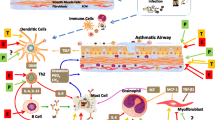
ABSTRACT
Gender differences in asthma incidence, prevalence and severity have been reported worldwide. After puberty, asthma becomes more prevalent and severe in women, and is highest in women with early menarche or with multiple gestations, suggesting a role for sex hormones in asthma genesis. However, the impact of sex hormones on the pathophysiology of asthma is confounded by and difficult to differentiate from age, obesity, atopy, and other gender associated environmental exposures. There are also gender discrepancies in the perception of asthma symptoms. Understanding gender differences in asthma is important to provide effective education and personalized management plans for asthmatics across the lifecourse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearly 40 million people (12.9 %) in the Unites States are diagnosed with asthma [1]. This chronic inflammatory airway disease results in ~ $50 billion per year cost to society in addition to the significant loss of productivity of individuals who suffer from asthma [1–4]. There is convincing evidence to support gender effects in the pathophysiology and severity of asthma [Table 1].
Epidemiological data show that asthma incidence, prevalence and severity differs according to gender. Asthma prevalence, severity, exacerbation rate, hospitalizations and mortality are higher among women than men overall; however, asthma related office and emergency room visits and hospitalizations are higher among boys than girls 0 to 14 years of age [3, 5–10]. The reasons for the gender difference are unknown but have been linked to immunological and hormonal factors, and/or to differences in gender-specific responses to environmental or occupational exposures [11–14]. For example, children living on farms have a lower incidence of asthma [15], which has been related to modulation of the immune system by early life exposures, differences in physical activity levels or eating habits [16, 17]. However gender influences the impact of this exposure, i.e. the cumulative asthma incidence is lower in girls as compared to boys raised on a farm [18]. Asthma in early childhood is generally associated with male gender, poor socioeconomic status, and exposure to soot, exhaust and/or household tobacco, wood, or oil smoke [19, 20]. Yet, asthma in early childhood is only associated with obesity in young girls, not in young boys in two large cross-sectional series from China and the Netherlands [21, 22], and in two longitudinal cohorts from the United Kingdom and Taiwan. The U.K. study followed children longitudinally until the age of 8 years and found that a higher body mass index (BMI) was associated with increased wheezing in girls but not in boys [OR (95CI): 1.52 (1.01, 2.28)] [23]. The Taiwanese study followed adolescents prospectively for 12 months, and reported that obese adolescent females but not males were at higher risk of developing asthma [24]. Interestingly, higher serum leptin levels were associated with obesity, female gender and asthma [25•]. Leptin, which plays a key role in body weight regulation, promotes Th1 immune responses and increases the production of pro-inflammatory mediators [25•]. The gender dimorphism of the obese-asthma phenotype is supported by the finding that asthma impairment related to obesity is highest among women 12 to 44 years of age [26, 27]. The European Network For Understanding Mechanisms of Severe Asthma (ENFUMOSA) and Severe Asthma Research Program (SARP) identified that there was a higher ratio of female to male gender (4.4:1) in severe asthma as compared to nonsevere asthma, and that BMI was higher among severe asthmatic women as compared to nonsevere asthmatic women, but was not different among nonsevere and severe asthmatic men [9, 28, 29]. Thus, most reports, but not all [30] , suggest a gender-obesity interaction in asthma, but it remains unclear whether the sex hormones or other gender-specific factors are responsible for such differences.
Interestingly, the lifetime diagnosis of asthma and current asthma was recently reported to be higher among same-sex partnered men and women. Such increased risk may be mediated by a higher prevalence of obesity among same-sex partnered women and by the higher prevalence of smoking among same-sex partnered men [31]. A higher prevalence of asthma is also found in women with attention deficit hyperactivity disorder (ADHD) as compared to men with ADHD, which has been related to higher prevalence of smoking and obesity [32] among individuals with ADHD. Thus, it is imperative to assess the role and impact of confounders before confirming or dismissing associations between gender and asthma.
Exposure to particulate matter or to second-hand smoking [33] are associated with lower Forced Expiratory Volume in the first second (FEV1) in women younger than 55 years of age, but not in men. The role of air pollution was recently assessed in 30,139 Chinese children aged 3 to 12 years. Among those children, respiratory symptoms in relation to air pollution were reported more frequently by girls with allergic tendency, whereas effects were more prominent in the non-allergic boys [34]. In this context, there is a higher incidence of non-atopic asthma in women during the reproductive period as compared to men, but no gender difference in the incidence of atopic asthma. [35, 36]. Altogether, there is evidence to suggest an interaction between gender, obesity and asthma type (atopic vs. nonatopic). This indicates an effect modification by age, BMI and gender on the response to risk factors and environmental exposures [19].
