Abstract
Asthma is a heterogeneous disease with a sexual dimorphism in prevalence that changes throughout life. As children, boys are more likely than girls to have asthma, but as adults women are more likely than men to have asthma. The prevalence of asthma in females increases around the age of menarche, suggesting a role for sex hormones in disease pathogenesis. In some women with asthma, increased symptoms are reported just prior to or during menstruation. Further, asthma symptoms during pregnancy and menopause also vary for women. In this chapter, we will discuss the findings from epidemiological, clinical, and molecular studies exploring the role of sex hormones and asthma. We will also discuss findings from animal models of asthma, focusing primarily on allergic asthma, and how these findings may be used to develop potential therapies or guide the personalized use of currently available asthma therapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Asthma is a multidimensional chronic disease that is characterized by reversible expiratory airflow limitation, airway hyperresponsiveness (AHR), and airway inflammation [1]. Asthma affects 5–10 % of the population in the United States and other developed countries, resulting in 300 million people worldwide having this disease [1]. A sex disparity , which changes with age, exists in asthma (Fig. 4.1) [2]. Before the age of 12, boys have an increased prevalence of asthma compared to girls [3], with approximately 11.9 % of boys having asthma compared to 7.5 % of girls having asthma [4]. The prevalence of asthma in adolescent girls increases at puberty, and as adults, 9.6 % of women have asthma compared to 6.3 % of men [4, 5]. This sex disparity in asthma is maintained until women approach menopause, when the prevalence in asthma declines [6].
Prevalence of asthma in females and males at various ages . Graph is adapted from Carey, M. et al. [2]
Women with asthma also have increased asthma-related mortality and morbidity , more severe asthma phenotypes, less responsiveness to corticosteroid medications, and more fluctuations in asthma symptoms, corresponding to the menstrual cycle, compared to men with asthma [5, 7, 8]. As children, boys are twice as likely as girls to be hospitalized for an asthma exacerbation (as reviewed in [9]). After the age of 15, however, girls and women are three times more likely than boys and men to be hospitalized for an asthma-related event [10–12]. The sex differences in hospitalizations of adult men and women with asthma are independent of controller medication use or length of time before seeking medical attention [9]. Combined, these data suggest that asthma prevalence and the severity of asthma symptoms and exacerbations of asthma are increased in women compared to men. In this chapter, we will discuss the findings from epidemiological, clinical, and molecular studies exploring the role of sex hormones and asthma.
Classifications Systems for Patients with Asthma
Asthma is a heterogeneous disease with many different phenotypes responsiveness to current therapies, and triggers for exacerbations [13]. Patients with asthma were classified as extrinsic (allergic) and intrinsic (nonallergic) based on clinical symptoms, IgE serum levels, atopy status, and age of onset [14]. Using this classification system, women had an increased prevalence in both allergic and nonallergic asthma compared to men. However, other phenotypic variables, such as forced expiratory volume in one second (FEV1) pre- and post-bronchodilator, blood and sputum eosinophil and neutrophil counts, responsiveness to corticosteroids, age of onset, and asthma triggers, were not included when categorizing patients [14]. Not accounting for multiple variables resulted in patients with varying clinical symptoms, pathophysiology, and molecular markers being classified as having either allergic or nonallergic asthma. This categorization system created difficulty in determining the appropriate clinical trial populations for therapeutics targeting a specific mechanism for airway inflammation .
Recently a more sophisticated systems biology approach utilizing cluster modeling classification systems was used to categorize patients with asthma. Cluster analysis uses algorithms and a predefined set of asthma variables from patients with asthma and healthy controls to define asthma phenotypes [15–19]. Common variables used in these studies are shown in Table 4.1, and several groups have reported a female dominance in the clusters listed below [15–19]:
-
1.
Early-onset atopic asthma
-
2.
Later onset atopic asthma
-
3.
Later onset asthma in obese individuals
-
4.
More severe asthma with increased sputum neutrophils and decreased lung function
As denoted in the list above, cluster analysis statistical approaches have categorized women-dominated asthma phenotypes spread across the asthma spectrum; from early-onset mild, intermittent asthma to late-onset, severe asthma. To better understand these cellular and molecular variables used in cluster analysis studies, we will first review the basic immune mechanisms and cell types involved in asthma and then discuss findings from the literature. Defining the mechanisms and properly phenotyping patients will target patient populations that may benefit from various therapeutics when conducting clinical trials and designing treatment regiments.
Host Immune Responses in Asthma
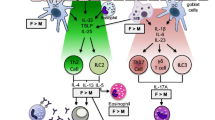
Sir William Osler described asthma in the 1892 edition of Principles and Practice of Medicine as a disease with a genetic component , originating early in life that resulted in spasms of the bronchial muscles, swelling of bronchial mucous membrane, inflammation in bronchioles, and various circumstances that induced symptoms [20]. Approximately 70–80 % of patients with asthma are sensitized to allergens, pollen, dust mites, cockroach antigen, mold, etc., and asthma symptoms and exacerbations are increased with exposure to these allergens in these patients. However, other environmental stimuli , including viral infections, cigarette smoke, and air pollution, also may result in asthma symptoms through different innate and adaptive immune responses . In this chapter, we will primarily discuss findings focused on the role of sex hormones in allergic asthma immune pathways (as shown in Fig. 4.2 ), but we will also introduce alternative, nonallergic immune pathways involved with asthma pathogenesis.
