Abstract
In recent years, the incidence of respiratory diseases such as asthma and pneumonia has increased significantly. However, the effect of lead (Pb) pollution on the respiratory system remains unclear. The aim of this study was to evaluate the effects of exposure to environmental and occupational Pb on respiratory health. Articles published in PubMed and Web of Science before September 2023 were systematically searched. The overall adjusted odds ratio (OR) and 95% confidence intervals (CIs) for the association between Pb exposure and respiratory diseases were extracted from each relevant article. The random effects model was applied to analyze the overall pooled effect estimates. Among the 36,373 search results, 36 related articles were screened for meta-analysis. The results of the meta-analysis suggested that Pb exposure increased the risk of respiratory diseases: OR = 1.12 (95% CIs: 1.05, 1.18). The funnel plot, Egger’s and Begg’s tests showed no publication bias. Sensitivity analysis confirmed that the meta-analysis was statistically reliable and stable. Environmental and occupational Pb exposure is associated with an increased risk of respiratory diseases including asthma. The study highlights the importance of further research on the harmful effects of Pb and the urgency of mitigating air pollution.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Air pollution is harmful to human health and increases the global economic burden of environmental diseases (Nunes et al. 2021). Long-term exposure to atmospheric particulate matter (PM), especially fine PM2.5 (aerodynamic diameter ≤ 2.5 μm) can lead to high mortality and morbidity (McGuinn et al. 2019; Shi et al. 2021). Although the content of trace elements in PM2.5 is low, some of them are usually highly toxic, non-degradable in the environment, and easy to bioaccumulate (Hua et al. 2023). Among them, lead (Pb), as a cumulative toxicant, is listed by the World Health Organization (WHO) as one of the top ten chemicals of public health concern (WHO 2022). WHO also estimates that Pb exposure caused 21.7 million years of disability and death globally in 2019 because of long-term health effects (WHO 2022). Pb in the environment mainly comes from sources such as non-ferrous metal mining and smelting, coal combustion, and past Pb-containing gasoline combustion (Cui et al. 2023). In addition, Pb is widely used in industries such as paints, batteries, plastics, printing, pigments, ceramics, and cosmetics (Tarvainen et al. 2023).
People are exposed to Pb through occupational and environmental exposures, which mainly include inhalation of Pb particles from burning Pb-containing materials and ingestion of Pb-contaminated dust, water, and food. Pb can be transported to various tissues and organs through blood circulation, which is known to affect almost all human organ systems (Liu et al. 2024). Pb that enters the body is absorbed through the digestive system and affects the function of the reproductive, liver, endocrine, immune, and gastrointestinal systems (Swaringen et al. 2022). Studies have shown that Pb poisoning leads to cognitive decline, growth retardation, and decreased immunity in children (Galiciolli et al. 2022).
At present, acute and chronic respiratory infections are the leading causes of morbidity and mortality worldwide (Reiner et al. 2019). Collaborators (2017) analyzed the global burden of disease system in 2015 and found that lower respiratory tract infections caused 20.74 million deaths and 103 million disability-adjusted life-years. Exposure to Pb-contaminated environments is significantly associated with respiratory infections in preschool children and increases the incidence of obstructive pulmonary disease and bronchial reactivity (Li et al. 2020; Rabito et al. 2011; Rokadia and Agarwal 2013). However, some studies have obtained different conclusions. For example, Lee et al. (2020) showed that chronic obstructive pulmonary disease (COPD) was not related with blood lead levels after adjusting for possible confounding variables such as smoking and occupation. The relationship between Pb exposure and respiratory diseases remains to be firmly determined. Therefore, more reliable estimates are required to assess the effects of Pb exposure on respiratory diseases.
A previous review analyzed the effects of Pb and zinc exposure on asthma in the general population from 2000 to 2018 (Darabi et al. 2023). However, only four published articles were reviewed in this review to assess the effect of blood Pb levels on asthma. It is difficult to establish a relationship between Pb exposure and respiratory disease because the available evidence is incomplete and there is only one endpoint for respiratory disease outcomes. Recently, there has been a rapid increase in the number of studies on Pb exposure and respiratory diseases and an increase in interest from the scientific community, which requires the latest review of the evidence. Therefore, we aimed to conduct a systematic review and meta-analysis of published evidence on the association between Pb exposure and respiratory disease-related outcomes in the general population. We followed all applicable PRISMA guidelines for system review and meta-analysis (Crawford et al. 2023).
Materials and methods
Search strategy
Literature on Pb exposure and respiratory diseases was searched in PubMed and Web of Science online databases. We limited the search to studies published up to September 29, 2023. Keywords such as Pb and respiratory diseases were used to retrieve relevant literature, as detailed in Table S1.
Eligibility criteria
After removing duplicate articles using automated identification, we screened titles and abstracts to filter articles that did not meet the inclusion criteria. The full text of the remaining literature was independently reviewed by two reviewers (CT and DKX). In case of inconsistent opinions, the third reviewer (WHH) reviews the manuscript based on the inclusion and exclusion criteria, and the final decision was made after discussion by the third reviewer. Details of the inclusion and exclusion criteria are given below:
Inclusion criteria
-
Cohort, cross-sectional, and case-control studies.