Sex hormones and Asthma
Asthma is more prevalent and severe in young boys [37, 38], but there is a gender-switch at puberty, which has been related to increase of sex hormones [9, 39, 40]. The transition from childhood to adulthood is characterized by a higher odds ratio of persistence of wheezing in females [41, 42], and by asthma improvement in males but asthma worsening in females [43]. In two cohorts of patient followed longitudinally until the age of 18 years, male gender was independently associated with asthma remission [13, 44, 45]. After age of 11 years, the provocative concentration of methacholine necessary to cause a 20 % decrement in FEV1 (PC20) increased in adolescent boys suggesting an improvement in airway responsiveness during puberty in boys but not in girls [30]. The male-specific increase in PC20 was seen after Tanner stage 2, and further increased with sexual maturation [30]. In contrast, adult women with stable well-controlled asthma decrease PC20 by more than half over the course of the menstrual cycle, with the lowest PC20 occurring at peak estrogen and progesterone levels in the luteal phase [46]. The cyclic changes in PC20 have been attributed to abnormal β2 adrenoceptor regulation in premenstrual asthma [46, 47]. It has been suggested that β2 adrenoceptors are influenced by ovarian sex-steroid hormones, and that this is the mechanism underlying gender differences in β2 bronchodilator responses [47]. This concept is supported by the paradoxical down regulation of β2 adrenoceptors when progesterone is given during the follicular phase to women with premenstrual asthma [47–49]. On the other hand, estrogen supplementation during the follicular phase had no effect on β2 adrenoceptor responses or airway reactivity [48, 50]. Interestingly, while higher progesterone to estrogen ratio occurs during the luteal phase of fertile cycles [51, 52], the opposite occurs during the menopausal transition, where women are exposed to unopposed estrogen stimulation [53]. Thus, it remains unclear as to whether progesterone and/or estrogen or a balance between the sex hormones are responsible for premenstrual worsening of asthma.
Sex hormones have a wide variety of effects beyond the β2 adrenoceptor. For example, sex hormones alter function of epithelial cells. The progesterone receptor is expressed in airway epithelium and progesterone inhibits the beat frequency of cilia, which may impact mucociliary clearance during menstrual cycle among women [54]. Genetic polymorphisms are also influenced by gender. Immunoglobulin E (IgE) levels and asthma have been associated with single nucleotide polymorphisms (SNPs) in thymic stromal lymphopoietin (TSLP). Two SNPs in TSLP (rs1837253 and rs2289276) are of particular interest. The first is associated with a lower risk of asthma in men, but the second is associated with a higher risk of asthma in women. Whether this differential effect is regulated by gender, or the sex-related differences in the hormonal profile is unknown [55]. Likewise, in a series of 1,261 children and adolescent with moderate to severe asthma, enrolled in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) cohort, IgE levels were higher among boys 6 to 17 years old compared to girls, but girls had higher IgE levels during puberty (12–14 years). A higher IgE level was associated with more symptoms triggered by dust, pollen and animals, and was associated with a lower FEV1/FVC ratio even after adjustment for age, gender and race [56]. Gender-specific differences in the antioxidant response to oxidative stress have been reported. In unadjusted analyses, the activities of superoxide dismutase, glutathione peroxidase, and glutathione reductase were higher among asthmatic women than men [57].
The Role of Puberty
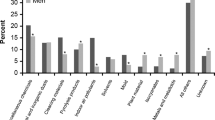
After puberty, a gender switch occurs, and asthma becomes more prevalent and severe in women [30, 40, 58]. Dysanapsis, which refers to the differences in the relative growth of airways and parenchyma, accounts for why boys, who have larger lungs, do not necessarily have larger airways as compared to girls. Later, this differential parenchymal-airway growth is reversed, and may in part be responsible for increased asthma severity in adult women [11, 59, 60]. The exact age at which the gender switch in asthma occurs is based mostly on cross-sectional data and varies among reports from 11 to 18 years of age [1, 4, 13, 40]. Nevertheless, the gender switch is consistent regardless whether information on asthma prevalence, asthma severity or cost to society are used to predict the age of the switch [1] [Fig. 1]. A recent report examined the role of sex hormones on asthma development longitudinally and found that the shift in the prevalence of asthma starts after the age of 11.1 years and remains until the age of 16.3 years. Many reports have linked female sex hormones to asthma severity. Women with premenstrual asthma are at are at higher risk for severe asthma, required more bursts of corticosteroids therapy, and have a higher risk for emergency room visits, hospitalization, and admission to the intensive care unit [61•]. Interestingly, asthmatic women receiving oral contraceptives have attenuated cyclical changes in airway reactivity in association with a suppression of the upsurge in progesterone and estradiol during the luteal phase [62]. Consistent with a detrimental role of sex hormone in women with premenstrual asthma, the risk of asthma increases by as much as two fold in girls who exhibited early menarche [63–65]. In multiparous women, asthma prevalence increases linearly with the number of births [66]. Girls who mature early, and pregnant women are likely to be exposed to higher estrogen levels, and greater cumulative hormonal exposure of sex hormones, which place them at higher risk for asthma development later in life. In contrast, oral contraceptive may be protective [66] and decrease the risk of exacerbation in asthmatic women [64].