The allergic airway immune response is characterized by increased CD4+ Th2 cells, eosinophils, mast cells, and basophils in the airway. Airway epithelial cells are the first point of contact for airway allergens, and airway epithelial cells are important for regulating antigen-presenting cells (APCs) . APCs, including dendritic cells (DCs), alveolar macrophages (AMΦ), and B lymphocytes, engulf antigens and process them for presentation to the T cells, initiating the adaptive immune response [21, 22]. Antigens processed by APCs, through the endocytic pathway, are presented by major histocompatibility complex (MHC) class II molecules to CD4+ T lymphocytes. Naïve CD4+ T lymphocytes are activated when two events occur: (1) the antigen/MHC class II complex on the APC engages the T cell receptor, and (2) co-stimulation molecules, such as CD80 or CD86, on the APC bind to co-stimulation molecules, such as CD28, on the CD4+ T cells. DCs are the most potent APCs in activating naïve T cells [23, 24], and DCs also secrete chemokines that attract CD4+ Th2 differentiated cells and eosinophils to the lung [25].
Many cell types are involved in the allergic response, but the key cell types in initiating and amplifying the allergen-induced inflammatory cascade are the antigen-presenting DC and T helper 2 (Th2) lymphocytes. Th2 lymphocytes are CD4+ T cells that are differentiated from activated naïve T cells. Initiation of Th2 cell differentiation is not completely understood, but interleukin-4 (IL-4) is also required for Th2 cell differentiation [26, 27]. Th2 cells, as well as mast cells and basophils, secrete IL-4, and IL-4 is required for B lymphocytes to isotype switch antibody production to IgE [26, 27]. Th2 cells additionally produce a variety of other pro-allergic inflammatory cytokines such as IL-5, IL-9, IL-10, and IL-13. IL-5 is an important eosinophil regulatory factor and increases eosinophil maturation and proliferation [28]. IL-13 is a central mediator in AHR and also induces mucus hypersecretion from goblet (mucous) cells in the airway [27–29]. IL-9 is important for mast cell growth, T cell proliferation, IgE secretion from B cells, and mucus hypersecretion from goblet cells [30]. IL-10 is an anti-inflammatory cytokine that is secreted by Th2 cells as well as other cells, including T regulatory cells and Th17 cells. In summary, IL-4, IL-5, IL-9, and IL-13 are important for establishing the allergic airway response, and IL-10 is an important anti-inflammatory cytokine in the airway allergen immune response.
The allergic response to an aerosolized allergen is also characterized by antigen-specific immunoglobulin E (IgE) production by B lymphocytes whereupon IgE can bind to the high-affinity IgE receptor, FcεR1, on tissue mast cells and peripheral blood basophils. Antigen cross-linking of the antigen-specific IgE/FcεR1 complex ignites degranulation of the mast cells and basophils, causing release of soluble mediators , such as histamine and tryptase [13, 31]. Other mediators, including prostaglandins and leukotrienes, are also released in response to an allergen [32, 33]. Release of these mediators causes vasodilation and recruitment of inflammatory cells into the airway, including eosinophils and lymphocytes. Therefore, antigen cross-linking to FcεR1 is an important component of the immune response to allergens.
Recently a new cell type, group 2 innate lymphoid cells (ILC2) , was described to be residing in the lung in small numbers and be important in airway immune responses. ILC2 are activated by IL-33, IL-25, and thymic stromal lymphopoietin (TSLP) which are secreted from airway epithelial cells and other immune cells, in response to allergens and other stimuli, such as infections with fungi, helminthes, and viruses [34–36]. ILC2 lack lineage markers for T cells, B cells, DCs, and macrophages [34, 37]. ILC2 express CD25 (IL-2Rα) and CD127 (IL-7Rα) as well as receptors for the ILC2 activating cytokines IL-25, IL-33, and/or TSLP [34, 36, 37]. Once activated, ILC2 quickly secrete large amounts of IL-13 and IL-5 compared to Th2 cells [37]. Therefore, while ILC2 are rare within the lung, ILC cytokine expression increases eosinophil infiltration into the lung and mucus production. Collectively, the immune response to aerosolized allergens causes an increase in airway inflammation, airway mucus hypersecretion by goblet cells (predominantly through IL-13), and increased airway smooth muscle constriction.
