies on long-term effect and safety are lacking. The aim of this paper is to review published real-life studies on effectiveness and side effects of omalizumab treatment in patients with chronic urticaria.
Literature Search
A systemic literature search was conducted in PubMed and EMBASE using the following search terms: “chronic urticaria,” “omalizumab,” “omalizumab AND urticaria,” and “CSU AND omalizumab.” All studies were reviewed by the first author, and retrospective and prospective case series and observational studies published since 2013 and including patients with chronic urticaria treated with omalizumab were included. Reference lists were scrutinized for completion.
Main Findings
The literature search identified a total of 13 studies; four prospective and nine retrospective studies published since 2013. The 13 studies comprised 505 patients (375 females and 130 males) with an age range of 7–82 years, who were all diagnosed with antihistamine refractory chronic urticaria and treated with omalizumab. Some of the patients had also received treatment with ciclosporine or montelukast before initiating treatment with omalizumab. Omalizumab was most often administrated as 300 mg once every fourth week or 150 mg once every second or fourth week. In some cases omalizumab was administrated with shorter or prolonged intervals, or in higher doses, according to the patients’ needs. The data of the studies was collected from 2006 till 2016. The patient characteristics and clinical outcomes of the identified studies are shown in Table 1.
The majority of the identified studies reported the response to omalizumab treatment based on overall physician assessment of the patients’ relief of symptoms as either “complete,” “partial,” or “none,” while three studies reported the response based on improvement in validated scoring systems such as UAS7 (Urticaria Activity Score in the past week), UCT (Urticaria Control Test), or DLQI (Dermatology Quality of Life Index). UAS7 is a reliable tool, which prospectively documents signs and symptoms of urticaria for 7 days. UAS7 is based on intensity of itch, which ranges from 0 (none) to 3 (severe), and number of wheals, which range from 0 (none) to 3 (>50 wheals per day). The score is summed over 7 days giving the UAS7 score of 0 to 42 points. A UAS7 score of 7–15 indicates mild urticaria; a score of 16–27 points indicates moderate urticaria, whereas severe urticaria is characterized by a UAS7 score of 28–42. The UCT is a retrospective four-item questionnaire, which documents control of urticaria in the past 4 weeks. Each question is scored from 0 to 4. A UCT score of >11 indicates well-controlled disease [21]. DLQI is based on ten questions and is a well-used questionnaire in dermatology for measuring quality of life [9]. The questions relate to symptoms, social activity, personal relationships, and treatment-associated problems. Each question in the DLQI is scored from 0, which indicates no impact to 3, which indicate very much impact [22].
The fraction of patients who obtained complete response to omalizumab in the reviewed studies was, on average, 64% (lowest 47% [13], highest 72% [18]), whereas the fraction of patients who obtained partial response was, on average, 25% (lowest 7% [17], highest 43% [4]). The proportion of patients with no or very limited response to omalizumab in the 13 identified studies was, on average 15% (lowest 2% [18], highest 33% [14, 16]).
A combined total of 15 patients from five studies reported adverse effects, primarily headache, nausea, fatigue, injection-site reactions, and palpitations. Two studies reported adverse effects but did not specify number of patients with adverse effects.
Effect of Omalizumab in Real Life for CSU
Prospective Studies
The four prospective studies identified comprised a total of 116 patients treated with omalizumab [10•, 11, 12, 22]. All four studies reported an overall very positive effect of omalizumab treatment; however, a Danish study by Lefévre et al. [22] observed that no patient had a complete response without the use of concomitant medications such as antihistamines or systemic immunosuppressants such as azathioprine. Likewise, Gómez-Vera et al. [12] also showed that no patient experienced complete response. However, both studies reported a significant reduction in UAS7 scores after treatment of, respectively, 31.1 to 8 and 32 to 6 that by far exceeded the minimal relevant clinical difference in UAS7, which has been estimated to be approximately 10 points [23].
A third prospective study by Sussman et al. [10•] included 61 patients with CSU. Patients from two different centers from Canada were included. The effect of 150 mg omalizumab was evaluated according to changes in UAS7 scores. The study reported a reduction in UAS7 from 32.2 to 5.7 in one subgroup and a reduction from 24.4 to 2.2 in the other subgroup after the last treatment. The fraction of complete responders in both subgroups was 68%, whereas the fraction reporting no response was only 3%.
