Opinion Statement
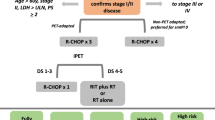
The seminal SWOG trial S8736 trial established the success of a short course of chemotherapy followed by involved field radiation in treating limited stage aggressive NHL lymphoma. Addition of rituximab offered a surprisingly modest improvement in this disease subset. Radioimmunotherapy could hold a slight advantage over rituximab, but that should be investigated in a randomized trial setting. The role of radiation therapy continues to be widely debated, with interpretation complicated by different trial populations, methods of assessing risk, as well as by differences in timing and dose of radiation. Prolonged course of chemotherapy followed by radiation is certainly not justified in all patients with limited stage disease. Three to four cycles of R-CHOP followed closely by IFRT/ISRT, or six cycles of R-CHOP chemoimmunotherapy (based on the MInT trial) are acceptable options. PET/CT scans may further limit radiation to minority of patients who have residual PET-positive masses. PET/CT-directed treatment strategy is being tested in a US intergroup trial. There is evidence that localized DLBCL has a different biology as compared to advanced stage disease. This relates to propensity of limited stage disease to be proportionately more germinal center B-cell like (GCB) and to have late relapses beyond 5 years. Both biology and imaging need to be integrated in the study of limited stage disease without presumption that it should be approached the same as advanced stage disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aggressive non-Hodgkin’s lymphomas (NHL) represent significant disease burden. This review will focus on the most common NHL, diffuse large B-cell Lymphoma (DLBCL), which accounts for approximately 28 % of all lymphoid neoplasms diagnosed in the USA [1]. Approximately, 25–30 % of these patients present with limited (also called localized or early) stage disease, commonly defined as stage I or non-bulky stage II disease. Typically, these have tumor size less than 10 cm in the greatest diameter, no B symptoms, and sites that can be encompassed in one radiation field. In the pre-rituximab era, the treatment of limited stage DLBCL was based on Southwest Oncology Group (SWOG) 8736 results [2]. In the light of long-term follow-up data from SWOG S8736, along with the advent of more effective systemic therapy, the role of radiation is being questioned. There is a paucity of randomized controlled trials in this population, with few dedicated trials to limited stage disease in the era of rituximab and PET/CT scan-based staging which have long enough follow-up to capture outcomes in this disease subset.
Treatment
A historical perspective
For more than 2 decades, the most commonly used treatment in this population was based on the landmark SWOG S8736 trial [2]. This trial enrolled patients with aggressive NHL histologies and demonstrated that over the first 5 years of follow-up, three cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy followed closely by involved field radiation therapy (CHOP3 + IFRT) was superior to eight cycles of CHOP alone (CHOP8) for patients with stage I, IE, and non-bulky stages II, and IIE disease. However, with additional follow-up PFS curves overlapped at 7 years and OS curves at 9 years [3]. Long-term analysis with a median 17-year follow-up showed that 10- and 15-year PFS estimates in the CHOP3 + IFRT group (54 and 40 %) were no different from the CHOP8 group (55 and 41 %; p = 0.91), with continued relapses observed beyond 5 years in both arms. Similarly, 10- and 15-year OS estimates in the CHOP3 + IFRT group (63 and 46 %) were comparable to the CHOP8 group (61 and 46 %; p = 0.66) [4••]. S8736 also established a stage-modified (Miller) international prognostic index (sm-IPI) that was prognostic and included age greater than 60 years, elevated serum LDH, stage II disease, and WHO performance status. Subsequent SWOG studies, except for the most recent study S1001, only enrolled patients with at least one risk factor, as patients with sm-IPI of 0 have excellent outcomes. Patients with bulky stage II disease have outcomes similar to advanced stage disease and should be treated as such.
CHOP with or without radiation: radiation considerations
Eastern Cooperative Oncology Group (ECOG) study 1484 tested whether radiation therapy was effective as consolidation after a full 8-cycle course of response-adapted radiotherapy [5]. Radiotherapy was given at 40 Gy to all patients in PR and was randomized at 30 Gy in patients with CR. It showed borderline better disease-free survival in the radiation arm, but similar OS (Table 1). It is difficult to compare this study to others due to response-adapted design, inclusion of bulky stage II disease, and lack of LDH which made sm-IPI assessment impossible. Furthermore, the role of radiation therapy after a full course of chemotherapy is controversial on its own, particularly for low-risk limited stage disease in complete remission.
