Opinion statement
Use of poly(ADP-ribose) polymerase (PARP) inhibitors has greatly increased over the past 5 years. With several new Food and Drug Administration (FDA) approvals, three PARP inhibitors have entered into standard of care treatment for epithelial ovarian cancer (including ovarian, fallopian tube, and primary peritoneal cancer). Olaparib and rucaparib currently have indications for treatment of recurrent BRCA mutant ovarian cancer. Olaparib, rucaparib, and niraparib all have indications for maintenance therapy in recurrent platinum-sensitive ovarian cancer after response to platinum-based therapy. In our practice, we use both olaparib and rucaparib in the recurrent setting, and all three PARP inhibitors in the maintenance setting. Choice of which PARP inhibitor to use in either setting is largely based upon baseline laboratory values, number of prior therapies, and presence of a BRCA mutation and/or homologous recombination deficiency (HRD). As (HRD) and other biomarker assessments continue to improve, we anticipate being able to better identify which patients might most benefit from PARP inhibitor therapy in the future. The clinically available PARP inhibitors are currently undergoing extensive investigations in clinical trials. Other newer agents such as talazoparib, veliparib, 2X-121, and CEP-9722 are in earlier stages of development. As more FDA-approved indications for PARP inhibitor therapy in ovarian cancer become available, we anticipate the decision of which PARP inhibitor to use will become increasingly complex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer remains the gynecologic malignancy associated with the highest number of deaths each year [1]. Until recently, cytotoxic chemotherapy, usually before or after surgery, was the mainstay of treatment in both the primary and recurrent setting. Although cytotoxic chemotherapy remains the standard of care for first-line treatment of ovarian cancer [2], several targeted therapeutics have achieved Food and Drug Administration (FDA) approval for patients with ovarian cancer. To date, poly(ADP-ribose) polymerase (PARP) inhibitors have the largest number of approvals in a single drug class for a targeted therapy in ovarian cancer (Table 1). Herein, we review the data for the current and potentially future uses of PARP inhibitor therapy in patients with ovarian cancer.
PARP inhibitors
PARP inhibitors are a class of drugs that target the ability of a cell to repair DNA. When single-strand DNA breaks occur in a normal cell, PARP1 and PARP2 bind to the DNA and aid in base-excision repair [3, 4]. When these proteins are inhibited, single-strand breaks accumulate and, ultimately, lead to double strand DNA breaks and cytotoxicity. Unlike PARP2, PARP1 can also repair double-strand DNA breaks and damage to replication forks [3]. Thus, inhibition of PARP1 also impairs these functions. Other mechanisms for PARP inhibitors have also been identified, including PARP1 trapping on damaged DNA and impaired recruitment of BRCA1 to damaged DNA [3, 5, 6].
For double-strand DNA breaks, cells heavily rely on homologous recombination repair mechanisms [7]. In patients whose tumors already lack this repair mechanism, PARP inhibition forces cells to activate non-homologous end joining (NHEJ) repair. NHEJ is an error prone mechanism for repairing damaged DNA and is therefore unable to effectively repair DNA damage on a large scale [5]. For this reason, both preclinical and clinical data support the hypothesis that tumors demonstrating homologous recombination deficiency (HRD) have an improved response to PARP inhibition [8, 9]. The most common clinical cause of HRD is a germ line mutation in the BRCA1 or BRCA2 gene [10]. However, somatic BRCA1 and BRCA2 mutations, as well as both germ line and somatic mutations in genes for other HRD proteins such as ATM, RAD51, and ATR, have also been shown to be associated with higher PARP inhibitor efficacy [8].
BRCA1/BRCA2 mutations and other HRD phenotypes have been identified in multiple different tumor types, including epithelial ovarian cancer [8]. Data from The Cancer Genome Atlas (TCGA) for ovarian cancer found that approximately 50% of the tumors evaluated had mutations in HR-associated genes [11]. Interestingly, only 20% of the tumors included in the TCGA cohort had a germ line or somatic BRCA mutation, suggesting that these other HR-associated genes comprise a clinically significant subset of epithelial ovarian cancer patients. In large part for this reason, ovarian cancer has been the leading tumor type to gain FDA approvals for PARP inhibitor therapy.
Recurrent ovarian cancer
As is common with many new drugs, PARP inhibitors were first explored in the recurrent setting for ovarian cancer. Thus, it is not surprising that the first FDA approval for PARP inhibitor use in ovarian cancer was for the treatment of recurrent ovarian cancer. In 2014, olaparib was approved for deleterious germ line BRCA-mutated advanced ovarian cancer based largely on data from a multicenter phase II trial of patients with multiple solid tumor types and a germ line BRCA1 or BRCA2 mutation [12, 13].