Tontini et al. [11] also evaluated patients prospectively. Eight patients were treated with 300 mg omalizumab and UAS7 was used to grade the response. Complete responders were defined as a decrease of at least 90% in UAS7 from baseline. A reduction in UAS7 from 34.4 to 1.4 was reported after 6 months of treatment.
Retrospective Studies
Two retrospective studies with a combined total of 64 patients treated with 150 or 300 mg omalizumab every fourth week [15, 17] used UAS7 to determine response rates. In a German population of patients with CSU, Metz et al. [23] observed complete response among 83% (at least 90% reduction in UAS7), which was a bit higher compared to the complete response rate (UAS7 = 0) of 61% among CSU patients from Thailand observed by Kulthanan et al. [17], possibly due to the difference in the definition of complete response between the two studies.
A large retrospective study from Denmark by Ghazanfar et al. [20] examined the effect of omalizumab in 137 patients with CSU. They found a complete or almost complete response among 67% of the patients (defined as 90% or more reduction in reported symptoms) and a partial response among 22% of the patients (defined as between 30 and 89% reduction in reported symptoms).
In contrast, a retrospective chart review of 41 patients from the USA by Clark et al. [19] found a significant response among 100% of the patients receiving 300 mg omalizumab every second week (defined as at least a 50% improvement in overall symptom severity) and among 66.7% of the patients receiving 300 mg omalizumab every fourth week.
A Brazilian study of 47 antihistamine refractory CSU patients investigated two different doses of omalizumab in two groups of patients—150 and 300 mg—every fourth week [18]. A complete response of 84.6% was seen among patients treated with 300 mg compared to 60% complete response in the group treated with 150 mg. Only one patient had no effect of either 150 or 300 mg omalizumab. Likewise, Rottem et al. also noted a higher complete response rate of 77% in patients treated with 300 mg compared to 36% in patients treated with 150 mg every 4 weeks [4].
Dosing Regimens
The licensed dose of omalizumab is 300 mg every 4 weeks; however, no formal recommendations exist for tapering or optimizing dosage when symptoms are well-controlled or recur. Tontini et al. [11] suggested that omalizumab treatment should be discontinued after induction of remission; however, they did not define an optimal timing for this. Also, if omalizumab cannot be completely withdrawn due to symptoms, then a long term patient-tailored management is possible, as no decrease in the efficacy of omalizumab was observed in their prospective study. Tontini et al. also observed that most CSU patients experienced flare-ups if administration of omalizumab was delayed more than 30 days [11].
Danish researchers have published an algorithm for treating chronic urticaria patients with omalizumab [24•]. The study included a total of 27 patients with a mean age of 34 years. The inclusion criteria of the study were a daily UAS score of 6. Omalizumab 150 mg was administrated every second week while the patients also continued on high dose of antihistamines. If the patients’ symptoms were reduced to a UAS of less than 2 then the dosing interval was prolonged successively to a maximum of 8 weeks. If the patients scored UAS 2 or more after 2–3 administrations of the same dose, the dosing interval was reduced to 1 week. If the patient still had a UAS of more than 3 after 2–3 doses of 150 mg omalizumab, the dose was then increased to 300 mg. Fifteen (55.5%) patients experienced a UAS of less than 2 after being treated with 150 mg omalizumab, while 12 patients (44.4%) continued treatment with 300 mg omalizumab and reached a UAS of less than 2. Three (11.1%) patients were completely symptom-free and were able to discontinue treatment with omalizumab. Concurrently, Arment-Carbo et al. [14] presented a case series of 15 patients treated with omalizumab of whom eight patients reported complete response after 6 months of treatment, although three out of the eight patients were only able to achieve complete response after an increase of omalizumab from 150 to 300 mg. Other real-life studies also suggested an individualized dosing regimen as the most efficient treatment plan [4, 18]. Notably, a retrospective study by Metz et al. [25••] of 25 patients with CSU and/or CINDU (mean age 45 years) investigated the response to omalizumab during retreatment. All patients experienced complete symptom control after a first round of omalizumab but 23 patients experienced relapse of disease despite antihistamine treatment within 2 to 8 weeks after the last injection of omalizumab. Two patients experienced relapse of symptoms after 4 and 7 months. Retreatment with omalizumab was successful and resulted in rapid and complete response after the first injection and all patients were able to stop antihistamine treatment. Ghazanfar et al. [20] also observed complete response to retreatment with omalizumab in patients who experienced relapse of symptoms after discontinuation of omalizumab.