Adapted from 2012 National Health Interview Survey (NHIS) Data [1]. Lifetime asthma prevalence (percent) stratified by age and gender, United States. The lifetime prevalence in asthma is higher in boys until the age of 14 and in girls after the after of 15 years
In pregnant women, female sex of the fetus is linked to risk of asthma severity. It is interesting to speculate that this may be related to a spillover of fetal sex hormone into the maternal circulation. While many reports have reported that carrying a female fetus is associated with increased asthma symptoms during pregnancy, a higher use of asthma medications and a higher risk of asthma related hospitalization [67–70], a larger Canadian study did not confirm the finding [71]. Recent reports have demonstrated sex-specific alterations in the expression of placental genes of pregnant women with asthma compared to placentae of non-asthmatic mothers. There were a small number of genes with altered expression (6 genes) in placentae of asthmatic women carrying male fetuses, as compared to 59 genes with altered expression in placentae of asthmatic mothers carrying female fetuses. The genes were linked to growth, inflammation and immune pathways and might contribute to the fetal-sex dimorphic differences in asthma severity and fetal growth during pregnancy [72••].
Perhaps some of the strongest evidence in favor of a role of sex hormones in asthma is the worsening asthma symptoms in the premenstrual period, which occurs in one third of women, and the beneficial role of oral contraceptives [61•, 66], which regulate and prevent the harmful effect of estrogen and progesterone fluctuation. The role of sex hormones in asthmatic women is also supported by the findings of abnormal sex hormone levels among asthmatic women compared to non-asthmatic [73] and by the cyclic changes in FEV1 and gas transfer during the menstrual cycle reaching peaks levels at the end of the luteal phase through the start of menstruation [74••].
The role of menopause
Equally important as the association of asthma to parity, early menarche (<12 years) and the use of oral contraceptives [63, 64, 66, 75], is the protective role of menopause and the deleterious effect of postmenopausal hormonal supplementation [76–78]. The independent effect of menopause on asthma severity may be confounded by the effect of age, obesity, asthma duration and age related comorbidities. Menopause is associated with increased respiratory symptoms and lower FEV1 [79], but another report suggests that menopause is protective [76]. The risk of severe asthma continues to increase in men after the age of 45 years, but not in women [80]. The drop in asthma severity between ages 50 to 65 in menopausal women compared to men supports the theory that asthma improves after menopause.
As sex hormones levels decrease with menopause, the age adjusted risk of asthma may drop in postmenopausal compared to premenopausal women [76, 77]. This protective effect of menopause is reversed by postmenopausal hormone replacement therapy [76, 77, 81, 82]. The highest risk of replacement therapy is in those women using conjugated estrogen [76, 83•], who have a low BMI [78, 82], and are non-smokers [83•]. It remains unknown whether low dose progesterone-based postmenopausal hormonal therapy is safer than the estrogen-progesterone preparations, or whether medroxy-progesterone acetate based combinations used mainly in the United States has different effects than other preparations used in Europe [83•]. It seems that estrogen, progesterone and androgens interact in a complex manner resulting in different airway responses and symptoms variation in patients with asthma, which occur over the lifecourse as well as at different stages within the menstrual cycle.
Menopausal women may not behave similarly, and the effect of perimenopause may be different than of menopause per se. Most women enter the menopausal transition after the age of 45 years once they start having menstrual cycles of variable length [84] as a result of altered ovarian function and exhaustion of the follicular pool. The perimenopausal hormonal abnormalities include hyperestrogenism, hypergonadotropism, and decreased luteal phase progesterone excretion [85]. These changes are associated with a myriad of symptoms including hot flashes, night sweats, depression and poor sleep hygiene [86, 87]. Perimenopausal symptoms get worse before they get better [84]. Furthermore, irregular ovulatory cycles during the perimenopausal period can result in luteal out-of-phase (LOOP) events, i.e. atypical estradiol secretion characterized by a second increase in estradiol during the mid- and late luteal phases [88]. Therefore, asthmatic women may experience different hormonal states as they transition through menopause. This transient hyper-estrogenic state [89–91] may explain the initial deterioration in symptoms and pulmonary function in women entering the menopause transition [79]. After menopause, FSH and LH levels are elevated and estrogen eventually drops to levels seen with surgical oophorectomy. Progesterone levels are also extremely low. This drop in female sex hormones may explain the later protective effect of menopause [76].