Rucaparib was evaluated in Study 10, which was a phase I/II clinical trial [14]. The phase I component that established the recommended phase II dose was completed in a cohort of patients with any advanced solid tumor that had progressed on standard treatment. Following this, the study enrolled patients with recurrent, platinum-sensitive, high-grade serous, endometrioid, mixed, or clear cell ovarian cancer with BRCA1 or BRCA2 mutations in a phase II expansion cohort. During dose escalation, no maximum tolerated dose was identified, and a recommended phase II dose of 600 mg twice daily was chosen based on the safety profile, pharmacokinetics, and antitumor activity. Forty-two patients were enrolled in the phase II component, and 83% achieved a RECIST/GCIG CA-125 response to treatment. Interestingly, 91% of patients had a dose reduction and/or a delay in treatment due to an adverse event [14].
The impact of a molecular signature on rucaparib efficacy was evaluated in ARIEL2 Part 1 [15]. This study enrolled women with recurrent platinum-sensitive high-grade ovarian cancer who had received at least one prior platinum therapy. Patients were enrolled regardless of BRCA mutation status. However, patients were stratified into three groups using the Foundation Medicine next-generation sequencing assay: BRCA mutant of germ line or somatic origin, BRCA wild type with high LOH, and BRCA wild type with low LOH. The primary outcome was progression-free survival (PFS). Of 204 patients treated on trial, 40 had BRCA mutations, 82 were wild type/LOH high, 70 were wild type/LOH low, and 12 were wild type/LOH unclassified. Median PFS was highest in the BRCA mutant group (12.8 months), compared with 5.7 months and 5.2 months in the LOH high and low groups, respectively [15]. In a combined analysis of 106 patients from Study 10 and ARIEL2, the overall response rate was found to be 53.8% (45.3% partial response, 8.5% complete response; 95% CI 43.8–63.5). These data demonstrated a median duration of response of 9.2 months (95% CI 6.6–11.6) [16•]. Based on these combined data, in 2016, the FDA-approved rucaparib for recurrent ovarian cancer patients with deleterious germ line or somatic BRCA mutations who had received at least two prior chemotherapies.
Although niraparib does not have FDA approval for the treatment of recurrent ovarian cancer, a phase I trial published in 2013 suggested antitumor efficacy [17]. The dose escalation cohort enrolled patients with advanced solid tumors, with enrichment for BRCA1 and BRCA2 mutations, and identified a recommended phase II dose of 300 mg per day. In a dose expansion study of women with platinum-resistant high-grade serous ovarian cancer with BRCA1 or BRCA2 mutations, 50% of the platinum-sensitive and 44% of the platinum-resistant patients achieved a clinical benefit. In women with ovarian or primary peritoneal cancers without a BRCA mutation, 67% of the platinum-sensitive patients and 32% of platinum-resistant patients received a clinical benefit.
Based on these data, a phase II study (QUADRA) evaluating single-agent niraparib in recurrent high-grade serous ovarian cancer who have received three or four prior chemotherapy regimens is currently ongoing (NCT02354586) [18]. All patients had evaluation for BRCA gene mutation and HRD status using MyChoice HRD test. Patients were deemed HRD positive if they had a germ line or somatic BRCA mutation or had an HRD score greater than or equal to 42. Preliminary data showed that among those with platinum-sensitive, HRD-positive tumors, the overall objective response rate was 27.5% (38.9% in patients with BRCA mutations and 21.2% in BRCA wild type but HRD-positive patients). Perhaps most interestingly, though, 18% of patients with platinum-resistant, HRD-negative tumors demonstrated clinical benefit [18].
Veliparib has not yet achieved FDA approval for the treatment of ovarian cancer. However, a phase II evaluation in patients with recurrent epithelial ovarian cancers with BRCA1 or BRCA2 mutations was published in 2015 [19]. A dose of 400 mg twice daily was administered, and the primary end point was objective tumor response. Hematologic and gastrointestinal side effects were most common. The overall response rate was 26%, and another 48% achieved stable disease. Objective responses were seen in patients with both platinum-resistant and platinum-sensitive disease.
In our practice, we use both olaparib and rucaparib in patients who meet the aforementioned FDA approval criteria. If patients are candidates for either olaparib or rucaparib therapy, the decision to use one over the other is largely provider dependent but may be based upon anticipated tolerability to known side effects as well as baseline laboratory studies. There are currently no head-to-head trials comparing efficacy of rucaparib vs. olaparib (or any other PARP inhibitor) in the recurrent setting, and thus, the decision to use one instead of the other is relatively subjective.
Maintenance treatment
The first FDA approval for use of PARP inhibitors as a maintenance therapy in patients with recurrent high-grade serous ovarian cancer in the recurrent setting was given to niraparib in 2017. This was based on data published in 2016 from the NOVA/ENGOT-OV16 trial [20••]. This was a phase III, double-blinded, randomized, placebo-controlled trial of women with recurrent, platinum-sensitive ovarian cancer with predominantly high-grade serous features. Participants must have had at least two prior chemotherapy regimens with response to last platinum-based regimen and must have been within 8 weeks of their last platinum-based regimen. Five hundred and fifty-three patients with (gBRCA cohort) and without germ line BRCA mutations (non-gBRCA cohort) were enrolled and randomized 2:1 to niraparib or placebo. The primary outcome was PFS. PFS was significantly longer in the niraparib group for all three subgroups: patients with germ line BRCA mutation (21.0 vs. 5.5 months, HR 0.27), patients without germ line BRCA mutations but with HRD-positive tumors (12.9 vs. 3.8 months, HR 0.38), and patients without germ line BRCA mutations (9.3 vs. 3.9 months, HR 0.45). Quality of life outcomes were similar between the two groups. The most common adverse events associated with niraparib use included nausea, thrombocytopenia, fatigue, and anemia [20••].