Prediction of Response to Omalizumab in Real Life
Unlike the recommendation for asthma, the administration of omalizumab for CSU is done without considering IgE levels and weight of the patients except for two of the identified studies [13, 16], which used dosing monograms for asthma. Both studies concluded that weight does not have an effect on the effectiveness of omalizumab. Romano et al. [16] found that patients with low levels of IgE responded poorly to omalizumab therapy. Further two studies [19, 20] also investigated whether there was a difference in response to omalizumab treatment based on predictors such as IgE, age, and gender. Both studies reported that there was no statistically significant difference in response when parameters such as age, gender, and IgE levels were included.
Ghazanfar et al. found that patients with a positive HR (histamine release) test (indicative of autoimmunity) had a lower rate of complete or almost complete response (27.3% compared to 77.3% among patients with a negative HR test) [20]. Accordingly, a retrospective chart review by Palacious et al. [26] of 41 patients with chronic urticaria concluded that having a negative basophil CD203c assay predicts a greater response to omalizumab. Among patients with a negative CD203c 87% responded to omalizumab compared to 50% among patients with a positive CD203c. These results signal that the presence of IgG anti-IgE (receptor) autoantibodies or other autoantibodies in CSU could be a predictor of poor or slower response to omalizumab. However, another retrospective study from the United States by Viswanathan et al. investigated the effectiveness of omalizumab in 19 patients with refractory CSU [13], where ten patients also had autoimmunity. Four patients without and five patients with autoimmunity responded completely to the treatment, while eight responded partially and only two patients had no response. Overall, no difference in response to omalizumab was found according to whether the patients had autoimmunity.
Safety
Seven out of the 13 studies reported side effects [4, 12, 13, 15, 16, 19, 20]. The most common were nausea, headache, dizziness, fatigue, and injection-site reactions, whereas no severe side effects were reported. This is in line with the adverse effect profile reported in the clinical trials [5••, 6,7,8]. Metz et al. reported one case of mild cutaneous angioedema [15], while Rottem et al. reported one patient with palpitations and weakness 2 h after administration of omalizumab, but no other complications were reported and the patient continued treatment [4]. In a recent case report, Konstantinou et al. reported transient hair loss as a possible side effect of omalizumab in three women with a mean age of 56.6 years treated successfully with omalizumab for refractory CSU; however, none of the patients discontinued their treatment. In one of the women, hair loss was visible by scalp inspection; however, this patient was also known with Hashimoto’s thyroiditis, which is commonly associated with hair loss [27•].
Further investigations regarding the safety of omalizumab are needed. Particularly, data on omalizumab usage during pregnancy and lactation is important, as the majority of the patients with CSU are female of childbearing age. A few reports of the use of omalizumab for CSU during pregnancy have been published. Notably, in a report of four female patients with CSU, omalizumab had a great effect on their urticarial activity and they experienced no complications in their pregnancy and had full term deliveries with no fetal complications [28•]. A similar case report was presented from Denmark [29•] on a female patient who was treated with omalizumab continuously through two consecutive pregnancies. The patient experienced no complications during any of her pregnancies and delivered two healthy babies. Likewise, Santos et al. [30] reported a female patient with three types of chronic urticaria (CSU, delayed pressure urticaria, and symptomatic dermographism) who was treated with omalizumab during pregnancy due to increased disease activity. The patient was treated with 150 mg omalizumab every 2 weeks. She experienced no complications during pregnancy or labor and she delivered a healthy baby. Furthermore, in a pregnancy register of omalizumab use in female asthmatic patients [31], a total of 191 asthmatic women were treated with one or more doses of omalizumab. No apparent increased birth prevalence of major anomalies was reported. However, further investigations regarding use of omalizumab during pregnancy in chronic urticaria patients are needed.
Effectiveness in CINDU
A prospective study by Sussman et al. [10•] investigated the response to omalizumab in six patients with cold urticaria. They observed that all patients became symptom-free on omalizumab with a significant decrease of their cold stimulation tolerance test results. Metz et al. [15] also investigated the response of omalizumab treatment in CINDU patients reporting 34 cases of different types of CINDU, of which 71% responded completely to omalizumab. However, Metz et al. also noted that a larger fraction of CINDU patients needed updosing to achieve complete response compared to the CSU patient group.