-
The outcome was symptoms of respiratory disease.
-
Studies reported the effect of Pb on respiratory diseases quantitatively, and outcomes included effect estimates, such as rate ratio (RR), odds ratio (OR), hazard ratio (HR), and 95% confidence intervals (CIs).
-
Peer-reviewed studies.
Exclusion criteria
-
Duplicate studies.
-
Conference abstracts, experimental studies, and grey literature.
-
Studies that did not report effect measure or 95% CIs, variance, or standard error.
-
Case report, case-control, time-series, and case-crossover designs.
-
Study populations that were limited by specific medical conditions.
-
research on animals.
Risk of bias
There are no commonly used tools to evaluate the risk of bias (RoB) at the level of systematic review of observational study of environmental exposure (Cai et al. 2021). We used the tool developed by the WHO to assess RoB in observational air pollution epidemiological studies (WHO 2020). The RoB assessment tool evaluates the following six areas: confounding, selection bias, exposure assessment, outcome assessment, missing data, and selective reporting. For each domain, RoB is evaluated as “low”, “moderate” or “high”. Each domain was divided into 1 to 4 subdomains, with a total of 13 subdomains. The RoB for each study is the highest rating for an individual subdomain. The RoB assessment for each qualified study was performed independently by two authors (CT and DKX). Differences were resolved through discussion. In case of disagreement, the third author (WHH) assists in the unification.
Data extraction and synthesis
Two authors jointly completed the data extraction, one author (CT) extracted the information, and the other author (DKX) checked the information. Information extracted included the main authors, publication year, study area, design, study population characteristics, exposure assessment method, exposure levels, health outcomes, effect estimates, correlation coefficients, and corresponding 95% CIs. For all the above indicators, we considered an article to be “unclear” if it did not contain an explicit conclusion. If there is no report in the article, we considered it “not reported”.
Meta-analysis
Meta-analysis was performed using the statistical software STATA 17. We extracted effect values and their associated 95% CIs as the base data to assess the effect of Pb exposure on respiratory diseases. Most articles used OR value and 95% CIs to indicate the effect. If the original article reported the β value and SE (standard error), the formulas exp(β) and exp(β ± 1.96*SE) were applied to calculate the OR and its 95% CIs (Borroni et al. 2022). Random effects model was used to obtain pooled OR and to calculate the heterogeneity of any study. P < 0.05 or I2 > 50% indicates heterogeneity (Cheng et al. 2022). Subgroup analyses were performed based on factors such as region, age, sample, and study design. Funnel plots, Egger’s and Begg’s tests were applied to assess publication bias (Wu et al. 2021). To evaluate potential unstable factors in the meta-analysis, sensitivity analysis were performed by removing one study at a time (Zhang et al. 2023).
Results
Search results
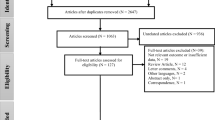
The PRISMA flowchart illustrates the literature screening process of this study (Fig. 1). Of the initial 36,373 studies, the titles and abstracts of 25,001 studies were considered. Subsequently, 1828 articles were carefully read, 76 articles were included in qualitative synthesis, and 36 articles were included in the meta-analysis. We list the main features and results of each study in Table 1. Of the 36 selected studies, 16 were from North America, 16 were from Asia, and 4 were from Europe. Twenty-two were cross-sectionally designs, followed by 10 cohort studies and 4 case-control studies.
Characteristics of the eligible studies
The 36 included studies had different study period, exposure characteristic, and population characteristics. There were 286,077 participants in 36 studies. The duration of investigation ranged from a few months (short-term) to a year or more (long-term). Twenty-four studies investigated long-term health indicators, 6 studies investigated short-term health outcomes, and 6 studies did not specify the study period.
The study evaluated the outcome of one or more different respiratory diseases, specifically: asthma (n = 26), wheezing (n = 8), cough (n = 4), dyspnea/shortness of breath (n = 3), respiratory infections/syncytial virus (n = 3), throat discomfort (n = 3), chronic bronchitis (n = 2), and obstructive lung disease (n = 2). Most of the studies measured blood Pb concentrations in the subjects. In addition, one study measured prenatal urinary Pb, three studies measured prenatal maternal blood Pb, two studies measured umbilical cord Pb, and two studies measured Pb in total suspended particulates.
Risk of bias assessment
Figure 2 shows the RoB assessment for the included studies. Table S2 shows the detailed analysis of the six areas based on RoB. It was found that 7 studies had high RoB, 6 studies had low RoB, and the other studies were assessed as having moderate RoB. In terms of RoB, confounding factor is the weakest domain. Among them, 16 studies did not adjust for BMI and 5 studies did not adjust for any confounding factors (Bener et al. 2001; Khazdair et al. 2012; Kuntawee et al. 2020; Mitsui-Iwama et al. 2019; Myers et al. 2002).