It is difficult to separate the physiological and hormonal effect of menopause from other associated behavioral, or psychological factors. Whether increased asthma-related symptoms reported during the early perimenopausal transition, is due to the effect of estradiol levels, or to the misconception and misinterpretation of the vasomotor symptoms remains unclear. Cluster analyses have identified a group of older obese women with difficult-to-manage asthma, who have frequent exacerbations [28]. Studies indicate that the obesity effect on asthma severity is modified by gender [92, 93]. Estrogen and leptin, with are both pro-inflammatory and produced by adipocytes, are higher in obese women as compared to non-obese women. Leptin is associated with increased airway hyper-responsiveness in mouse models [94•], but a direct role in human asthma is unclear and confounded by the respiratory effects of obesity itself [81]. Taking all this into consideration, peri-menopausal women may be at higher risk for severe asthma, and adjustment for age and BMI are necessary to identify the independent role menopause may play in older women.
Differences in perception and behavior
Asthma in women is reported to be more severe and associated with higher health care use [9, 95]. Intriguingly, direct costs due to hospitalization and medication are higher in women compared to men, even after adjusting for the same degree of asthma severity [95]. These findings suggest that women may have a different perception, and take distinct actions regarding their symptoms and asthma control.
The perception of asthma symptoms and airflow obstruction is different between men and women and may be age dependent. While asthmatic women have worse asthma related quality of life, a higher perception of dyspnea, a higher asthma-related healthcare utilization, a higher rate of depression, more rescue inhalers use, and more physical limitation compared to asthmatic men, they have better lung function and similar asthma severity [96, 97, 98•, 99, 100, 101•, 102]. A higher rate of anxiety, excessive daytime sleepiness, and insomnia are reported among women with current asthma compared to men [103]. In line with this, physician-diagnosed asthma is equally prevalent among female and male swimmers (19 %), but women report more respiratory symptoms than men during swimming [104•]. Understanding and using strategies that target this gender-specific difference in disease response and symptom profiles may result in improved asthma related quality of life and health of asthmatic women [105].
Similarly, household and environmental and occupational discrepancies have also been reported among working asthmatics. While working women smoke less than men (18.3 % vs. 22.8 %), they have a higher odds ratio of 2.2 of having diagnosis of asthma [106]. Even with the lower cumulative smoking exposure (number of pack years), asthmatic women with smoking report more wheezing compared to men [107]. Adolescent girls with asthma, but not boys, have more physical tobacco dependence than their nonasthmatic counterparts [108•]. The different workplace exposures between men and women should be taken into consideration when considering gender effects on asthma. Women with work-related asthma are more likely to work in healthcare, educational services, retail trade, and education [109]. While all asthmatics overall are more likely to own a furry pet compared to nonasthmatics (49.9 % versus 44.8 %), and to frequently allow them into the bedroom (68.7 %), asthmatic women have a higher chance of owning a pet compared to asthmatic men [110]. These gender-specific considerations are important to educating asthmatics about asthma control and how to avoid triggers.
Adherence to medications continues to be a major issue in asthma management. While women were much more likely to be educated about asthma control and management and more likely to carry a rescue inhaler than men (61 % vs 30 %) [111, 112], personality traits and the misconception for medication need [113, 114] are additional factors associated with medication adherence. Emergency department visits for acute asthma by adults who ran out of their inhaled medication are more common in men as compared to women [102, 115•, 116]. On the other hand, the higher rate of unscheduled office visits in women might suggest a lower threshold for outpatient healthcare contact [8]. Women need more encouragement and education that men regarding the correct use of peak flow meters [117], and adolescent boys are twice more likely to receive spirometry testing during office visits in a series of 40,528 asthmatic Italian children 6 to 17 years of age [118]. Thus, gender differences in asthma may also be related to behaviors of male and female asthmatics, their adherence to treatment guidelines and to medications and the response of their caregivers to male or female patients.
Conclusion
There is sound evidence in favor of gender effects on asthma incidence and severity throughout the life course. While the clinical and epidemiological data support the role of sex hormones on asthma incidence and severity, the data are confounded by many internal and external factors, such as aging, obesity, atopy, and gender differences in behavior and exposures. Further work is needed to establish the gender impact on asthma in utero, in early-life, at puberty, adulthood and the menopause transition. These studies will also generally reveal novel information on the genesis of asthma and provide new insights for pathway based therapies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
CDC. National Health Interview Survey (NHIS) data. http://www.cdc.gov/asthma/nhis/2012/data.htm. Accessed Dec 2.