Since this approval, trials of other PARP inhibitors as maintenance therapy have been completed. Several months after the niraparib approval, olaparib was approved by the FDA for maintenance treatment in patients with recurrent epithelial ovarian cancer who have had a complete or partial response to platinum-based chemotherapy. This was based on phase II and phase III data. In 2012, Study 19 demonstrated improved PFS in a randomized, double-blind, placebo-controlled phase 2 trial of 265 platinum-sensitive recurrent high-grade serous ovarian cancer patients (8.4 vs. 4.8 months, p < 0.001) [21]. The dose of olaparib used was 400 mg capsules twice daily. In a planned subgroup analysis, patients with BRCA1/BRCA2 mutations demonstrated the greatest improvement [22]. Follow-up data published in 2016 did not demonstrate a statistically significant improvement in overall survival [23]. Importantly, Study 19 data published in 2016 also demonstrated no significant difference in quality of life between patients receiving olaparib or placebo on trial [24].
In 2017, phase III data of olaparib in the maintenance setting was published (SOLO 2) [25••]. SOLO 2 enrolled only women with platinum-sensitive high-grade serous or endometrioid ovarian cancer who had a mutation in BRCA1 or BRCA2. Participants had received at least two prior lines of chemotherapy and must have had a complete or partial response to the most recent treatment. The olaparib dose was 300 mg tablets twice daily, and patients were randomized 2:1 to olaparib or placebo treatment. The primary end point was PFS. PFS was significantly longer in women receiving olaparib than those receiving placebo (19.1 vs. 5.5 months, p < 0.0001). There were no statistically significant differences in quality of life measures. The most common adverse events were nausea, vomiting, diarrhea, and fatigue. Grade 3 or higher anemia was also more common in the olaparib group, although there were no differences in grade 3 or higher neutropenia or thrombocytopenia.
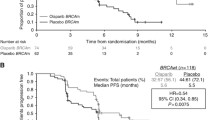
In 2017, data for rucaparib in the maintenance setting were published as ARIEL3 in Lancet Oncology [26••]. This was a randomized, double-blind, placebo-controlled phase III trial of patients with high-grade serous or endometrioid ovarian cancer. Participants had received at least two prior platinum-based regimens and must have had either a partial or complete response to the last treatment. They were randomized to either rucaparib 600 mg twice daily or placebo (2:1 randomization). Treatment must have been initiated within 8 weeks of the last dose of platinum chemotherapy. Patients were enrolled regardless of BRCA mutation status, but randomization was stratified by the presence of a BRCA1/BRCA2 mutation, mutation in another non-BRCA HR-associated gene, or no mutation in an HR-associated gene. The primary outcome was PFS. There were 564 participants enrolled (375 to rucaparib, 189 to placebo). In the intention to treat analysis, the median PFS was significantly improved in the rucaparib compared with the placebo group (10.8 months vs. 5.4 months, p < 0.0001).
This difference was more pronounced in the comparison of patients with tumors demonstrating HRD, with a PFS of 13.6 months compared with 5.4 months in the rucaparib and placebo groups, respectively (p < 0.0001) [26••]. In contrast to the above studies, in this subanalysis, HRD was defined using both HR-associated genes and genomic loss of heterozygosity (LOH). LOH was assessed using the Foundation Medicine T5 NGS Assay (Cambridge, MA, USA) with a prespecified cutoff of at least 16% to designate high genomic LOH. Overall survival analyses from ARIEL3 are not yet mature. However, based on these available data, the FDA-approved rucaparib in 2018 for maintenance therapy of recurrent ovarian cancer who have had a complete or partial response to platinum-based chemotherapy.
A summary of the trials evaluating maintenance PARP inhibitor use in the recurrent setting is shown in Table 2. In our practice, similar to considerations for use in the recurrent setting, decisions for which PARP inhibitor to use are multifactorial and largely provider dependent. Considerations may include baseline laboratory values and patient concerns about specific side effects, but there are also no head-to-head studies comparing any of the approved PARP inhibitors in the maintenance setting.
Front-line treatment
Currently, there are no FDA-approved PARP inhibitors for primary treatment of ovarian cancer. Preliminary data from a phase I study of veliparib given in combination with IV carboplatin/paclitaxel/bevacizumab or IV/IP cisplatin/paclitaxel/bevacizumab was presented in 2015 (NCT00989651) [27]. Twice daily veliparib was administered at increasing dose levels for cycles 1–6. Bevacizumab was initiated in cycle 2 and was continued as single-agent therapy for cycles 7–22. In this study of 189 patients, the recommended phase II dose of veliparib was found to be 150 mg twice daily. Based on these data, a phase III trial of this regimen is currently underway (NCT02470585). A smaller Japanese phase I trial of veliparib used in combination with carboplatin and paclitaxel in nine patients was published in 2017 [28]. Similarly, these data demonstrated that the recommended phase II dose of veliparib was 150 mg twice daily given with carboplatin and weekly paclitaxel. All of the five patients who were able to be assessed had an objective response: four had a partial response, and one had a complete response. To our knowledge, no other trials of adjuvant or neoadjuvant treatment with PARP inhibitors have been published for ovarian cancer.