Results of the meta-analysis
Figure 3 and Table S3 show the pooled effect estimations and heterogeneity of each endpoint. Although heterogeneity existed across studies and varied by study area and evaluation method, the results showed that Pb exposure was associated with respiratory diseases (OR = 1.12, 95% CIs: 1.05, 1.18). Figure S1 shows the results of sensitivity analysis. Exclusion of individual studies did not significantly affect the results, revealing that the analysis was statistically reliable and stable.
For the different endpoints, Pb exposure had no significant effect on wheeze (OR = 1.05, 95% CIs: 0.93, 1.20), cough (OR = 1.11, 95% CIs: 0.87, 1.41), respiratory symptoms (OR = 1.69, 95% CIs: 0.81, 3.54), shortness of breath (OR = 1.66, 95% CIs: 0.61, 4.50), throat discomfort (OR = 1.53, 95% CIs: 0.78, 3.01), bronchial symptoms (OR = 0.95, 95% CIs: 0.70, 1.28), and lung disease (OR = 1.59, 95% CIs: 0.97, 2.58). Pb exposure was associated with asthma (OR = 1.12, 95% CIs: 1.02, 1.23).
Figure 4 shows the subgroup analyses. Significant associations of Pb exposure with respiratory diseases were observed in North America (OR = 1.11, 95% CIs: 1.02, 1.22) and Asia (OR = 1.16, 95% CIs: 1.05, 1.28). Regarding the age subgroup analyses, Pb pollution was associated with increase respiratory disease in the age groups < 6 years (OR = 1.17, 95% CIs: 1.00, 1.38) and > 18 years (OR = 1.15, 95% CIs: 1.03, 1.29). For the different Pb samples, the OR was 1.27 (95% CIs: 1.03, 1.56) for Pb in PM2.5, 1.31 (95% CIs: 1.04, 1.66) for maternal blood Pb, and 1.12 (95% CIs: 1.05, 1.19) for blood Pb. Subgroup analysis of the study design showed that Pb pollution was significantly associated with respiratory diseases (OR = 1.18, 95% CIs: 1.10, 1.26) in the cross-sectional.
Publication bias
The funnel plots of all included studies were visually symmetrical (Figure S2), indicating no publication bias. The Egger’s (P = 0.07) and Begg’s (P = 0.81) tests were both greater than 0.05, confirming the absence of publication bias for the endpoint of respiratory diseases.
Discussion
In this meta-analysis, 36 population-based articles were identified to assess the impact of Pb exposure on respiratory disease. In general, the available evidence indicates that Pb exposure is significantly associated with respiratory diseases (OR = 1.12, 95% CIs: 1.05, 1.18). Twenty-six studies investigating the association between Pb exposure and asthma indicated some evidence of a positive relationship. The authors conducted a large population-based birth cohort study and found that pregnant woman and infant cumulative exposure to low levels of Pb may increase pediatric asthma risk (Hsieh et al. 2021). Similarly, Koh et al. (2019) found that serum Pb levels were associated with self-reported asthma and atopic dermatitis in subjects at least 19 years of age. Farkhondeh et al. (2015) summarized the possible mechanisms of Pb-induced asthma, including Pb-induced inflammation and oxidative stress, as well as effects on the immune system.
Eight studies have investigated the association between Pb exposure and the incidence of wheezing. A birth cohort study in Mexico City found a significant association between Pb exposure and ever wheeze (McRae et al. 2022). Conversely, they did not find an association between prenatal Pb exposure and early childhood wheeze (OR = 1.24, 95% CIs: 0.69, 2.25) (Ruan et al. 2022). There were four studies that explored the effect of long-term exposure to Pb on the risk of cough. Khazdair et al. (2012) suggested a significant positive effect of Pb exposure on cough, whereas 3 associations were non-significant. Regarding the outcome endpoints of respiratory symptoms, shortness of breath, throat discomfort, bronchial symptoms, and lung disease, no clear associations with Pb exposure were found in our meta-analyses. Evidence on some of these outcome endpoints is very sparse, reported in only two studies.