Understanding the basic immune pathways in allergic asthma is a first step in determining the role of sex hormones on asthma. Another important step is determining the expression of estrogen receptors (ERs), progesterone receptors (PRs), and androgen receptors (ARs) on immune cells. Nuclear ERs, PRs, and ARs are readily expressed in inflammatory cells involved with asthma (as reviewed in [38]). Membrane-bound ERs are not found on T cells or cells of hematopoietic origin, but membrane-bound ERs are found on other cell types [38]. Since sex hormone receptors are found on many different immune cells, sex hormones may affect several steps in allergic airway disease, including antigen uptake, antigen processing and presentation, adaptive immune effector function, antibody isotype switching to IgE, and mast cell degranulation. Throughout this chapter we will discuss the role of sex hormones in the allergic immune response.
Changes in Sex Hormones during Puberty, Pregnancy, and Menopause Adversely Affect a Subset of Women with Asthma
As demonstrated in Fig. 4.1 the prevalence of asthma increases about the age of menarche, suggesting a role for sex hormones in asthma prevalence. Salam and colleagues reported girls who reached menarche prior to age 12 had a 2.08 higher risk for asthma compared to girls who reached menarche at age 12 or later [39]. These data suggest that ovarian hormones increase the risk for development of asthma. In the following subsections, we will discuss how various stages in life and fluctuations of hormones affect asthma prevalence and severity.
Pre- and Perimenstrual Asthma Symptoms
Twenty to thirty-five percent of women with asthma have pre- or perimenstrual worsening of asthma, but asthma exacerbations were independent of cyclic hormonal changes [40–44]. Estrogen and progesterone concentrations oscillate throughout with menstrual cycle, and these fluctuations in hormonal concentrations are thought to be potentially linked to changes in asthma symptoms. As shown in Fig. 4.3, estrogen concentrations peak on day 13 of a 28-day menstrual cycle, just prior to ovulation, and progesterone concentrations peak at days 22–24, four to six days prior to menses. Asthma symptoms are increased in the premenstrual phase of the cycle (days 25–28) when both estrogen and progesterone concentrations are low (Fig. 4.3). Clinical studies tracking asthma symptoms and peak expiratory flow rates in women through at least two menstrual cycles found increased symptoms and decreased peak flow rates in the morning during the premenstrual and perimenstrual phases of the cycle compared to other phases of the cycle [40, 43]. Women with premenstrual asthma symptoms had increased fractional exhaled nitric oxide (FeNO) , a noninvasive measure of epithelial induced nitric oxide that correlates with eosinophilic inflammation [45], and eosinophils in the sputum in the premenstrual phase compared to the seventh day of their cycle [46]. However, no difference in the phase of the menstrual cycle of women requiring emergency department visits for asthma symptoms was reported [44]. Combined, these studies suggest a subset of women with asthma have increased premenstrual asthma symptoms and decreased peak expiratory flow rates, but no change in asthma exacerbations during premenstrual phase of their cycle.
Use of certain hormonal birth controls pills reduced the fluctuations of hormone levels and could potentially reduce pre- and perimenstrual asthma symptoms in women with asthma.
However, only a few studies, with small patient sizes, have been conducted to determine the effect of hormonal birth control medications on asthma symptoms through the menstrual cycle. One study followed 18 women with asthma through two menstrual cycles. Women not taking oral contraceptives (n = 9) had increased AHR to adenosine monophosphate during the luteal phase and reduced diurnal peak expiratory flow rates. However, women taking oral contraceptives medications (n = 9) did not have a significant increase in AHR to adenosine monophosphate or significant changes in morning and afternoon peak flow rates [47]. Another study followed 28 women with asthma for 12 weeks (2–4 menstrual cycles) and found no difference in asthma symptoms in women taking oral contraceptives versus women not taking oral contraceptives [48]. Therefore, additional studies, with larger patient populations that stratify different types of hormonal birth control medications, are needed to fully delineate if asthma symptoms are reduced in women taking hormonal contraceptives.
Pregnancy and Asthma
Asthma is a common chronic condition present during pregnancy with a 3.7–8.4 % prevalence among pregnant women [49]. Variations in asthma symptoms and asthma control during pregnancy are common. Two studies from the late 1980s reported that in pregnant women with asthma approximately one-third had decreased asthma symptoms, one-third had increased asthma symptoms, and one-third had no change in asthma symptoms [50, 51]. Since that time, there has been little improvement on predicting if asthma symptoms will increase, decrease, or remain the same in pregnant women. Researchers have shown that compared to women with mild asthma phenotypes, women with severe asthma were more likely to experience increased asthma symptoms later in the pregnancy, in the second and third trimesters [52]. Pregnant women with asthma were also more susceptible to respiratory viral infections, including rhinovirus and influenza, and viral infections increase asthma exacerbations [53, 54]. Variations in asthma symptoms and control in pregnant women are not fully understood, but are likely related to various phenotypes of asthma, hormonal changes during pregnancy, and adherence to asthma medications. Many pregnant women with asthma decide, either individually or as advised by their medical provider, to reduce or stop asthma medications to prevent adverse side effects on the fetus. However, National Heart , Lung, and Blood Institute and the Global Initiative for Asthma (GINA) guidelines indicate that pregnant women should maintain their current regimen of asthma medications, including inhaled corticosteroids, long-acting beta agonist, leukotriene modifiers, theophylline, and oral corticosteroids [55, 56]. Use of asthma medications during pregnancy was not associated with fetal abnormalities [57], and maintaining asthma control during pregnancy is important. More severe asthma, poorly controlled asthma, and asthma exacerbations during pregnancy are associated with increased risk for development of preeclampsia and gestational diabetes in the mother and preterm birth, low birth weight, and perinatal mortality for the baby [55, 57, 58].