Sullivan PW et al. The burden of adult asthma in the United States: evidence from the Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2011;127(2):363–9. e1-3.
Moorman JE et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat. 2012;3(35):1–67.
Akinbami LJ, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012(94): p. 1–8.
Patel M et al. Predictors of Severe Exacerbations, Poor Asthma Control, and beta-Agonist Overuse for Patients with Asthma. J Allergy Clin Immunol Pract. 2014;2(6):751–8. e1.
Melero Moreno C et al. Factors related with the higher percentage of hospitalizations due to asthma amongst women: the FRIAM study. Arch Bronconeumol. 2012;48(7):234–9.
Tomita K et al. Association between episodes of upper respiratory infection and exacerbations in adult patients with asthma. J Asthma. 2012;49(3):253–9.
Heaney LG et al. Refractory asthma in the UK: cross-sectional findings from a UK multicentre registry. Thorax. 2010;65(9):787–94.
The ENFUMOSA. cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J. 2003;22(3):470–7.
Davidson R et al. Influence of maternal and perinatal factors on subsequent hospitalisation for asthma in children: evidence from the Oxford record linkage study. BMC Pulm Med. 2010;10:14.
Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54(12):1119–38.
Almqvist C et al. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63(1):47–57.
Vink NM et al. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents' Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126(3):498–504. e1-6.
Melgert BN et al. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep. 2007;7(2):143–50.
Fuchs O et al. Farming environments and childhood atopy, wheeze, lung function, and exhaled nitric oxide. J Allergy Clin Immunol. 2012;130(2):382–8. e6.
von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10(12):861–8.
Kosti RI et al. The association between leisure-time physical activities and asthma symptoms among 10- to 12-year-old children: the effect of living environment in the PANACEA study. J Asthma. 2012;49(4):342–8.
Genuneit J. Sex-specific development of asthma differs between farm and nonfarm children: a cohort study. Am J Respir Crit Care Med. 2014;190(5):588–90.
Balmes JR et al. Annual average ambient particulate matter exposure estimates, measured home particulate matter, and hair nicotine are associated with respiratory outcomes in adults with asthma. Environ Res. 2014;129:1–10.
Hafkamp-de Groen E et al. Socioeconomic and sociodemographic factors associated with asthma related outcomes in early childhood: the Generation R Study. PLoS One. 2013;8(11):e78266.
Wang D et al. Gender-specific differences in associations of overweight and obesity with asthma and asthma-related symptoms in 30 056 children: result from 25 districts of Northeastern China. J Asthma. 2014;51(5):508–14.
Willeboordse M et al. Sex differences in the relationship between asthma and overweight in Dutch children: a survey study. PLoS One. 2013;8(10):e77574.
Murray CS et al. Body mass index in young children and allergic disease: gender differences in a longitudinal study. Clin Exp Allergy. 2011;41(1):78–85.
Ho WC et al. Higher body mass index may induce asthma among adolescents with pre-asthmatic symptoms: a prospective cohort study. BMC Public Health. 2011;11:542.
Quek YW et al. Associations of serum leptin with atopic asthma and allergic rhinitis in children. Am J Rhinol Allergy. 2010;24(5):354–8. This paper demonstrates that in overweight girls higher serum leptin level was strongly associated with mildto-moderate persistent asthma.
Lang JE et al. Does age impact the obese asthma phenotype? Longitudinal asthma control, airway function, and airflow perception among mild persistent asthmatics. Chest. 2011;140(6):1524–33.
Scott HA et al. Relationship between body composition, inflammation and lung function in overweight and obese asthma. Respir Res. 2012;13:10.
Moore WC et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–23.
Jarjour NN et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185(4):356–62.
Tantisira KG et al. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. Am J Respir Crit Care Med. 2008;178(4):325–31.
Blosnich JR et al. Asthma disparities and within-group differences in a national, probability sample of same-sex partnered adults. Am J Public Health. 2013;103(9):e83–7.
Fasmer OB et al. Adult attention deficit hyperactivity disorder is associated with asthma. BMC Psychiatry. 2011;11:128.
Comhair SA et al. Detrimental effects of environmental tobacco smoke in relation to asthma severity. PLoS One. 2011;6(5):e18574.
Dong GH et al. Gender differences and effect of air pollution on asthma in children with and without allergic predisposition: northeast Chinese children health study. PLoS One. 2011;6(7):e22470.
Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175(7):661–6.