The harm of Pb on the respiratory system should not be ignored, especially for children under 6 years old (OR = 1.17, 95% CIs: 1.00, 1.38) and adults older than 18 years (OR = 1.15, 95% CIs: 1.03, 1.29). Similarly, Taylor et al. (2019) found that Pb emissions from smelting posed a significant risk of harm to children’s health, including respiratory diseases, IQ, academic performance, and social behavior problems. Asthmatic children with high blood Pb were more likely to have eosinophilia, higher total IgE levels (83.3%), and more severe asthma symptoms (Mohammed et al. 2015). Zheng et al. (2013) found that children in Guiyu Town, one of the largest e-waste disposal centers in China, had significantly lower forced vital capacity (FVC) than the lowest age group in the reference region. Prenatal Pb exposure in pregnant women can also lead to a significant increase in the incidence of asthma, wheezing, and obstructive bronchitis in children (Palkovicova et al. 2004). Children are a more vulnerable and sensitive group due to their physical incompetence compared to adults. In addition, children have unique exposure pathways (mother-to-child transmission), high-risk behaviors (hand-to-mouth contact), larger surface areas of respiratory capacity and body weight, and lower toxin clearance rates, resulting in a high risk of exposure (Grant et al. 2013). The sequelae of childhood Pb exposure may persist into adolescence and adulthood (David et al. 2022). Jurdziak et al. (2015) and Khazdair et al. (2012) found that Pb exposure is more likely to lead to respiratory symptoms such as chest pressure, sputum, wheezing, and decreased vital capacity parameters (e.g., FVC) in adults. Adults can be exposed to Pb through a variety of routes, including occupational settings, smoking, transportation, and environmental exposures. Long-term bioaccumulation and high-dose exposure to Pb may lead to mitochondrial damage and lipid accumulation in lung cells, thereby accelerating lung tissue damage (Lin et al. 2024). In addition, Wen et al. (2023) found a positive correlation between blood lead and blood eosinophil counts in adults with asthma. Higher blood eosinophil counts have been shown to be a risk factor for future asthma attacks in adults with persistent asthma.
A growing number of reports provide preliminary evidence for the mechanisms of Pb toxicity to the respiratory system. Pb may indirectly damage interstitial lung cells and tissues by triggering inflammatory response and contribute to COPD (Cabral et al. 2015). Dobrakowski et al. (2016a) investigated the correlation between occupational Pb accumulation and oxidative stress in 36 males. Dobrakowski et al. (2016b) found that occupational Pb can lead to changes in the activity of enzymes related to the antioxidant defense system, thereby inducing increased oxidative stress. However, it is unclear whether a slight increase in oxidative stress markers increases the risk of future clinical outcomes. Zeng et al. (2017) further confirmed that heavy metal Pb can produce reactive oxygen species (ROS), resulting in lung cell damage, alveolar collapse, atelectasis, and alveolar ventilation dysfunction. Oxidants can also cause airway inflammation and airway hyperresponsiveness, which is a major symptoms of asthma (Duan et al. 2022). Wei et al. (2020) in a 4-year longitudinal follow-up of 1243 workers, found that high Pb exposure increased biomarkers of oxidative stress, leading to lipid peroxidation and redox imbalance, as well as changes in the morphology and function of lung epithelial cells.
Recent articles have indicated that Pb interferes with the redox balance of the body and that immunotoxicity may be the causes of respiratory diseases. The pathological mechanisms of Pb immunotoxicity are mainly characterized by changes in the concentration of Th1 lymphocytes, IgE, and some cytokines (Jurdziak et al. 2015). The imbalance between Th1 and Th2 lymphocytes under Pb exposure may be responsible for the stimulation of production, thereby increasing the risk of atopic response and asthma (Gao et al. 2007). Kalahasthi et al. (2022) reviewed 40 studies that found significantly higher levels of impaired immune and inflammatory markers in occupational Pb-exposed populations. High-quality articles are required to strengthen the understanding of Pb in the mechanism of immunotoxins and to reveal its association with respiratory diseases. Many gaps remain in the understanding of Pb exposure and respiratory function in children. Zeng et al. (2017) found that children living in exposed areas had higher blood Pb and lower levels of hemoglobin, hematocrit, and lung function. However, the effects and mechanisms of low hemoglobin on respiratory diseases are still unclear.
Despite the current ban on leaded gasoline and paint, Pb pollution continues to increase the burden of disease, especially in low- and middle-income countries (Attina Teresa and Trasande 2013). The main sources of Pb include deteriorating paint, dust, soil, air, drugs, cosmetics, toys, adulterated food, and workplace etc. (Swaringen et al. 2022). Emissions from Pb smelters can contaminate aerosols, soil, and dust, resulting in increased concentrations of Pb in human blood, which is the main source of Pb pollution. Currently, recycling of lead-acid batteries and poorly controlled electronics are becoming major sources of Pb pollution (Earl et al. 2016). A survey by the Indian Chamber of Commerce and Industry revealed that 76% of e-waste workers suffer from respiratory diseases (including asthma, breathing difficulties, and coughing) (Sharma 2015). Due to lower labor costs and weak government regulation, approximately 80% of e-waste from developed countries is illegally exported to developing countries, especially China and India (Awasthi et al. 2016). Previous literature has indicated that heavy metal pollution from e-waste in India has spread to the surrounding environment through informal activities (Awasthi et al. 2016). Even if Pb emissions are controlled, the historical accumulation of Pb pollution in aerosols, soil, and dust can lead to elevated blood Pb levels (Taylor et al. 2019). In the subgroup study of study regions, Pb exposure levels in North America and Asia were associated with respiratory diseases. It is recommended to use special masks to filter PM2.5 in areas with Pb pollution to minimize direct oral inhalation.