Menopause and Asthma
As described above, ovarian hormones are associated with asthma. Therefore, one would predict that the decline in circulating ovarian hormones that occurs with menopause leads to decreased asthma in women. However, studies in menopausal women report variable findings, with asthma symptoms increasing, decreasing, or remaining the same when compared to premenopausal women or postmenopausal women on hormone replacement therapy (HRT) (as reviewed in [59]). The US Nurses’ Health Study was a prospective study which followed 41,202 premenopausal women and 23,035 postmenopausal women from 1980 until 1990 [6]. Women were reclassified as pre- or postmenopausal every 2 years based on questionnaire responses. At the end of the study, 582,135 person-years were documented with 726 new cases of asthma. The US Nurses’ Health Study determined that postmenopausal women who never used HRT had a significant decrease in the risk of developing asthma compared to premenopausal women [6]. Further, postmenopausal women who used HRT had an increased age-adjusted risk for asthma compared to postmenopausal women who never used HRT [6]. The European Community Respiratory Healthy Surveys (ECRHS I) also conducted a study and reported no difference in self-reported asthma between premenopausal and postmenopausal women not taking HRT [60]. However, the ECRHSII cross-sectional study reported increased asthma symptoms in women during the menopause transition (amenorrhea for 6+ months) compared to premenopausal women [61]. Further, the ECRHSII study reported increased asthma severity in women diagnosed with asthma during or after menopause [62]. Combined, these studies suggest menopause and HRT are involved in asthma pathogenesis, but the mechanisms are not completely understood. Co-variables, including BMI, genetic factors, estrogen production from adipose tissue, and insulin sensitivity, need to also be considered in future study design.
Summary
Changes in circulating sex hormones occur throughout life for women and therefore it is challenging to design clinical studies that determine the roles of sex hormones in asthma pathogenesis. Further complicating clinical trials is sex hormones regulate AHR, airway inflammation, and metabolism, important aspects in asthma pathogenesis. Therefore to define the mechanisms by which sex hormones regulate asthma pathogenesis researchers use asthma-like rodent (mainly mouse) models. We will discuss animal models of asthma in the next section.
Sex Hormones in Mouse Models of Asthma
Animal models which mimic asthma with increased airway inflammation, AHR, and mucus hypersecretion are vital for determining the mechanisms associated with asthma pathogenesis. Mouse models are used most readily due to reagent availability, knockout strains of mice, and genetically similar immune systems within inbred mouse strains. Sex differences and the role of sex hormones in mouse models of asthma have variable findings based on mouse strain, protocol used for inducing allergic airway inflammation, and endpoints analyzed. The subsections below describe the different findings in mice with regard to sex hormones at baseline (prior to inducing inflammation), during allergic airway inflammation, and during nonallergic airway inflammation.
Baseline AHR and Airway Physiology in Female and Male Mice
At baseline naïve mice have very few lymphocytes, neutrophils, and eosinophils in the bronchoalveolar lavage (BAL) fluid [63], and there is no sexual dimorphism in the percentages of baseline leukocytes in naïve mice [64]. However, baseline AHR is increased in male mice compared to female mice in both the BALB/c and C57BL/6 strains of mice [64]. ER-α-deficient mice (ER-α KO) have increased AHR compared to WT female mice [65, 66], and using an estrogen antagonist increased AHR compared to vehicle control [67]. Conversely, administering 17β-estradiol to male mice decreased AHR compared to vehicle control [67].
Researchers speculated that the increased AHR in male mice is due to male mice having decreased numbers of alveoli and decreased alveolar surface area compared to female mice [68]. To test this hypothesis, female mice were ovariectomized just after weaning (approximately 21 days) and similar alveolar structures were seen in ovariectomized female and male mice, suggesting sex hormones were important in the development of alveoli [68, 69]. Additional studies in ER-α and ER-β KO mice showed ER-α and ER-β signaling is required for fully developing alveolar structures in female mice, and that ER-β KO mice have decreased elastic recoil compared to WT female mice [69]. Increased numbers of alveoli and alveolar surface area may be responsible for the observed sex difference in AHR, and normalization for lung volume and size in a statistical model resulted in no difference in AHR between males and females.