There are also no FDA-approved PARP inhibitors for maintenance after completion of primary treatment of ovarian cancer. However, two trials are currently ongoing which are addressing this question: SOLO1 and PRIMA. The SOLO1 trial is a multi-institutional double-blinded placebo-controlled randomized trial of olaparib (NCT01844986) [29]. Patients are eligible if they have high-grade serous or endometrioid ovarian cancer and have a known or suspected BRCA mutation. They must have stage III-IV disease and have had a complete or partial response to first-line platinum therapy. The primary outcome is PFS and the dose of olaparib is 300 mg tablets twice daily. SOLO1 is no longer accruing, and a June 2018 press release reported a statistically significant improvement in PFS in the olaparib compared with the placebo group. The initial results are expected to be presented this year.
PRIMA is a multi-institutional phase 3 double-blinded placebo-controlled randomized trial of niraparib (NCT02655016). Patients are eligible if they have stage III-IV high-grade predominantly serous or endometrioid ovarian cancer and have just completed first-line platinum based chemotherapy. They must have had a complete or partial response to chemotherapy but are only eligible if they either are stage IV or have stage III disease with visible residual disease after primary debulking. Patients who were inoperable with stage III or IV are also eligible to enroll. The primary outcome is PFS.
In our practice, we are not yet using PARP inhibitors in the upfront setting for patients with ovarian cancers. However, as more data become available, we will need to assess whether PARP inhibitors should be used in this setting, and if so, how this fits together with other primary maintenance options that are currently available or actively being studied (e.g., bevacizumab, immune checkpoint inhibitors).
Combination therapy with PARP inhibitors
Given the success of single agent PARP inhibitors, studies evaluating combinations with PARP inhibitors have begun to emerge. Several trials have studied the combination of olaparib and a cytotoxic chemotherapy agent. A phase I study of olaparib and liposomal doxorubicin in solid tumors demonstrated that the combination was tolerable, and did find some early signal for anti-tumor efficacy [30]. This cohort of 44 patients included 28 patients with ovarian cancer and found a maximum tolerated dose of olaparib 400 mg twice daily (capsule) and liposomal doxorubicin 40 mg/m2. Interestingly, 3/12 platinum-resistant patients and 10/14 platinum-sensitive patients had a complete or partial response [30]. Following that, Kummar et al. investigated veliparib in combination with oral cyclophosphamide in a randomized phase II trial in ovarian cancers [31]. They found that the combination of veliparib and oral cyclophosphamide was not significantly better than oral cyclophosphamide alone, and the trial was stopped early as per the protocol due to lack of improved efficacy. Another phase I study of veliparib and weekly topotecan that included 45 ovarian cancer patients found that the combination was well tolerated [32]. The authors also found that patients with BRCA1, BRCA2, or RAD51D mutations showed longer duration of response than those without HRD mutations [32].
A randomized phase II study evaluated the combination of carboplatin, paclitaxel, and olaparib [33•]. Patients with platinum-sensitive, recurrent ovarian cancer who had received a maximum of three prior lines were enrolled and randomized to either carboplatin/paclitaxel/olaparib or carboplatin/paclitaxel alone. The primary outcome was PFS, and patients were not required to have a BRCA mutation. PFS was significantly longer in the olaparib containing group, with a median of 12.2 vs. 9.6 months, respectively (HR 0.51, p = 0.0012). This difference was even more pronounced in the subgroup of patients with BRCA mutations (HR 0.21, p = 0.0015).
More recently, a phase I/IB dose escalation study of olaparib and carboplatin was completed in women with ovarian (78%), breast (18%), or uterine cancer (4%) [34]. This study found a maximum-tolerated dose of olaparib 200 mg tablets every 12 h and carboplatin with an AUC of 4 given in differing sequences. The objective response rate for the study was 46%; notably, 15 platinum-resistant ovarian or primary peritoneal cancers achieved partial responses with this regimen. Interestingly, these data suggested that carboplatin administered prior to olaparib may result in intracellular olaparib accumulation and increased olaparib clearance, which may improve clinical benefit.
Trials evaluating combinations of PARP inhibitors with novel agents have also been successfully completed. Matulonis et al. recently published a phase I study of the combination of BKM120, a PI3K pathway inhibitor in combination with olaparib [35]. The study included 46 women with ovarian cancer (26 of whom were platinum-resistant) and 24 women with breast cancer. Among the ovarian cancer patients, 22 had a partial response or stable disease. This included 17 patients with platinum-resistant disease. In the ovarian cancer patients with germ line BRCA mutations (n = 28), 29% had a partial response and 46% had stable disease. Interestingly, treatment responses were also observed in the ovarian cancer patients who were germ line BRCA wild type (n = 8), with 12% and 62% having a partial response or stable disease, respectively.