The health effects of Pb poisoning are irreversible, so it is important to control sources of Pb to minimize Pb exposure. In 2008, the United States Environmental Protection Agency developed a stricter standard that the average Pb concentration in the air should not exceed 0.15 µg/m3 over a rolling period of three months. However, even blood Pb levels < 5 µg/dL (the reference level for initiating public health action in the United States) can have deleterious health effects in children (Caldwell et al. 2017). The WHO report states that the safety level of Pb exposure is not clear (WHO 2018).
Pb poisoning remains a major environmental pollution issue and there are many measures to reduce Pb exposure. In addition to policies restricting the use of Pb in industry, measures at the individual and household-level mainly include preventing exposure to Pb paint and reasonable disposal of Pb-acid batteries (Jahir et al. 2021). In addition, changing nutritional status and increasing dietary iron and calcium levels and intake of vitamin B1, B2, B6, and B9 can offset the adverse effects of Pb (Reuben et al. 2017). In the case of severe Pb poisoning, medical and follow-up treatment are needed and chelating therapy is commonly used (Tirima et al. 2018). Previous studies have shown that public education on knowledge of Pb poisoning is an effective preventive measure (Nussbaumer-Streit et al. 2016). Active prevention combined with case management measures can effectively eliminate the increase of blood Pb level. Many efforts have been made to prevent and control Pb hazards, but there is still a lack of effective strategies to control the consumption of Pb-contaminated products (Pfadenhauer et al. 2016).
Strengths and limitations
As we know, this is the first meta-analysis of the relationship between Pb exposure and respiratory diseases. All respiratory outcomes, including respiratory diseases, respiratory symptoms, and respiratory function assessments, were included in the analysis. The evidence of this study is comprehensive. The review followed the guidelines for systematic reviews and meta-analysis and developed stricter inclusion and exclusion criteria, so the evidence and results of this study are reliable. The included studies covered most age groups of the population and covered 12 countries. Some of these studies had large sample sizes and were conducted over a long research time, which increases the credibility of the research evidence.
There are some limitations to this study. First, the included studies were published in English, which may have led to reporting bias. Second, the number of studies on the outcome endpoints such as chronic bronchitis and obstructive lung disease is small, which did not allow us to perform meta-regression to test the effects of several regulatory factors on the set effect and the heterogeneity explained by these characteristics (Ziou et al. 2022). Third, medical record review may only register severe respiratory diseases. In the study of minors, some questionnaires and interviews were derived from adult agents or self-reports, and memories and misunderstandings of the questions may lead to deviations in the results. Most of the included studies were cross-sectional studies and did not allow causal inferences.
Conclusion
Our systematic review and meta-analysis found some evidence of the relationship between exposure to Pb-contaminated environments and respiratory diseases. The results showed that Pb exposure increased the risk of respiratory diseases (OR = 1.12, 95% CIs: 1.05, 1.18). Subgroup analysis indicated that Pb exposure was significantly associated with respiratory diseases in the Asian and North American studies, but not in the European studies. Pb exposure increased the risk of respiratory diseases in the age groups under 6 years and over 18 years. Our meta-analysis provides evidence for a positive association between Pb exposure and the development of respiratory diseases. However, the existing literature is limited, with most of them being cross-sectional studies lacking high-quality exposure measures. Further studies are needed to strengthen the evidence system and to determine the exact mechanism by which Pb exposure affects the functioning of respiratory disease.
Data availability
Not applicable.
References
Attina Teresa M, Trasande L (2013) Economic costs of childhood lead exposure in low- and Middle-Income Countries. Environ Health Perspect 121(9):1097–1102
Awasthi AK, Zeng X, Li J (2016) Environmental pollution of electronic waste recycling in India: a critical review. Environ Pollut 211:259–270
Bener A, Almehdi AM, Alwash R et al (2001) A pilot survey of blood lead levels in various types of workers in the United Arab Emirates. Environ Int 27(4):311–314
Borroni E, Pesatori AC, Bollati V et al (2022) Air pollution exposure and depression: a comprehensive updated systematic review and meta-analysis. Environ Pollut 292:118245