Other factors including variations in vagal nerve-mediated pathways and smooth muscle contractility may also contribute to sex differences in AHR after stimulation. Vagal nerve-mediated pathways are increased in response to methacholine and carbachol, chemicals known to increase smooth muscle contractility, in male mice compared to female mice [70]. Gonadectomized male mice also had similar vagal nerve responses as intact female mice, and restoring androgens to gonadectomized male mice increased the vagal nerve response and AHR to methacholine [70], suggesting androgens increased the vagal nerve response. However, no difference in ex vivo smooth muscle contractility assays to carbachol was reported in tracheal rings from male and female mice. Further, ER-α KO mice and WT female mice had similar smooth muscle contractility to carbachol [70]. Combined, these results suggest sex hormones affect baseline AHR in mice independent of smooth muscle constriction.
Dryness of the throat and vaginal dryness are symptoms reported by women during menopause. These symptoms suggest that sex hormones may affect mucous cell metaplasia and mucus production in the airway and the reproductive system. Therefore, researchers became interested in the role of estrogen and progesterone on mucus production in the nasal and airway epithelial cells . In 1975, Helmi and colleagues administered 10 μg/day ethynyl estradiol, a downstream hormone of the 17β-estradiol, to guinea pigs and measured mucous cell hyperplasia and metaplasia in nasal epithelial cells by histochemical staining. Guinea pigs administered ethynyl estradiol had increased mucous cell hyperplasia and metaplasia compared to vehicle-treated animals [71]. In vitro studies in human airway or nasal epithelial cells also showed that estrogen or progesterone increased mucus production and the expression of the mucus proteins, Muc5AC and Muc5B, compared to vehicle-treated cells [72, 73]. In summary, sex hormones regulate baseline airway responsiveness to methacholine as well as mucus production. Acknowledging baseline differences is vital for experiment design and interpretation of data as normalizing experimental results to percent baseline may not provide the most accurate interpretation of data.
Establishment of Allergic Airway Inflammation in Mice
During ongoing allergic airway inflammation the role of sex hormones varies based on the protocol used to induce allergic airway inflammation and the endpoint measured. The results discussed in the paragraphs below are summarized in Table 4.2. Two widely used models of allergic airway inflammation are: (1) ovalbumin (OVA) sensitization followed by OVA challenge and (2) exposure to house dust mite (HDM) antigen. Both these models increase airway inflammation, AHR, and mucus hypersecretion through a CD4+ Th2 cell-mediated mechanism. Female mice undergoing the OVA sensitized and challenged protocol or the HDM protocols had increased eosinophilic infiltration and IgE serum levels compared to male mice undergoing the same protocol [74–76]. Female mice with OVA-induced allergic airway inflammation also had significantly increased lung IL-4, IL-5, and IL-13 protein expression, mucous cell metaplasia, and airway remodeling compared to male mice [74–76]. Further, castration of male mice prior to OVA sensitization and challenge increased eosinophils and lymphocytes in the BAL fluid, mucous cell metaplasia, and airway inflammation compared to sham-operated mice [75]. However, an ovariectomy prior to OVA sensitization decreased OVA-induced airway inflammation and AHR compared to intact female BALB/c mice [77]. These results suggest that ovarian sex hormones increased, while testosterone decreased, allergic airway inflammation, AHR, and mucous cell metaplasia.
However, Carey and colleagues induced OVA-specific allergic airway inflammation with sensitization and challenge in WT, ER-α KO, and ER-β KO female C57BL/6 mice [66]. ER-α KO mice had a significant increase in AHR compared to WT and ER-β KO mice [66]. One potential explanation is AHR varies in different strains of mice, with C57BL/6 mice having decreased AHR compared to BALB/c mice [78]. Therefore, when interpreting these results, strain variations in AHR, allergic airway inflammation, and mucus production should be considered.
While OVA and HDM are two commonly used models for allergic airway inflammation, exposures to other stimuli, including tobacco smoke, also exacerbate allergic asthma symptoms. Seymour and colleagues determined the role of sex and progesterone in OVA-sensitized and challenged mice exposed to tobacco smoke from birth. Similar to the studies discussed previously, OVA-sensitized and challenged female mice in ambient air since birth had increased serum IgE levels, increased eosinophils, and increased IL-4, IL-5, and IL-13 protein expression in the lung compared to OVA-sensitized and challenged male mice in ambient air since birth [79]. Exposure to tobacco smoke since birth followed by OVA sensitization and challenge starting at 6 weeks of age increased OVA-specific IgE serum levels compared to OVA sensitization and challenge in female mice only. Cytokine expression of IL-4, IL-5, and IL-13 was significantly increased in male mice administered tobacco smoke followed by the OVA protocol compared to male mice in ambient airway undergoing the OVA protocol. Combined these data suggest gender differences exist in the allergic inflammatory response with ambient air or being exposed to tobacco smoke at birth. These data are very important for girls and boys exposed to secondhand smoke from birth who develop allergic airway inflammation.