Ma J, Xiao L. Association of general and central obesity and atopic and nonatopic asthma in US adults. J Asthma. 2013;50(4):395–402.
Wijga A et al. Sex differences in asthma during the first 8 years of life: the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) birth cohort study. J Allergy Clin Immunol. 2011;127(1):275–7.
Dawson B et al. A survey of childhood asthma in Aberdeen. Lancet. 1969;1(7599):827–30.
Leynaert B et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax. 2012;67(7):625–31.
Schatz M, Camargo Jr CA. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol. 2003;91(6):553–8.
Sears MR et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349(15):1414–22.
Sekerel BE et al. Are risk factors of childhood asthma predicting disease persistence in early adulthood different in the developing world? Allergy. 2006;61(7):869–77.
Kjellman B, Gustafsson PM. Asthma from childhood to adulthood: asthma severity, allergies, sensitization, living conditions, gender influence and social consequences. Respir Med. 2000;94(5):454–65.
Arshad S, Raza A, Lau L, Bawakid K, Karmaus W, Zhang H, et al. Pathophysiological characterization of asthma transitions across adolescence. Respir Res. 2014;15(1):153.
Andersson M et al. Remission and persistence of asthma followed from 7 to 19 years of age. Pediatrics. 2013;132(2):e435–42.
Tan KS, McFarlane LC, Lipworth BJ. Loss of normal cyclical beta 2 adrenoceptor regulation and increased premenstrual responsiveness to adenosine monophosphate in stable female asthmatic patients. Thorax. 1997;52(7):608–11.
Wheeldon NM et al. Influence of sex-steroid hormones on the regulation of lymphocyte beta 2-adrenoceptors during the menstrual cycle. Br J Clin Pharmacol. 1994;37(6):583–8.
Tan KS, McFarlane LC, Lipworth BJ. Paradoxical down-regulation and desensitization of beta2-adrenoceptors by exogenous progesterone in female asthmatics. Chest. 1997;111(4):847–51.
Tan KS et al. Effects of exogenous female sex-steroid hormones on lymphocyte beta 2-adrenoceptors in normal females. Br J Clin Pharmacol. 1996;41(5):414–6.
Lieberman D et al. Influence of estrogen replacement therapy on airway reactivity. Respiration. 1995;62(4):205–8.
Laufer N, Navot D, Schenker JG. The pattern of luteal phase plasma progesterone and estradiol in fertile cycles. Am J Obstet Gynecol. 1982;143(7):808–13.
Owen Jr JA. Physiology of the menstrual cycle. Am J Clin Nutr. 1975;28(4):333–8.
O'Connor KA et al. Total and unopposed estrogen exposure across stages of the transition to menopause. Cancer Epidemiol Biomarkers Prev. 2009;18(3):828–36.
Jain R et al. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol. 2012;46(4):446–53.
Hunninghake GM et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy. 2010;65(12):1566–75.
Haselkorn T et al. Allergy, total serum immunoglobulin E, and airflow in children and adolescents in TENOR. Pediatr Allergy Immunol. 2010;21(8):1157–65.
Malling TH et al. Differences in associations between markers of antioxidative defense and asthma are sex specific. Gend Med. 2010;7(2):115–24.
Venn A et al. Questionnaire study of effect of sex and age on the prevalence of wheeze and asthma in adolescence. BMJ. 1998;316(7149):1945–6.
Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis. 1980;121(2):339–42.
Hibbert M et al. Gender differences in lung growth. Pediatr Pulmonol. 1995;19(2):129–34.
Rao CK et al. Characteristics of perimenstrual asthma and its relation to asthma severity and control: data from the Severe Asthma Research Program. Chest. 2013;143(4):984–92. This paper demonstrates that premenstrual asthma (PMA) is common in women with severe uncontrolled asthma and is independently associated with Aspirin sensitivity and lower lung function. This paper suggests that alterations in prostaglandins may contribute to PMA.
Tan KS, McFarlane LC, Lipworth BJ. Modulation of airway reactivity and peak flow variability in asthmatics receiving the oral contraceptive pill. Am J Respir Crit Care Med. 1997;155(4):1273–7.
Al-Sahab B et al. Early menarche predicts incidence of asthma in early adulthood. Am J Epidemiol. 2011;173(1):64–70.
Salam MT, Wenten M, Gilliland FD. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol. 2006;117(5):1001–7.
Fida NG, Williams MA and Enquobahrie DA. Association of Age at Menarche and Menstrual Characteristics with Adult Onset Asthma among Reproductive Age Women. Reprod Syst Sex Disord. 2012. 1(3).
Jenkins MA et al. Parity and decreased use of oral contraceptives as predictors of asthma in young women. Clin Exp Allergy. 2006;36(5):609–13.