Cabral M, Toure A, Garçon G et al (2015) Effects of environmental cadmium and lead exposure on adults neighboring a discharge: evidences of adverse health effects. Environ Pollut 206:247–255
Cai Y, Ramakrishnan R, Rahimi K (2021) Long-term exposure to traffic noise and mortality: a systematic review and meta-analysis of epidemiological evidence between 2000 and 2020. Environ Pollut 269:116222
Caldwell KL, Cheng P-Y, Jarrett JM et al (2017) Measurement challenges at low blood lead levels. Pediatrics 140(2), e20170272
Cheng S, Jin Y, Dou Y et al (2022) Long-term particulate matter 2.5 exposure and dementia: a systematic review and meta-analysis. Public Health 212:33–41
Collaborators GL (2017) Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the global burden of Disease Study 2015. Lancet Infect Dis 17(11):1133–1161
Cornwell CR, Egan KB, Zahran HS et al (2020) Associations of blood lead levels with asthma and blood eosinophils in US children. Pediatr Allergy Immunol 31(6):695–699
Crawford L, Halperin SA, Dzierlenga MW et al (2023) Systematic review and meta-analysis of epidemiologic data on vaccine response in relation to exposure to five principal perfluoroalkyl substances. Environ Int 172:107734
Cui Q, Li L, Cao Y et al (2023) Trends in elemental pb concentrations within atmospheric PM2.5 and associated risk to human health in major cities of China. Environ Pollut 320:121036
Darabi B, Kalvandi G, Najafi R et al (2023) The effects of lead and zinc on Asthma: a systematic review and Meta-analysis. Iran J Pediatr 33(1), e121732
David JR, Sally MB, Frances B et al (2022) Lead exposure in children. BMJ 377, e063950
Dobrakowski M, Pawlas N, Hudziec E et al (2016a) Glutathione, glutathione-related enzymes, and oxidative stress in individuals with subacute occupational exposure to lead. Environ Toxicol Pharmacol 45:235–240
Dobrakowski M, Pawlas N, Kasperczyk A et al (2016b) Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum Exp Toxicol 36(7):744–754
Duan Q, Zhou Y, Yang D (2022) Endoplasmic reticulum stress in airway hyperresponsiveness. Biomed Pharmacother 149:112904
Earl R, Burns N, Nettelbeck T et al (2016) Low-level environmental lead exposure still negatively associated with children’s cognitive abilities. Australian J Psychol 68(2):98–106
Farkhondeh T, Samarghandian S, Sadighara P (2015) Lead exposure and asthma: an overview of observational and experimental studies. Toxin Reviews 34(1):6–10
Feiler MO, Pavia CJ, Frey SM et al (2021) Early life blood lead levels and asthma diagnosis at age 4–6 years. Environ Health Prev Med 26(1):108
Galiciolli MEA, Lima LS, da Costa NdS et al (2022) IQ alteration induced by lead in developed and underdeveloped/developing countries: a systematic review and a meta-analysis. Environ Pollut 292:118316
Gao D, Mondal TK, Lawrence DA (2007) Lead effects on development and function of bone marrow-derived dendritic cells promote Th2 immune responses. Toxicol Appl Pharmcol 222(1):69–79
Grant K, Goldizen FC, Sly PD et al (2013) Health consequences of exposure to e-waste: a systematic review. Lancet Global Health 1(6):e350–e361
Hsieh C-Y, Jung C-R, Lin C-Y et al (2021) Combined exposure to heavy metals in PM < sub > 2.5 and pediatric asthma. J Allergy Clin Immunol 147(6):2171–2180e2113
Hua C, Ma W, Zheng F et al (2023) Health risks and sources of trace elements and black carbon in PM2.5 from 2019 to 2021 in Beijing. Journal of Environmental Sciences
Huang X, Xie J, Cui X et al (2016) Association between concentrations of metals in urine and adult asthma: a case-control study in Wuhan, China. PLoS ONE 11(5), e0155818
Jahir T, Pitchik HO, Rahman M et al (2021) Making the invisible visible: developing and evaluating an intervention to raise awareness and reduce lead exposure among children and their caregivers in rural Bangladesh. Environ Res 199:111292
Joseph Christine LM, Havstad S, Dennis O, R., et al (2005) Blood lead level and risk of Asthma. Environ Health Perspect 113(7):900–904
Jurdziak M, Gać P, Martynowicz H et al (2015) Function of respiratory system evaluated using selected spirometry parameters in persons occupationally exposed to lead without evident health problems. Environ Toxicol Pharmacol 39(3):1034–1040
Kalahasthi R, Nagaraju R, Balachandar R et al (2022) Association between occupational lead exposure and immunotoxicity markers: a systematic review and meta-analysis. Toxicology 465:153047
Kang SY, Kim C-K (2019) Association between lead exposure and increased risk of bronchial asthma in Korean adolescents. Allergy Asthma Respir Dis 7(1):37–43
Khazdair MR, Boskabady MH, Afshari R et al (2012) Respiratory symptoms and pulmonary function testes in lead exposed workers. Iran Red Crescent Med J 14(11):737–742