Groupe d’Etude des Lymphomes de l’Adulte (GELA, now part of LYSA) trial LNH 93-1 assessed chemotherapy intensification in the low-risk group with patients younger than 61 years of age [7]. They randomized 329 patients to receive CHOP3 + IFRT (IFRT started a month after last CHOP at a dose of 40 Gy), and 318 patients to aggressive dose-intense chemotherapy [doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (ACVBP)] given at 2-week intervals followed by sequential consolidation with methotrexate, etoposide, ifosfamide, and cytarabine. The patients had low-risk disease and no age adjusted international prognostic index (IPI) risk factors. ACVBP based regimen achieved higher response rates, but was associated with greater grade 3–4 toxicity, and was more complex. Due to these factors and lack of vindesine availability, the regimen has not gained traction in the USA, particularly in the presence of perceived success of and experience with R-CHOP.
In an often-quoted trial to justify omission of radiation therapy, GELA trial LNH 93-4 [8] randomized patients older than 60 years (median age was 68 years) to four cycles of CHOP alone (277 patients) or followed by 40 Gy of IFRT (299 patients). Ninety-five percent had a 0 IPI risk score and 65 % stage I disease, so that 65 % had one stage-modified IPI risk factor (older age) and 35 % had two stage-modified IPI risk factors (older age and stage II disease). Five-year EFS was 61 % in the CHOP × 4 arm and 64 % in CHOP × 4 + IFRT arm (P = 0.6), whereas 5-year OS was 72 and 68 %, respectively. However, IFRT was administered at a median of 35 days after last chemotherapy cycle, as opposed to 24 days in SWOG studies. Additionally, 12 % of patients did not receive radiation therapy. In the light of this, it is interesting to see that the in-field-only relapse rate on this study was 34 % (28 % in LNH 93-1), as opposed to razor-sharp 17–18 % rate seen in SWOG, ECOG, and BCCA studies. This could have accounted for lack of difference in outcomes between the radiation and observation arms. Therefore, it seems important that if treatment relies on short course of chemotherapy and radiation, the radiation needs to start within 3–4 weeks of chemotherapy and be given as prescribed.
There is significant fear of radiation therapy due to increased rate of secondary malignancies that persist as far as 30 years after treatment completion. However, a lot of those studies were performed on younger patients with Hodgkin lymphoma, with larger radiation fields and higher doses than in the standard practice now. The risks of radiation therapy in a population with median age of 65 may be much different than that in a population with median age of 30, due to greater sensitivity of younger tissue to radiation damage and competing risks of death in older patients. In a long-term follow-up of S8736, estimates of 5- and 10-year cumulative incidence of second cancer were 6 and 12 % in CHOP3 + IFRT arm, versus 9 and 11 % in CHOP8 arm, which was not significant (p = 0.44) [4]. Regardless of the perception of radiation risk, further studies are using PET/CT scans to direct radiation therapy to those that truly require it (see below).
Rituximab-based treatment
The rituximab era in limited stage DLBCL began with SWOG S0014, a phase II trial of four doses of rituximab plus CHOP × 3 + IFRT in high risk limited stage disease [9]. Sixty eligible patients were treated, with median age of 69, bulky disease in 5 %, and stage I disease in 57 %. Stage-modified IPI was 1 in 70 %, 2 in 20 %, and 3 in 10 % of patients. Radiation at 40–46 Gy was delivered as planned in 91 % of the patients, with median of 24 days after last R-CHOP. With a median follow-up of 5.3 years, 2-year outcomes were 93 % for PFS and 95 % for OS, dropping to 88 and 92 %, respectively, at 4 years. Only one of six relapses occurred in the radiation field, and seven patients developed solid tumors, but none in the radiation field. These results compared favorably to a matched cohort from SWOG S8736, which showed 4-year PFS of 78 % and OS of 88 % [10]. This trial now has a median follow-up of 12 years. There is a similar pattern of continued relapse as the SWOG 8736 with estimated 10-year PFS of 60.8 % (95 % CI: 47.1, 72.0 %) and 10-year OS of 69.3 % (95 % CI: 55.8, 79.5 %) [4].