Ghazanfar et al. [20] studied a total of 17 patients with different forms of CINDU (cold, delayed pressure, symptomatic dermographism, or cholinergic urticaria) and observed a complete response rate of omalizumab in 53%. Four patients experienced limited or no effect. None of the CINDU patients in this study reported any side effects.
Future Perspectives
Gimenez-Arnau et al. [32] present a real-life guideline for the use of omalizumab in CSU, which suggests a starting dose of 300 mg once every 4 weeks and advice that clinical efficacy should be monitored with validated tools such as UAS7 and UCT, as this will make the comparison between real-life results easier. Furthermore, it is suggested that patients should be treated until they are completely symptom-free and the disease is under control, then it is recommended to prolong the treatment intervals instead of terminating the treatment completely or reduce the dose to avoid relapse of severe symptoms. However, it is possible to terminate the treatment completely and retreat the patient if needed. If the patient experiences no effect after 6 months of treatment with 300 mg omalizumab every month, then the dose can be increased to 450 or 600 mg once a month, but if no response is seen after an additional 3 months on high dose treatment, then the patient should be considered a non-responder.
Conclusion
The reviewed real-life evidence showed that the far majority of patients with CSU show great response to omalizumab with the fraction of patients responding completely or almost completely to therapy being around 64% and a further 25% responding partially. This translates into an overall beneficial effect of omalizumab in almost 90% of patients with CSU. However, it is difficult to compare studies due to differences in definition of effect with some studies reporting response based on overall physician judgment and other using validated scoring systems such as UAS7.
No serious side effects or complications were encountered in any of the reviewed studies; however, mild side effects of treatment may occur, particularly nausea, headache, dizziness, fatigue, and injection-site reactions. However, larger real-life studies on long-term administration of omalizumab in CSU patients are still needed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Saini S, Rosen KE, Hsieh HJ, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol Pract. 2011;128:567–73.
• Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA(2) LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868–87. This is the internationally endorsed and clinically recommended guideline for management of urticaria.
Büyüköztürk S, Gelincik A, Demirtürk M, et al. Omalizumab markedly improves urticarial activity scores and quality of life scores in chronic spontaneous urticarial patients: a real life survey. J Dermatol. 2012;39:439–42.
Rottem M, Segal R, Kivity S, et al. Omalizumab therapy for chronic spontaneous urticaria: the Israeli experience. Isr Med Assoc J. 2014;16:487–90.
•• Maurer M, Rosen K, Hsieh H, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–35. A large phase 3 clinical trial of omalizumab in chronic urticaria.
Kaplan A, Ledford D, Ashby M, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132:101–9.
Saini S, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75
Maurer M, Altrichter S, Bieber T, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202–9.
Lefévre AC, Deleuran M, Vestergaard C. A long term case series study of the effect of omalizumab on chronic spontaneous urticaria. Ann Dermatol. 2013;25:242–4.
• Sussman G, Hébert J, Barron C, et al. Real-life experience with omalizumab for the treatment of chronic urticaria. Ann Allergy Asthma Immunol. 2014;112:170–4. A large prospective study of the effectiveness of omalizumab in a real-life setting.
Tontini C, Marinangeli L, Cognigi M, et al. Omalizumab in chronic spontaneous: patient-tailored tapering or planned discontinuation? Ann Allergy Asthma Immunol. 2015;115:147–62.
Gómez-Vera J, Gutiérrez-Avila SA, Acosta-Gutiérrez DN, et al. Omalizumab in the treatment of antihistamine-resistant chronic urticaria in adults. Ann Allergy Asthma Immunol. 2016;117:192–211.
Viswanatha RK, Moss MH, Mathur SK. Retrospective analysis of the efficacy of omalizumab in chronic refractory urticaria. Allergy Asthma Proc. 2013;334:446–52.
Armengot-Carbo M, Velasco-Pastor M, Rodrigo-Nicholas B, et al. Omalizumab in chronic urticaria: a retrospective series of 15 cases. Dermatol Ther. 2013;26:257–9.