Koh HY, Kim TH, Sheen YH et al (2019) Serum heavy metal levels are associated with asthma, allergic rhinitis, atopic dermatitis, allergic multimorbidity, and airflow obstruction. J Allergy Clin Immunology: Pract 7(8):2912–2915e2912
Kuntawee C, Tantrakarnapa K, Limpanont Y et al (2020) Exposure to Heavy Metals in Electronic Waste Recycling in Thailand
Lee E, Park B, Chung WY et al (2020) Blood lead levels in relation to smoking and chronic obstructive pulmonary disease (COPD): a study from Korean National Health and Nutrition Examination Survey (KNHANES). J Thorac Disease 12(6):3135–3147
Li X-N, Liu Y, Huang N et al (2020) The Association between environmental lead exposure and recurrent respiratory infections in children aged 3–7 years in Shenyang, China. Indian Pediatr 57(11):1023–1025
Lin H-W, Lee H-L, Shen T-J et al (2024) Pb(NO3)2 induces cell apoptosis through triggering of reactive oxygen species accumulation and disruption of mitochondrial function via SIRT3/SOD2 pathways. Environ Toxicol 39(3):1294–1302
Liu Z-H, Ai S, Xia Y et al (2024) Intestinal toxicity of pb: structural and functional damages, effects on distal organs and preventive strategies. Sci Total Environ 931:172781
McGuinn LA, Schneider A, McGarrah RW et al (2019) Association of long-term PM2.5 exposure with traditional and novel lipid measures related to cardiovascular disease risk. Environ Int 122:193–200
McRae N, Gennings C, Rivera Rivera N et al (2022) Association between prenatal metal exposure and adverse respiratory symptoms in childhood. Environ Res 205:112448
Mendy A, Gasana J, Vieira ER (2012) Urinary heavy metals and associated medical conditions in the US adult population. Int J Environ Health Res 22(2):105–118
Min J-Y, Min K-B, Kim R et al (2008) Blood lead levels and increased bronchial responsiveness. Biol Trace Elem Res 123(1):41–46
Mitsui-Iwama M, Yamamoto-Hanada K, Fukutomi Y et al (2019) Exposure to paraben and triclosan and allergic diseases in Tokyo: a pilot cross-sectional study. Asia Pac Allergy 9(1)
Mohammed AA, Mohamed FY, El-Okda E-S et al (2015) Blood lead levels and childhood asthma. Indian Pediatr 52(4):303–306
Motosue AM, Petronella S, Sullivan J et al (2009) Lead exposure risk is Associated with Asthma in a low-income Urban Hispanic Population: results of the communities Organized against Asthma and lead (COAL) project. J Allergy Clin Immunol 123(2):S20
Myers SN, Rowell B, Binns HJ (2002) Lead poisoning and asthma: an examination of Comorbidity. Arch Pediatr Adolesc Med 156(9):863–866
Nguyen TT, Higashi T, Kambayashi Y et al (2016) A Longitudinal Study of Association between Heavy Metals and itchy eyes, coughing in chronic cough patients. Related with Non-Immunoglobulin E Mediated Mechanism
Nunes RAO, Alvim-Ferraz MCM, Martins FG et al (2021) Estimating the health and economic burden of shipping related air pollution in the Iberian Peninsula. Environ Int 156:106763
Nussbaumer-Streit B, Yeoh B, Griebler U et al (2016) Household interventions for preventing domestic lead exposure in children. Cochrane Database of Systematic Reviews (10)
Oktapodas Feiler M, Caserta MT, van Wijngaarden E et al (2020) Environmental lead exposure and influenza and respiratory Syncytial Virus diagnoses in Young Children. A Test-Negative Case-Control Study
Palkovicova L, Reichrtova E, Rausova K et al (2004) PRENATAL EXPOSURE TO LEAD AND ASTHMA RESPIRATORY SYMPTOMS IN EARLY CHILDHOOD. Epidemiology 15(4)
Park S, Lee E-H, Kho Y (2016) The association of asthma, total IgE, and blood lead and cadmium levels. J Allergy Clin Immunol 138(6):1701–1703e1706
Pesce G, Sesé L, Calciano L et al (2021) Foetal exposure to heavy metals and risk of atopic diseases in early childhood. Pediatr Allergy Immunol 32(2):242–250
Pfadenhauer LM, Burns J, Rohwer A et al (2016) Effectiveness of interventions to reduce exposure to lead through consumer products and drinking water: a systematic review. Environ Res 147:525–536
Pugh Smith P, Nriagu JO (2011) Lead poisoning and asthma among low-income and African American children in Saginaw, Michigan. Environ Res 111(1):81–86
Rabito FA, Perry S, Salinas O et al (2011) A longitudinal assessment of occupation, respiratory symptoms, and blood lead levels among latino day laborers in a non-agricultural setting. Am J Ind Med 54(5):366–374
Rabito FA, Horter L, Langlois EC et al (2013) Blood lead and Pediatric Asthma. Epidemiology 24(3)
Reiner RC, Welgan CA, Casey DC et al (2019) Identifying residual hotspots and mapping lower respiratory infection morbidity and mortality in African children from 2000 to 2017. Nat Microbiol 4(12):2310–2318
Reuben A, Caspi A, Belsky DW et al (2017) Association of Childhood blood lead levels with cognitive function and socioeconomic status at Age 38 years and with IQ Change and Socioeconomic mobility between Childhood and Adulthood. JAMA 317(12):1244–1251
Rokadia HK, Agarwal S (2013) Serum heavy metals and Obstructive Lung Disease: results from the National Health and Nutrition Examination Survey. Chest 143(2):388–397