Another phase I trial evaluating two different olaparib-containing PI3K combinations in women with recurrent ovarian cancer, endometrial cancer, or triple negative breast cancer was also recently completed. Both novel agents targeted the PI3K pathway: vistusertib (AZD2014), which is an mTOR inhibitor; and AZD5363, which is an AKT inhibitor. Preliminary results are now available for all of the study arms. In patients who received the combination of olaparib and AZD5363, the study demonstrated that the combination was well tolerated and that several ovarian cancer patients had durable responses [36]. In the olaparib and vistusertib arms, the response rate was 20% for women with ovarian cancer [37]. Of note, the majority (95%) of these patients were platinum-resistant and BRCA negative (84%).
The anti-angiogenesis drug cediranib was also recently paired with olaparib in a randomized phase 2 study of women with recurrent high-grade serous or endometrioid ovarian cancer [38•]. Ninety women were randomized on trial, stratified by BRCA mutation status (mutated vs. wild type vs. unknown). Interim analysis found a significantly improved PFS of 17.7 months in the combination arm compared with 9.0 months for women receiving olaparib alone. Notably, in a subanalysis evaluating patients who were BRCA wild type or unknown, the PFS for the combination was 16.5 months compared with 5.7 months in the olaparib alone arm. This difference was notably larger than among those patients with BRCA mutations (19.4 months for the combination vs. 16.5 months for olaparib alone). Unfortunately, there was no arm with single-agent cediranib to also act as a comparator to the combination arm [38•]. Overall survival data have not yet been published, but a recently presented update of the PFS data showed sustained improvement in the combination arm [39]. Other studies of similar combinations are currently ongoing for both cediranib (e.g., NCT02446600, NCT02446600), as well as for other anti-angiogenic therapies (e.g., NCT02477644).
More recently, several studies have begun investigating the utility of a combination PARP inhibitor and an immunotherapy agent. Results from the TOPACIO study have recently been presented [40]. Platinum-resistant or secondarily platinum-refractory ovarian cancer patients were included and received the combination of niraparib 200 mg once daily with pembrolizumab 200 mg IV every 21 days. Preliminary efficacy data showed that 13 of the 49 platinum-resistant or platinum-refractory patients had responses to the combination, and adverse events were similar to prior single-agent studies. Interestingly, 77% of patients enrolled were BRCA wild type, and 52% were HRD negative. Even in these two populations, the objective response rates were 24% and 27%, respectively, suggesting that this combination has activity in populations who do not typically respond to PARP inhibition.
Another phase II trial, the MEDIOLA trial (NCT02734004), evaluated the combination of olaparib and durvalumab and was recently presented at the 2018 Society of Gynecologic Oncology Annual Meeting [41]. This was based on a phase I trial of the combination in women with ovarian, triple negative breast, cervical, or uterine cancer which demonstrated tolerability of the combination, as well as early markers of efficacy [42]. In the phase II study, platinum-sensitive, known or suspected germ line BRCA1/2 mutant, recurrent ovarian cancer patients were enrolled as one of four cohorts; the other cohorts included ATM-negative gastric cancer, small-cell lung cancer, and germ line mutated HER2-negative breast cancer [43]. Preliminary data from the 34 ovarian cancer patients demonstrated an objective response rate of 72% and that the combination was well tolerated [41].
Future directions
Although the above studies have moved PARP inhibitor treatment of ovarian cancer further into the spotlight, many questions remain unanswered. Numerous clinical trials are currently underway, both for the currently available PARP inhibitors and for PARP inhibitors that have not yet achieved an FDA approval. Hopefully, these studies will help clarify some of the above clinical scenarios. Another PARP inhibitor, talazoparib, is thought to be more potent than currently available PARP inhibitors and is also currently being studied in several clinical trials. It is not unreasonable to expect that this and other novel PARP inhibitors may similarly demonstrate efficacy in the above spaces. The future challenge will be differentiating which PARP inhibitor should be used at any given time. The clearest way to evaluate this question would be larger phase III trials with head-to-head comparisons of different PARP inhibitors, although admittedly, these studies are unlikely to be funded by pharmaceutical companies. Considerations will need to include not only potential differences in efficacy but also differences in side effects and tolerability.
Clinical trials for novel PARP inhibitor-containing combinations are also ongoing, and many preclinical studies have been published that continue to support the potential utility of PARP inhibitor combinations [44,45,46,47]. One practical consideration for the use of these combinations will be adequately identifying and managing the side effects that come not only with novel agents, but also with new combinations of novel agents. This challenge will be greater for drugs with which oncologists have less experience but will still be important even when including standard cytotoxic chemotherapy agents.