Serum IgE levels were also increased in female mice compared to male mice after allergic sensitization and challenge [80]. Mast cells numbers in the uterine tissue of mice also fluctuate with the estrus cycle and estrogen and progesterone have been shown to cause mast cell degranulation [81]. Based on these findings, sex hormones were speculated to regulate mast cells during allergic airway inflammation. Estrogen treatment of bone marrow-derived mast cells increased degranulation, and this was inhibited in mast cells from ER-α KO mice. Further, in a human mast cell line (HMC-1), incubation with 17β-estradiol or progesterone significantly increased synthesis of β-tryptase, a serine proteinase found in the secretory granules of mast cells [81]. Combined these data suggest that sex hormones may be important for mast cell differentiation and degranulation and provide a piece to the puzzle of increased asthma prevalence and severity in women compared to men.
Sexual dimorphism exists in allergic asthma, but the mechanisms are not fully defined. Further research in mouse models and patients with allergic asthma is needed to determine the roles of sex hormones at the various steps in the immune response during allergic inflammation. Utilizing gonadectomized mice with restored sex hormones prior to initiation of allergic airway inflammation will aid in determining the mechanisms associated with the sex differences observed in asthma throughout life.
Other Triggers of Asthma and the Role of Sex Hormones on Immune Cells
Viral infections, environmental stimuli, obesity, stress, and exposure to toxins are additional stimuli that cause asthma exacerbation. Viral infections are the most frequent cause of asthma exacerbations, and therefore determining the role of sex hormones on viral-induced airway inflammation, AHR, and mucus production is important and remains largely unknown. Recent findings in either female mice or male mice showed increased IL-13 and IL-5 protein expression from ILC2 in response to influenza A or Alternaria alternata, a fungus known to cause airway inflammation [82]. Further, IL-17A protein expression from γδ T cells, Th17 cells, and ILC3 cells is reported to be increased in response to fungal or viral infections [83, 84]. Future experiments, incorporating mice of both sexes, gonadectomized mice, and mice deficient in estrogen signaling, are required to determine the role of sex hormones on ILC2, ILC3, γδ T cell, and Th17 cell cytokine expression and increased AHR, airway inflammation, and mucus production in mouse models.
Summary of Sex Hormones and Asthma
A gender disparity exists in asthma, and at puberty the prevalence of asthma increases in females compared to males. It is a very complicated, high-regulated process. Epidemiologic and clinical studies vary on the role of estrogen and progesterone in increasing asthma symptoms and severity, but the literature suggests that ovarian hormones increase asthma prevalence, symptoms, and severity. Accounting for sex differences in mouse models of asthma is the first step for determining the role of sex hormones in immune mechanisms associated with asthma. Further, designing clinical trials to include patients with various phenotypes of asthma, at the correct sex ratio, is important for determining if a therapeutic will work and if differential responses in asthma symptoms will be seen in women and men.
Abbreviations
- AHR:
-
Airway hyperresponsiveness
- AMΦ:
-
Alveolar macrophage
- APCs:
-
Antigen-presenting cells
- AR:
-
Androgen receptor
- BAL:
-
Bronchoalveolar lavage
- BMI:
-
Body mass index
- DCs:
-
Dendritic cells
- ER:
-
Estrogen receptor
- FEV1 :
-
Forced exhaled volume in one second
- HDM:
-
House dust mite
- HRT:
-
Hormone replacement therapy
- IgE:
-
Immunoglobulin E
- IL:
-
Interleukin
- ILC2s:
-
Group 2 innate lymphoid cells
- ILC3s:
-
Group 3 innate lymphoid cells
- KO:
-
Knockout
- MHC:
-
Major histocompatibility complex
- NK:
-
Natural killer cells
- OVA:
-
Ovalbumin
- PR:
-
Progesterone receptor
- SARP:
-
Severe Asthma Research Panel
- TSLP:
-
T helper (Th) thymic stromal lymphopoietin
References
Prevention FtGSfAMa. Global Initiative for Asthma (GINA). http://www.ginasthma.org/. 2014 2014. Report No.
Carey MA, Card JW, Voltz JW, Arbes Jr SJ, Germolec DR, Korach KS, et al. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007;18(8):308–13.
Almqvist C, Worm M, Leynaert B, Working group of GALENWPG. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63(1):47–57.
Centers for Disease Control and Prevention, Vital Signs, May 2011. 2013.
Moorman JE, Zahran H, Truman BI, Molla MT. Current asthma prevalence—United States, 2006–2008. MMWR Surveill Summ. 2011;60(Suppl):84–6.
Troisi RJ, Speizer FE, Willett WC, Trichopoulos D, Rosner B. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1183–8.
Gibbs CJ, Coutts II, Lock R, Finnegan OC, White RJ. Premenstrual exacerbation of asthma. Thorax. 1984;39(11):833–6.
Skobeloff EM, Spivey WH, Silverman R, Eskin BA, Harchelroad F, Alessi TV. The effect of the menstrual cycle on asthma presentations in the emergency department. Arch Intern Med. 1996;156(16):1837–40.