In the Mabthera International Trial (MInT), 824 patients with age 18–60 with bulky stage I or stage II–IV disease and 0–1 age adjusted IPI risk factors were randomized to six cycles of CHOP-like chemotherapy with or without rituximab [11]. Patients with bulky disease received radiation therapy, but the definition of bulky disease varied between 5, 7.5, and 10 cm. Based on varying definitions of bulk 40 % of patients received 30–40 Gy of IFRT, which made impact of radiation therapy difficult to analyze. Addition of rituximab improved complete remission, PFS, EFS, and OS, as expected. However, the impact of rituximab on limited stage disease could not be discerned since the trial did not analyze them separately, but rather looked at IPI and tumor bulk. A favorable cohort of 101 patients was defined, which received rituximab, had non-bulky disease and IPI of 0, with 6-year EFS of 84.3 %, PFS of 89.6 %, and OS of 94.9 %. In the initial report, bulky disease negatively affected all outcomes measured (EFS, PFS, and OS), regardless of rituximab assignment and having received radiation therapy implying that radiation therapy after full dose of rituximab-containing chemotherapy did not eliminate unfavorable risk of bulky disease. However, a 6-year follow-up on the study recently published compared the MInT population with one IPI risk factors with the respective population in the GELA LNH03-2B trial [12, 13]. The 3-year event-free, progression-free, and overall survival obtained with six cycles of any R-CHOP-like chemotherapy (or with six cycles of R-CHOP-21 alone) in the MInT trial was substantially better than those obtained with eight cycles of R-CHOP-21 in the GELA LNH03-2B trial. Indeed, the results obtained with six cycles of R-CHOP-21 or six cycles of any CHOP-like chemotherapy plus rituximab used in the MInT trial appeared to be comparable to the results in the French trial obtained with the intensive R-ACVBP regimen, which is substantially more toxic than the R-CHOP-21 regimen. The authors in the MInT study attributed this to the use of radiation therapy. This conclusion was in contradiction to an earlier analysis of bulky disease as mentioned before.
Recently, LYSA/GOELAMS group presented an abstract with preliminary results of randomized phase III trial comparing R-CHOP with or without Radiotherapy (RT) in non-bulky limited stage DLBCL [14••]. Three hundred one patients with a median age of 56 years, majority with normal LDH (82 %), performance status of 0 (80 %), and no B symptoms (96 % of cases) were evaluated. Patients achieving CR by PET/CT scan after 4 cycles of R-CHOP, and who had IPI of at least 0, were randomized to observation or 40 Gy of RT; those who had CR but IPI of at least 1 received 2 additional cycles of R-CHOP before same randomization; those who achieved PR by PET/CT scan all received 2 additional cycles of R-CHOP and 40 Gy of RT. One hundred fifty patients were randomized to the R-CHOP arm and 151 to the R-CHOP + RT arm. After 4 cycles, 253 patients (84 %) were in CR and 43 in PR (14 %). Thirty-four patients (79 %) out of the 43 partial responders received 2 additional cycles of R-CHOP followed by RT (including 12 patients not initially allocated to RT arm). At the end of treatment, CR and PR rate were 94 and 3 %, respectively. EFS and OS were not statistically different between the two arms. In an intent to treat analysis, 5-year EFS was reported as 87 % in the R-CHOP arm versus 91 % in the R-CHOP + RT arm (p = 0.13), and 5-year OS was 90 % in the R-CHOP arm versus 95 % in the R-CHOP + RT arm (p = 0.32). For patients in complete response after the 4 cycles of R-CHOP (84 % of the patients), 5-year EFS was 89 % in the R-CHOP arm versus 91 % in the R-CHOP + RT arm (p = 0.24). The RT arm did not have any in-field relapses, while little less than half of patients in chemotherapy arm had in-field relapses, suggesting that RT was quite effective in controlling local recurrence. Overall, this study enrolled an exceptionally favorable cohort of patients, with majority having sm-IPI of 0, which is reflected in excellent outcomes. Many patients also received R-CHOP6, which would be considered adequate for advanced stage disease, without the use of additional RT (by extrapolation from the MInT study). The role of additional 2 cycles of R-CHOP and RT for patients achieving PR cannot be assessed separately since it was administered in a non-randomized fashion. Also, a median follow-up of 51 months may not be adequate to capture late relapses that occur in limited stage disease.
In 2013, the non- radiotherapy arms of the German UNFOLDER study of younger patients with low risk DBLCL of all stages were closed early due to excessive local relapses. Similarly, several retrospective series also looked at outcomes with radiation therapy in the rituximab era, none of which favored longer chemotherapy course without radiation.