Another challenge for all targeted therapeutics and novel agents in general is the idea of predictive biomarkers. Although PARP inhibitors are one of a few number of therapeutics with a well-accepted predictive biomarker (e.g., BRCA1/2 germ line mutation) [48], there are many more biomarkers that show promise. This challenge will be even greater as we start looking at PARP inhibitor combinations. Additionally, although efficacy seems to be greatest in biomarker-positive ovarian cancer patients, efficacy analyses from some studies of PARP inhibitor combinations have suggested that there may still be significant benefit in biomarker-negative patients (such as cediranib and olaparib [38•]). Thus, limiting the use of PARP inhibitor therapy only to patients who demonstrate a given biomarker may miss an important subset of the population who could potentially benefit from these therapies.
Interestingly, preclinical data have also demonstrated that PARP inhibitor combinations with novel agents may have improved efficacy in tumors with biomarkers that are not associated with HRD, such as a KRAS mutation for the combination of a MEK inhibitor and a PARP inhibitor [44]. It will continue to be critical for exploratory and translational end points to be incorporated into early-phase clinical trials for single-agent and combination therapies with PARP inhibitors.
The path forward for PARP inhibitor therapy in ovarian cancer is likely to be multifaceted. First, in populations of patients who already benefit from PARP inhibition, we must make PARP inhibitor therapy even more effective. This will likely be through identification of better biomarkers, novel combinations, or new ways to maintain sensitivity to PARP inhibitors. Next, we must find a way to overcome resistance to PARP inhibitors. This is important not only for patients whose tumors seem to be intrinsically resistant to PARP inhibition but also for patients who acquire resistance during treatment with a PARP inhibitor. Last, we must find more effective ways to consistently manage the adverse events that occur with PARP inhibition so as to ensure that once a PARP inhibitor is initiated, patients are able to receive the appropriate dose for the longest period of time. Addressing these issues is likely to guarantee that PARP inhibitors move into the forefront of ovarian cancer treatment.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Network NCC. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer (version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed 29 June 2018.
Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016;8:362):362 ps17. https://doi.org/10.1126/scitranslmed.aaf9246.
Ronson GE, Piberger AL, Higgs MR, Olsen AL, Stewart GS, McHugh PJ, et al. PARP1 and PARP2 stabilise replication forks at base excision repair intermediates through Fbh1-dependent Rad51 regulation. Nat Commun. 2018;9(1):746. https://doi.org/10.1038/s41467-018-03159-2.
Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33(12):1397–406. https://doi.org/10.1200/jco.2014.58.8848.
Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72(21):5588–99. https://doi.org/10.1158/0008-5472.can-12-2753.
Dudas A, Chovanec M. DNA double-strand break repair by homologous recombination. Mutat Res. 2004;566(2):131–67. https://doi.org/10.1016/j.mrrev.2003.07.001.
McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66(16):8109–15. https://doi.org/10.1158/0008-5472.can-06-0140.
Lheureux S, Lai Z, Dougherty BA, Runswick S, Hodgson DR, Timms KM, et al. Long-term responders on olaparib maintenance in high-grade serous ovarian cancer: clinical and molecular characterization. Clin Cancer Res. 2017;23(15):4086–94. https://doi.org/10.1158/1078-0432.ccr-16-2615.
Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer (Oxford, England: 1990). 2016;60:49–58. https://doi.org/10.1016/j.ejca.2016.03.005.
The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. https://doi.org/10.1038/nature10166.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib monotherapy in patients with advanced cancer and a germ line BRCA1/2 mutation. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(3):244–50. https://doi.org/10.1200/jco.2014.56.2728.
Kim G, Ison G, McKee AE, Zhang H, Tang S, Gwise T, et al. FDA Approval summary: olaparib monotherapy in patients with deleterious germ line BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21(19):4257–61. https://doi.org/10.1158/1078-0432.ccr-15-0887.
Kristeleit R, Shapiro GI, Burris HA, Oza AM, LoRusso P, Patel MR, et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germ line BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095–106. https://doi.org/10.1158/1078-0432.ccr-16-2796.
Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87. https://doi.org/10.1016/s1470-2045(16)30559-9.
• Oza AM, Tinker AV, Oaknin A, Shapira-Frommer R, McNeish IA, Swisher EM, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germ line or somatic BRCA1 or BRCA2 mutation: integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol. 2017;147(2):267–75. https://doi.org/10.1016/j.ygyno.2017.08.022 The integrated analysis of two phase II studies of rucaparib in recurrent ovarian cancer patients with BRCA mutations. This provided the basis for the rucaparib FDA approval.
Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–92. https://doi.org/10.1016/s1470-2045(13)70240-7.
Moore KN, Secord AA, Geller MA, Miller DS, Cloven NG, Fleming GF, et al. QUADRA: A phase 2, open-label, single-arm study to evaluate niraparib in patients (pts) with relapsed ovarian cancer (ROC) who have received ≥ 3 prior chemotherapy regimens. J Clin Oncol. 2018;36(15_suppl):5514.
Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germ line BRCA1 or BRCA2 mutation—an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;137(3):386–91. https://doi.org/10.1016/j.ygyno.2015.03.042.
•• Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–64. https://doi.org/10.1056/NEJMoa1611310 The phase III study of niraparib in platinum-sensitive, recurrent ovarian cancer patients. This study served as the basis for the FDA approval.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92. https://doi.org/10.1056/NEJMoa1105535.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–61. https://doi.org/10.1016/s1470-2045(14)70228-1.
Ledermann JA, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17(11):1579–89. https://doi.org/10.1016/s1470-2045(16)30376-x.
Ledermann JA, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Quality of life during olaparib maintenance therapy in platinum-sensitive relapsed serous ovarian cancer. Br J Cancer. 2016;115(11):1313–20. https://doi.org/10.1038/bjc.2016.348.
•• Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–84. https://doi.org/10.1016/s1470-2045(17)30469-2 The phase III study of olaparib in platinum-sensitive, recurrent ovarian cancer patients with BRCA mutations. This study served as the basis for the FDA approval.
•• Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England). 2017;390(10106):1949–61. https://doi.org/10.1016/s0140-6736(17)32440-6 The phase III study of rucaparib in platinum-sensitive, recurrent ovarian cancer patients. This study served as the basis for the FDA approval.
Bell-McGuinn KM, Brady WE, Schilder RJ, Fracasso PM, Moore KN, Walker JL, et al. A phase I study of continuous veliparib in combination with IV carboplatin/paclitaxel or IV/IP paclitaxel/cisplatin and bevacizumab in newly diagnosed patients with previously untreated epithelial ovarian, fallopian tube, or primary peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2015;33(15_suppl):5507.
Nishio S, Takekuma M, Takeuchi S, Kawano K, Tsuda N, Tasaki K, et al. Phase 1 study of veliparib with carboplatin and weekly paclitaxel in Japanese patients with newly diagnosed ovarian cancer. Cancer Sci. 2017;108(11):2213–20. https://doi.org/10.1111/cas.13381.
Moore KN, DiSilvestro P, Lowe ES, Garnett S, Pujade-Lauraine E. SOLO1 and SOLO2: randomized phase III trials of olaparib in patients (pts) with ovarian cancer and a BRCA1/2 mutation (BRCAm). J Clin Oncol. 2014;32(15_suppl):TPS5616-TPS. https://doi.org/10.1200/jco.2014.32.15_suppl.tps5616.
Del Conte G, Sessa C, von Moos R, Vigano L, Digena T, Locatelli A, et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer. 2014;111(4):651–9. https://doi.org/10.1038/bjc.2014.345.
Kummar S, Oza AM, Fleming GF, Sullivan DM, Gandara DR, Naughton MJ, et al. Randomized trial of oral cyclophosphamide and veliparib in high-grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin Cancer Res. 2015;21(7):1574–82. https://doi.org/10.1158/1078-0432.ccr-14-2565.
Wahner Hendrickson AE, Menefee ME, Hartmann LC, Long HJ, Northfelt DW, Reid JM, et al. A phase I clinical trial of the poly(ADP-ribose) polymerase inhibitor veliparib and weekly topotecan in patients with solid tumors. Clin Cancer Res. 2018;24(4):744–52. https://doi.org/10.1158/1078-0432.ccr-17-1590.
• Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16(1):87–97. https://doi.org/10.1016/s1470-2045(14)71135-0 One of the first phase II trials evaluating the combination of a PARP inhibitor with standard of care chemotherapy.
Lee JM, Peer CJ, Yu M, Amable L, Gordon N, Annunziata CM, et al. Sequence-specific pharmacokinetic and pharmacodynamic phase I/Ib study of olaparib tablets and carboplatin in women’s cancer. Clin Cancer Res. 2017;23(6):1397–406. https://doi.org/10.1158/1078-0432.ccr-16-1546.
Matulonis UA, Wulf GM, Barry WT, Birrer M, Westin SN, Farooq S, et al. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann Oncol. 2017;28(3):512–8. https://doi.org/10.1093/annonc/mdw672.
Westin S, Litton J, Williams R, Soliman P, Frumovitz M, Schmeler K, et al. 391P Phase I expansion of olaparib (PARP inhibitor) and AZD5363 (AKT inhibitor) in recurrent ovarian, endometrial and triple negative breast cancer. Ann Oncol. 2017;28(suppl_5):mdx367.025-mdx367.025. https://doi.org/10.1093/annonc/mdx367.025.
Westin SN, Litton JK, Williams RA, Shepherd CJ, Brugger W, Pease EJ, et al. Phase I trial of olaparib (PARP inhibitor) and vistusertib (mTORC1/2 inhibitor) in recurrent endometrial, ovarian and triple negative breast cancer. J Clin Oncol. 2018;36(15_suppl):5504. https://doi.org/10.1200/JCO.2018.36.15_suppl.5504.
• Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. The Lancet Oncology. 2014;15(11):1207–14. https://doi.org/10.1016/s1470-2045(14)70391-2 One of the most promising published clinical trials of the combination of a PARP inhibitor and a novel agent. The combination had more notable improvement in efficacy in BRCA wild type patients.