Metz M, Ohanyan T, Church MK, et al. Omalizumab is an effective and rapidly acting therapy in difficult to treat chronic urticaria: a retrospective clinical analysis. J Dermatol Sci. 2014;73:57–62.
Romano C, Sellitto A, De Fanis U, et al. Omalizumab for difficult-to-treat dermatological conditions: clinical and immunological features from a retrospective real-life experience. Clin Drug Investig. 2015;35:159–68.
Kulthanan K, Tuchinda P, Chularojanamontri L, et al. Omalizumab therapy for treatment of recalcitrant chronic spontaneous urticaria in an Asian population. J Dermatolog Treat. 2016;
Ensina LF, Valle SOR, Juliani AP, et al. Omalizumab in chronic spontaneous urticaria: a Brazilian real-life experience. Int Arch Allergy Immunol. 2016;169:121–4.
Clark JJ, Secrest AM, Hull CM, et al. The effect of omalizumab dosing and frequency in chronic idiopathic urticaria: retrospective chart review. J Am Acad Dermatol. 2016;74:1274–5.
Ghazanfar MN, Sand C, Thomsen SF. Effectiveness and safety of omalizumab in chronic spontaneous or inducible urticaria: evaluation of 154 patients. Br J Dermatol. 2016;175:404–6.
Weller K, Groffik A, Church MK, et al. Development and validation of the urticaria control test: a patient-reported outcome instrument for assessing urticaria control. J Allergy Clin Immunol. 2014;133:1365–72.
Basra MKA, Fenech R, Gatt RM, et al. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159:997–1035.
Mathias SD, Crosby RD, Zazzali JL, et al. Evaluating the minimally important difference of the urticaria activity score and other measures of disease activity in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2012;108:20–4.
• Uysal P, Eller E, Mortz CG, et al. An algorithm for treating chronic urticaria with omalizumab: dose interval should be individualized. J Allergy Clin Immunol. 2014;133:914–5. An important study on how dosing interval should be individualized in the treatment of urticaria with omalizumab.
•• Metz M, Ohanyan T, Church MK, et al. Retreatment with omalizumab results in rapid remission in chronic spontaneous and inducible urticaria. JAMA Dermatol. 2014;15:288–90. This study provides relevant information on retreatment with omalizumab.
Palacious T, Stillman L, Borish L, et al. Lack of basophil CD203c-upregulating activity as an immunological marker to predict response to treatment with omalizumab in patients with symptomatic chronic urticaria. J Allergy Clin Immunol Pract. 2016;
• Konstantinou GN, Chioti AG, Danilidis M. Self-reported hair loss in patients with chronic spontaneous urticaria treated with omalizumab: an under-reported, transient side effect? Eur Ann Allergy Clin Immunol. 2016;48:205–7. An interesting case report on omalizumab and transient hair loss as side effect.
• Cuervo-Padro L, Barcena-Blanch M, Radojicic C. Omalizumab use during pregnancy for CIU: a tertiary care experience. Eur Ann Allergy Clin Immunol. 2016;48:145–6. An interesting case report on omalizumab use during pregnancy.
• Ghazanfar MN, Thomsen SF. Successful and safe treatment of chronic spontaneous urticaria with omalizumab in a woman during two consecutive pregnancies. Case Rep Med. 2015:Article ID 368053. An interesting case report on successful treatment with omalizumab during two pregnancies
Vieira Dos Santos R, Locks Bidese B, Rabello de Souza J, et al. Omalizumab in a patient with three types of chronic urticaria. Br J Dermatol. 2014;170:469–71.
Namazy J, Cabana A, Scheuerle E, et al. The Xolair pregnancy registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol. 2015;135:407–12.
Giménez-Arnau AM, Toubi E, Marsland AM, et al. Clinical management of urticaria using omalizumab: the first licensed biological therapy available for chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2016;30:25–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Misbah Noshela Ghazanfar declares no conflicts of interest.
Simon Francis Thomsen is a speaker and investigator and has received consultant fees and research grants from Novartis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cutaneous Drug Reactions
Rights and permissions
About this article
Cite this article
Ghazanfar, M.N., Thomsen, S.F. Omalizumab for Chronic Urticaria: Aftermarket Reports of Efficacy and Side Effects. Curr Derm Rep 6, 48–54 (2017). https://doi.org/10.1007/s13671-017-0182-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-017-0182-9