Ruan F, Zhang J, Liu J et al (2022) Association between prenatal exposure to metal mixtures and early childhood allergic diseases. Environ Res 206:112615
Shaheen M, Pan D (2019) 3469 age and racial variation in the relation between blood lead level and asthma in children: data from National Health and Nutrition Examination Survey 1999–2016. J Clin Translational Sci 3(s1):32–33
Shaheen SO, Newson RB, Henderson AJ et al (2004) Umbilical cord trace elements and minerals and risk of early childhood wheezing and eczema. Eur Respir J 24(2):292
Sharma DC (2015) Emissions from e-waste recycling threaten workers’ health. Lancet Respiratory Med 3(11):847–848
Shi L, Steenland K, Li H et al (2021) A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States. Nat Commun 12(1):6754
Swaringen BF, Gawlik E, Kamenov GD et al (2022) Children’s exposure to environmental lead: a review of potential sources, blood levels, and methods used to reduce exposure. Environ Res 204:112025
Tarvainen T, Lehtonen M, Lahaye Y et al (2023) Analytical workflow to trace lead sources in fill-derived soils in Turku, Southwest Finland. Appl Geochem 156:105735
Taylor MP, Isley CF, Glover J (2019) Prevalence of childhood lead poisoning and respiratory disease associated with lead smelter emissions. Environ Int 127:340–352
Tirima S, Bartrem C, von Lindern I et al (2018) Food contamination as a pathway for lead exposure in children during the 2010–2013 lead poisoning epidemic in Zamfara, Nigeria. J Environ Sci 67:260–272
Wang IJ, Karmaus WJJ, Yang C-C (2017) Lead exposure, IgE, and the risk of asthma in children. J Expo Sci Environ Epidemiol 27(5):478–483
Wei W, Wu X, Bai Y et al (2020) Lead exposure and its interactions with oxidative stress polymorphisms on lung function impairment: results from a longitudinal population-based study. Environ Res 187:109645
Wells EM, Bonfield TL, Dearborn DG et al (2014) The relationship of blood lead with immunoglobulin E, eosinophils, and asthma among children: NHANES 2005–2006. Int J Hyg Environ Health 217(2):196–204
Wen J, Giri M, Xu L et al (2023) Association between exposure to selected heavy metals and Blood Eosinophil counts in asthmatic adults. Results from NHANES, pp 2011–2018
WHO (2020) Risk of bias assessment instrument for systematic reviews informing WHO global air quality guidelines (2020)
WHO, W.H.O (2018) Lead poisoning and health
WHO, W.H.O (2022) Lead poisoning
Wu K-G, Chang C-Y, Yen C-Y et al (2019) Associations between environmental heavy metal exposure and childhood asthma: a population-based study. J Microbiol Immunol Infect 52(2):352–362
Wu H, Wang J, Xiang Y et al (2021) Effects of tetrabromobisphenol A (TBBPA) on the reproductive health of male rodents: a systematic review and meta-analysis. Sci Total Environ 781:146745
Yang G, Sun T, Han Y-Y et al (2019) Serum cadmium and lead, current wheeze, and lung function in a nationwide study of adults in the United States. J Allergy Clin Immunology: Pract 7(8):2653–2660e2653
Yau W-H, Chen S-C, Wu D-W et al (2023) Blood lead (pb) is associated with lung fibrotic changes in non-smokers living in the vicinity of petrochemical complex: a population-based study. Environ Sci Pollut Res 30(30):75225–75234
Zammit C, Bilocca D, Ruggieri S et al (2020) Association between the concentration and the Elemental composition of Outdoor PM2.5 and respiratory diseases in Schoolchildren. A Multicenter Study in the Mediterranean Area
Zeng X, Xu X, Zheng X et al (2016) Heavy metals in PM2.5 and in blood, and children’s respiratory symptoms and asthma from an e-waste recycling area. Environ Pollut 210:346–353
Zeng X, Xu X, Boezen HM et al (2017) Decreased lung function with mediation of blood parameters linked to e-waste lead and cadmium exposure in preschool children. Environ Pollut 230:838–848
Zhang L, Liang J, Gao A (2023) Contact to perfluoroalkyl substances and thyroid health effects: a meta-analysis directing on pregnancy. Chemosphere 315:137748
Zheng G, Xu X, Li B et al (2013) Association between lung function in school children and exposure to three transition metals from an e-waste recycling area. J Expo Sci Environ Epidemiol 23(1):67–72
Ziou M, Tham R, Wheeler AJ et al (2022) Outdoor particulate matter exposure and upper respiratory tract infections in children and adolescents: a systematic review and meta-analysis. Environ Res 210:112969
Funding
This work was supported by the National Key Research and Development Program of China (2022YFC3703002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent for publish
The authors approve the manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, T., Dai, K. & Wu, H. Effect of lead exposure on respiratory health: a systematic review and meta-analysis. Air Qual Atmos Health (2024). https://doi.org/10.1007/s11869-024-01619-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11869-024-01619-x