Liu JF, Barry WT, Birrer MJ, J-m L, Buckanovich RJ, Fleming GF, et al. Overall survival and updated progression-free survival results from a randomized phase 2 trial comparing the combination of olaparib and cediranib against olaparib alone in recurrent platinum-sensitive ovarian cancer. J Clin Oncol. 2017;35(15_suppl):5535. https://doi.org/10.1200/JCO.2017.35.15_suppl.5535.
Konstantinopoulos PA, Waggoner SE, Vidal GA, Mita MM, Fleming GF, Holloway RW, et al. TOPACIO/Keynote-162 (NCT02657889): A phase 1/2 study of niraparib + pembrolizumab in patients (pts) with advanced triple-negative breast cancer or recurrent ovarian cancer (ROC)—Results from ROC cohort. J Clin Oncol. 2018;36(15_suppl):106.
Drew Y, De Jonge M, Hong SH, Park YH, Wolfer A, Brown J et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in germ line BRCA-mutated (gBRCAm) platinum-sensitive relapsed (PSR) ovarian cancer (OC). Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; March 26, 2018; New Orleans, Louisiana2018.
Lee JM, Cimino-Mathews A, Peer CJ, Zimmer A, Lipkowitz S, Annunziata CM, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1–3 inhibitor cediranib in women’s cancers: a dose-escalation, phase I study. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(19):2193–202. https://doi.org/10.1200/jco.2016.72.1340.
Domchek S, Bang YJ, Coukos G, Kobayashi K, Baker N, McMurtry E, et al. MEDIOLA: A phase I/II, open-label trial of olaparib in combination with durvalumab (MEDI4736) in patients (pts) with advanced solid tumours. Annals of Oncology. 2016;27(suppl_6):1103TiP-TiP. https://doi.org/10.1093/annonc/mdw378.56.
Sun C, Fang Y, Yin J, Chen J, Ju Z, Zhang D, et al. Rational combination therapy with PARP and MEK inhibitors capitalizes on therapeutic liabilities in RAS mutant cancers. Sci Transl Med. 2017;9(392). https://doi.org/10.1126/scitranslmed.aal5148.
Kaufmann SH, Hendrickson AEW, Harrell MI, Menefee ME, Tanner EJ, Mutch DG et al. Abstract IS04: PARP inhibitor combinations for the treatment of ovarian cancer. Clin Cancer Res. 2017;23(11_Suppl):IS04.
Wilson AJ, Thompson J, Osman A, Saskowski J, Khabele DK. Abstract 5056: The bromodomain inhibitor JQ1 sensitizes homologous recombination proficient ovarian cancer cells to the PARP inhibitor olaparib. Cancer Res. 2017;77(13_Suppl):5056.
Brill E, Yokoyama T, Nair J, Yu M, Ahn YR, Lee JM. Prexasertib, a cell cycle checkpoint kinases 1 and 2 inhibitor, increases in vitro toxicity of PARP inhibition by preventing Rad51 foci formation in BRCA wild type high-grade serous ovarian cancer. Oncotarget. 2017;8(67):111026–40. https://doi.org/10.18632/oncotarget.22195.
Michels J, Vitale I, Saparbaev M, Castedo M, Kroemer G. Predictive biomarkers for cancer therapy with PARP inhibitors. Oncogene. 2014;33(30):3894–907. https://doi.org/10.1038/onc.2013.352.
Funding Source
This study is supported by NIH T32 Training Grant (5 T32 CA101642 02) NIH SPORE in Ovarian Cancer (NIH 2P50 CA109298), NIH MD Anderson Cancer Center Support Grant (P30CA016672), and Judy Reis/Al Pisani Ovarian Cancer Research Fund, CPRIT RP120214; SNW is supported by the Andrew Sabin Family Fellowship; RLC is supported by the Ann Rife Cox Chair in Gynecology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Katherine Kurnit declares that she has no conflict of interest.
Robert L. Coleman has received research funding through grants from the National Institutes of Health (NIH), the Gateway Fondation, and the V Foundation; has also received research support from AstraZeneca, Merck, Clovis, Genmab, Roche/Genentech, and Janssen; and has received compensation from AstraZeneca, Tesaro, Medivation, Clovis, GamaMabs, Genmab, Roche/Genentech, Janssen, Agenus, Regeneron, and OncoQuest for service as a consultant.
Shannon N. Westin has received research funding through grants from the NIH and the Andrew Sabin Family Fellowship; has also received research support from AstraZeneca, Tesaro, and Cotinga Pharmaceuticals; and has received compensation from AstraZeneca, Merck, Tesaro, Medivation, Clovis, Casdin Capital, Ovation, and Roche/Genentech for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Gynecologic Cancers
Rights and permissions
About this article
Cite this article
Kurnit, K.C., Coleman, R.L. & Westin, S.N. Using PARP Inhibitors in the Treatment of Patients With Ovarian Cancer. Curr. Treat. Options in Oncol. 19, 1 (2018). https://doi.org/10.1007/s11864-018-0572-7
Published:
DOI: https://doi.org/10.1007/s11864-018-0572-7