Kynyk JA, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med. 2011;17(1):6–11.
Chen Y, Stewart P, Johansen H, McRae L, Taylor G. Sex difference in hospitalization due to asthma in relation to age. J Clin Epidemiol. 2003;56(2):180–7.
Hyndman SJ, Williams DR, Merrill SL, Lipscombe JM, Palmer CR. Rates of admission to hospital for asthma. BMJ. 1994;308(6944):1596–600.
Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA. 1992;268(24):3437–40.
Borish L, Culp JA. Asthma: a syndrome composed of heterogeneous diseases. Ann Allergy Asthma Immunol. 2008;101(1):1–8.
Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–25.
Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–23.
Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–24.
Siroux V, Garcia-Aymerich J. The investigation of asthma phenotypes. Curr Opin Allergy Clin Immunol. 2011;11(5):393–9.
Wu W, Bleecker E, Moore W, Busse WW, Castro M, Chung KF, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133(5):1280–8.
Newby C, Heaney LG, Menzies-Gow A, Niven RM, Mansur A, Bucknall C, et al. Statistical cluster analysis of the British thoracic society severe refractory asthma registry: clinical outcomes and phenotype stability. PLoS One. 2014;9(7), e102987.
Osler W. The principles and practice of medicine: designed for the use of practitioners and students of medicine. New York: D. Appleton and Company; 1892. xvi, 2, 1079, 7, 8 p.
Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402(6760 Suppl):B18–23.
Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402(6760 Suppl):B2–4.
Hammad H, Lambrecht BN. Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy. 2011;66(5):579–87.
Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8(3):193–204.
Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–70.
Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–93.
Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282(5397):2258–61.
Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–81.
Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–90.
Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186(6):3283–8.
Busse WW, Lemanske Jr RF. Asthma. N Engl J Med. 2001;344(5):350–62.
Moore ML, Peebles Jr RS. Update on the role of prostaglandins in allergic lung inflammation: separating friends from foes, harder than you might think. J Allergy Clin Immunol. 2006;117(5):1036–9.
Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy, Asthma & Immunol Res. 2014;6(4):288–95.
Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36(3):451–63.
Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12(7):631–8.
Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–62.
Scanlon ST, McKenzie AN. Type 2 innate lymphoid cells: new players in asthma and allergy. Curr Opin Immunol. 2012;24(6):707–12.
Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol. 2013;13(1):92–9.
Salam MT, Wenten M, Gilliland FD. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol. 2006;117(5):1001–7.
Agarwal AK, Shah A. Menstrual-linked asthma. J Asthma. 1997;34(6):539–45.
Eliasson O, Scherzer HH, DeGraff Jr AC. Morbidity in asthma in relation to the menstrual cycle. J Allergy Clin Immunol. 1986;77(1 Pt 1):87–94.
Hanley SP. Asthma variation with menstruation. Br J Dis Chest. 1981;75(3):306–8.
Shames RS, Heilbron DC, Janson SL, Kishiyama JL, Au DS, Adelman DC. Clinical differences among women with and without self-reported perimenstrual asthma. Ann Allergy, Asthma Immunol. 1998;81(1):65–72.
Brenner BE, Holmes TM, Mazal B, Camargo Jr CA. Relation between phase of the menstrual cycle and asthma presentations in the emergency department. Thorax. 2005;60(10):806–9.
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15.
Oguzulgen IK, Turktas H, Erbas D. Airway inflammation in premenstrual asthma. J Asthma. 2002;39(6):517–22.
Tan KS, McFarlane LC, Lipworth BJ. Modulation of airway reactivity and peak flow variability in asthmatics receiving the oral contraceptive pill. Am J Respir Crit Care Med. 1997;155(4):1273–7.
Murphy VE, Gibson PG. Premenstrual asthma: prevalence, cycle-to-cycle variability and relationship to oral contraceptive use and menstrual symptoms. J Asthma. 2008;45(8):696–704.
Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol. 2003;13(5):317–24.
Schatz M, Harden K, Forsythe A, Chilingar L, Hoffman C, Sperling W, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol. 1988;81(3):509–17.
Stenius-Aarniala B, Piirila P, Teramo K. Asthma and pregnancy: a prospective study of 198 pregnancies. Thorax. 1988;43(1):12–8.
Schatz M. Interrelationships between asthma and pregnancy: a literature review. J Allergy Clin Immunol. 1999;103(2 Pt 2):S330–6.
Forbes RL, Gibson PG, Murphy VE, Wark PA. Impaired type I and III interferon response to rhinovirus infection during pregnancy and asthma. Thorax. 2012;67(3):209–14.
Forbes RL, Wark PA, Murphy VE, Gibson PG. Pregnant women have attenuated innate interferon responses to 2009 pandemic influenza A virus subtype H1N1. J Infect Dis. 2012;206(5):646–53.