Beecroft N, Cochrane GM, Milburn HJ. Effect of sex of fetus on asthma during pregnancy: blind prospective study. BMJ. 1998;317(7162):856–7.
Dodds L, Armson BA, Alexander S. Use of asthma drugs is less among women pregnant with boys rather than girls. BMJ. 1999;318(7189):1011.
Kwon HL et al. Effect of fetal sex on airway lability in pregnant women with asthma. Am J Epidemiol. 2006;163(3):217–21.
Bakhireva LN et al. Fetal sex and maternal asthma control in pregnancy. J Asthma. 2008;45(5):403–7.
Firoozi F et al. Effect of fetal gender on maternal asthma exacerbations in pregnant asthmatic women. Respir Med. 2009;103(1):144–51.
Osei-Kumah A et al. Sex-specific differences in placental global gene expression in pregnancies complicated by asthma. Placenta. 2011;32(8):570–8. This paper demonstrates that placental gene expression in influenced by the sex of the fetus and may contribute to the sexually dimorphic difference in fetal growth in response to maternal asthma.
Rubio Ravelo L et al. Comparative study of progesterone, estradiol and cortisol concentrations in asthmatic and non-asthmatic women. Allergol Immunopathol (Madr). 1988;16(4):263–6.
Farha S et al. Effects of the menstrual cycle on lung function variables in women with asthma. Am J Respir Crit Care Med. 2009;180(4):304–10. This paper demonstrate that while, airflow and gas transfer vary over the menstrual cycle physiologic, the physiologic mechanisms are different in women with asthma compared with healthy women.
Lieberoth S et al. Age at menarche and risk of asthma: systematic review and meta-analysis. J Asthma. 2014;51(6):559–65.
Troisi RJ et al. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1183–8.
Lange P et al. Exogenous female sex steroid hormones and risk of asthma and asthma-like symptoms: a cross sectional study of the general population. Thorax. 2001;56(8):613–6.
Gomez Real F et al. Hormone replacement therapy, body mass index and asthma in perimenopausal women: a cross sectional survey. Thorax. 2006;61(1):34–40.
Real FG et al. Lung function, respiratory symptoms, and the menopausal transition. J Allergy Clin Immunol. 2008;121(1):72–80. e3.
Zein J. et al. The Distinctive Role Of Age And Asthma Duration On Asthma Severity, in B13. Mechanisms and Treatment Considerations for Severe Asthma. 2014, American Thoracic Society. p. A2425-A2425.
Newson RB et al. The association of asthma, nasal allergies, and positive skin prick tests with obesity, leptin, and adiponectin. Clin Exp Allergy. 2014;44(2):250–60.
Jarvis D, Leynaert B. The association of asthma, atopy and lung function with hormone replacement therapy and surgical cessation of menstruation in a population-based sample of English women. Allergy. 2008;63(1):95–102.
Romieu I et al. Postmenopausal hormone therapy and asthma onset in the E3N cohort. Thorax. 2010;65(4):292–7. This study demonstrates that the use oestrogen alone in postmenopausal women, particularly in never smokers , was associated with an increased incidence of asthma.
Mishra GD, Dobson AJ. Using longitudinal profiles to characterize women's symptoms through midlife: results from a large prospective study. Menopause. 2012;19(5):549–55.
Santoro N et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81(4):1495–501.
Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women's lives. Am J Med. 2005;118(Suppl 12B):14–24.
Mishra GD, Kuh D. Health symptoms during midlife in relation to menopausal transition: British prospective cohort study. BMJ. 2012;344:e402.
Hale GE et al. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16(1):50–9.
Longcope C et al. Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas. 1986;8(3):189–96.
Miro F et al. Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: the FREEDOM Study. J Clin Endocrinol Metab. 2004;89(10):4910–5.
Shideler SE et al. Ovarian-pituitary hormone interactions during the perimenopause. Maturitas. 1989;11(4):331–9.
Loerbroks A et al. Obesity and adult asthma: potential effect modification by gender, but not by hay fever. Ann Epidemiol. 2008;18(4):283–9.
Chen Y et al. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol. 2002;155(3):191–7.
Shore SA et al. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115(1):103–9. This paper demonstrates that serum leptin augmented allergic airway responses in mice which may play a role in the relationship between obesity and asthma.
Serra-Batlles J et al. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12(6):1322–6.
Lee JH et al. Gender differences in IgE-mediated allergic asthma in the epidemiology and natural history of asthma: Outcomes and Treatment Regimens (TENOR) study. J Asthma. 2006;43(3):179–84.