National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program Asthma and Pregnancy Working Group. NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment-2004 update. J Allergy Clin Immunol. 2005;115(1):34–46.
(GINA) GIfA. Global Strategy for Asthma Management and Prevention. 2014.
Lim A, Stewart K, Konig K, George J. Systematic review of the safety of regular preventive asthma medications during pregnancy. Ann Pharmacother. 2011;45(7–8):931–45.
Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax. 2006;61(2):169–76.
Ticconi C, Pietropolli A, Piccione E. Estrogen replacement therapy and asthma. Pulm Pharmacol Ther. 2013;26(6):617–23.
Gomez Real F, Svanes C, Bjornsson EH, Franklin KA, Gislason D, Gislason T, et al. Hormone replacement therapy, body mass index and asthma in perimenopausal women: a cross sectional survey. Thorax. 2006;61(1):34–40.
Real FG, Svanes C, Omenaas ER, Anto JM, Plana E, Jarvis D, et al. Lung function, respiratory symptoms, and the menopausal transition. J Allergy Clin Immunol. 2008;121(1):72–80. e3.
Balzano G, Fuschillo S, De Angelis E, Gaudiosi C, Mancini A, Caputi M. Persistent airway inflammation and high exacerbation rate in asthma that starts at menopause. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace/Fondazione clinica del lavoro, IRCCS [and] Istituto di clinica tisiologica e malattie apparato respiratorio, Universita di Napoli, Secondo ateneo. 2007;67(3):135–41.
Walters DM, Wills-Karp M, Mitzner W. Assessment of cellular profile and lung function with repeated bronchoalveolar lavage in individual mice. Physiol Genomics. 2000;2(1):29–36.
Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177(1):621–30.
Card JW, Zeldin DC. Hormonal influences on lung function and response to environmental agents: lessons from animal models of respiratory disease. Proc Am Thorac Soc. 2009;6(7):588–95.
Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, et al. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. Am J Respir Crit Care Med. 2007;175(2):126–35.
Matsubara S, Swasey CH, Loader JE, Dakhama A, Joetham A, Ohnishi H, et al. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol. 2008;38(5):501–8.
Massaro GD, Mortola JP, Massaro D. Sexual dimorphism in the architecture of the lung’s gas-exchange region. Proc Natl Acad Sci U S A. 1995;92(4):1105–7.
Massaro D, Massaro GD. Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1154–9.
Card JW, Voltz JW, Ferguson CD, Carey MA, DeGraff LM, Peddada SD, et al. Male sex hormones promote vagally mediated reflex airway responsiveness to cholinergic stimulation. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L908–14.
Helmi AM, El Ghazzawi IF, Mandour MA, Shehata MA. The effect of oestrogen on the nasal respiratory mucosa. An experimental histopathological and histochemical study. J Laryngol Otol. 1975;89(12):1229–41.
Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. Estradiol increases mucus synthesis in bronchial epithelial cells. PLoS One. 2014;9(6), e100633.
Choi HJ, Chung YS, Kim HJ, Moon UY, Choi YH, Van Seuningen I, et al. Signal pathway of 17beta-estradiol-induced MUC5B expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2009;40(2):168–78.
Blacquiere MJ, Hylkema MN, Postma DS, Geerlings M, Timens W, Melgert BN. Airway inflammation and remodeling in two mouse models of asthma: comparison of males and females. Int Arch Allergy Immunol. 2010;153(2):173–81.
Hayashi T, Adachi Y, Hasegawa K, Morimoto M. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand J Immunol. 2003;57(6):562–7.
Takeda M, Tanabe M, Ito W, Ueki S, Konnno Y, Chihara M, et al. Gender difference in allergic airway remodelling and immunoglobulin production in mouse model of asthma. Respirology. 2013;18(5):797–806.
Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy. 2007;37(3):459–70.
Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;281(2):L394–402.
Seymour BW, Friebertshauser KE, Peake JL, Pinkerton KE, Coffman RL, Gershwin LJ. Gender differences in the allergic response of mice neonatally exposed to environmental tobacco smoke. Dev Immunol. 2002;9(1):47–54.
Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, et al. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy. 2005;35(11):1496–503.
Jensen F, Woudwyk M, Teles A, Woidacki K, Taran F, Costa S, et al. Estradiol and progesterone regulate the migration of mast cells from the periphery to the uterus and induce their maturation and degranulation. PLoS One. 2010;5(12), e14409.
McSorley HJ, Blair NF, Smith KA, McKenzie AN, Maizels RM. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014;7:1068–78.
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–76.
Newcomb DC, Peebles Jr RS. Th17-mediated inflammation in asthma. Curr Opin Immunol. 2013;25(6):755–60.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Newcomb, D.C. (2016). Sex, Gender, and Asthma. In: Hemnes, A. (eds) Gender, Sex Hormones and Respiratory Disease. Respiratory Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-23998-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-23998-9_4
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-23996-5
Online ISBN: 978-3-319-23998-9
eBook Packages: MedicineMedicine (R0)