Sinclair AH, Tolsma DD. Gender differences in asthma experience and disease care in a managed care organization. J Asthma. 2006;43(5):363–7.
McCallister JW et al. Sex differences in asthma symptom profiles and control in the American Lung Association Asthma Clinical Research Centers. Respir Med. 2013;107(10):1491–500. This review show that women with poorly controlled asthma reported similar overall asthma control compared to men, but they scored worse on the asthma-related QOL questionnaire and they had a unique symptom profile. They reported more nocturnal awakenings, activity limitations, and shortness of breath.
Chhabra SK, Chhabra P. Gender differences in perception of dyspnea, assessment of control, and quality of life in asthma. J Asthma. 2011;48(6):609–15.
de Miguel Diez J et al. Psychiatric comorbidity in asthma patients. J Asthma. 2011;48(3):253–8.
Lisspers K et al. Sex-differences in quality of life and asthma control in Swedish asthma patients. J Asthma. 2013;50(10):1090–5. This paper demonstrates a gender difference in asthma related symptoms and quality of life in younger but not older asthmatics. While younger women had lower quality of life and worse asthma control compared to younger men, there were sex differences in older asthmatics. Such differences was related to sex hormones.
Woods SE, Brown K, Engel A. The influence of gender on adults admitted for asthma. Gend Med. 2010;7(2):109–14.
Sundberg R et al. Asthma in men and women: treatment adherence, anxiety, and quality of sleep. Respir Med. 2010;104(3):337–44.
Paivinen MK, Keskinen KL, Tikkanen HO. Swimming and Asthma: Differences between Women and Men. J Allergy (Cairo). 2013;2013:520913. This report demonstrates that while gender difference in prevalence of asthma was not found in swimmers, asthmatic females had significantly more symptoms especially cough.
Clark NM et al. From the female perspective: Long-term effects on quality of life of a program for women with asthma. Gend Med. 2010;7(2):125–36.
Syamlal G, Mazurek JM, Dube SR. Gender differences in smoking among U.S. working adults. Am J Prev Med. 2014;47(4):467–75.
Bjerg A et al. Higher risk of wheeze in female than male smokers. Results from the Swedish GA 2 LEN study. PLoS One. 2013;8(1):e54137.
Guo SE et al. The gender-specific association between asthma and the need to smoke tobacco. Heart Lung. 2014;43(1):77–83. This study demonstrate a gender difference in tobacco dependence with women developping a quicker physical dependence. This report supports the importance of smoking cessation mainly in asthmatic women.
White GE et al. Gender differences in work-related asthma: surveillance data from California, Massachusetts, Michigan, and New Jersey, 1993–2008. J Asthma. 2014;51(7):691–702.
Downes MJ et al. Factors associated with furry pet ownership among patients with asthma. J Asthma. 2010;47(7):742–9.
Lindner PS, Lindner AJ. Gender differences in asthma inhaler compliance. Conn Med. 2014;78(4):207–10.
Zahran HS et al. Predictors of asthma self-management education among children and adults–2006-2007 behavioral risk factor surveillance system asthma call-back survey. J Asthma. 2012;49(1):98–106.
Axelsson M, Brink E, Lotvall J. A personality and gender perspective on adherence and health-related quality of life in people with asthma and/or allergic rhinitis. J Am Assoc Nurse Pract. 2014;26(1):32–9.
Axelsson M. Personality and reasons for not using asthma medication in young adults. Heart Lung. 2013;42(4):241–6.
Hasegawa K et al. Emergency department visits for acute asthma by adults who ran out of their inhaled medications. Allergy Asthma Proc. 2014;35(3):42–50. The study shows that the risk factors for running out of an asthma medication were male sex, non-Hispanic black race, Hispanic ethnicity, no insurance, lower household income, and use of EDs as the preferred source of asthma prescriptions.
Pai S et al. Characteristics of asthmatic patients with and without repeat emergency department visits at an inner city hospital. J Asthma. 2014;51(6):627–32.
Self TH et al. Gender differences in the use of peak flow meters and their effect on peak expiratory flow. Pharmacotherapy. 2005;25(4):526–30.
Bianchi M et al. Spirometry testing in a population of Italian children: age and gender differences. Respir Med. 2012;106(10):1383–8.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Serpil C. Erzurum was supported in part by the Alfred Lerner Memorial Chair in Innovative Biomedical Research. Joe G. Zein declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Asthma
Rights and permissions
About this article
Cite this article
Zein, J.G., Erzurum, S.C. Asthma is Different in Women. Curr Allergy Asthma Rep 15, 28 (2015). https://doi.org/10.1007/s11882-015-0528-y
Published:
DOI: https://doi.org/10.1007/s11882-015-0528-y