A retrospective analysis from the M. D. Anderson Cancer Center (MDACC) on the benefit of consolidative RT in patients with DLBCL treated with chemotherapy showed 103 (54 %) of 190 stage I and II patients who received IFRT after at least 6 cycles of R-CHOP or other chemotherapy, 49 of which patients had bulky disease that, patients who received RT had a significantly better outcome [15]. The 5-year OS and PFS rates for stage I and II disease treated with RT were 92 and 82 %, respectively, whereas the OS and PFS rates for those not treated with RT were 73 % (P = .0007) and 68 % (P = .0003), respectively. It is important to note that this is a retrospective single center experience which included patients of all stages, the definition of bulky disease was not conventional, and some patients got other more intense regimens like hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and examethasone (Hyper-CVAD).
The Osaka Lymphoma Study Group did a retrospective analysis of 137 patients with stage I of stage II non bulky (<10 cm) [16]. Out of 137 patients, 83 had 6 to 8 cycles of R-CHOP like immunochemotherapy (Chemo group), and 28 had 3 to 4 cycles of R-CHOP like immunochemotherapy followed by radiotherapy (Chemo + RT group). The 3-year OS were 85.5 % in Chemo group and 96.2 % in Chemo + RT group, respectively (P = 0.225). The 3-year PFS were 74.3 % in Chemo group and 89.7 % in Chemo + RT group, respectively (P = 0.185).
Vargo et al. recently published a retrospective analysis of the National Cancer Data Base of more than 59,000 patients with stage I and II DLBCL who were treated with either chemotherapy or chemotherapy and radiation [17•]. Fifty-four percent of patients were stage I, 42 % had extra-nodal disease, and 58 % were older than 60 years. There was a decline in the use of consolidative RT in patients between the peak in year 2000 to nadir in 2012, yet patients receiving RT fared better. The 5-year survival was 75 and 82 % and the 10-year survival was 55 % and 64 % (P < 0.001) in chemotherapy and chemotherapy and RT group. Several analyses of SEER database came to similar conclusions.
Kumar et al. reported a retrospective analysis of 261 patients with newly diagnosed, limited stage DLBCL from Memorial Sloan Kettering Cancer Center (MSKCC) and compared outcomes associated with different treatment programs including 3–4 cycles of R-CHOP ± IFRT and 6 cycles of R-CHOP ± IFRT [18•]. The median age was 58 years, 30 % of patients were stage I, 37 % stage IE, 18 % stage II, and 18 5 stage IIE. The outcomes of all four groups were similar (Table 1). Again, local relapses occurred only in patients who did not receive IFRT.
Radioimmunotherapy consolidation
Radioimmunotherapy is an approach of delivering short path length radiation using CD20 antibody as carrier. There are currently two compounds approved for low grade non-Hodgkin lymphoma, ibritumomab tiuxetan (Zevalin), and tositumomab (Bexxar). However, tositumomab is no longer available in the USA.
SWOG S0313 was a phase II trial that tested the efficacy of ibritumomab tiuxetan in limited stage DLBCL, by administering it after CHOP3 + IFRT [19•]. Forty-six patients were eligible and all finished treatment, with 42/46 patients completing all therapy as planned. Thirty-five percent of patients had >1 adverse risk factor, with 57 % being at least 60 years of age, 52 % with stage II disease, 37 % with elevated LDH, and only 4 % with World Health Organization performance status of 2 or higher. Twenty percent of patients had systemic symptoms. With a median follow-up of 7.3 years, the 2-year PFS estimate was 89 % (95 % confidence interval [CI], 81–97 %), the 5-year PFS was 82 % (95 % CI, 71–94 %), and the 7-year PFS estimate was 75 %. The 2-year OS estimate was 91 % (95 % CI, 83–99 %), 5-year OS was 87 % (95 % CI, 77–97 %), and the 7-year OS estimate was 82 %. When compared to similarly matched patients from prior trials (i.e, SWOG S8736 and S0014), PFS at 5 years was 72 % (95 % CI, 61–83 %) on S8736, 78 % (95 % CI, 68–89 %) on S0014, and 82 % (95 % CI, 71–94 %) on S0313. For OS, the 5-year estimate was 79 % (95 % CI, 70–89 %) on S8736, 83 % (95 % CI, 74–93 %) on S0014, and 87 % (95 % CI, 77–97 %) on S0313. Although this trial showed slightly more favorable 7-year PFS as compared to the previous SWOG trial, it was not a randomized comparison and thus it may be possible that other patient factors influenced the outcome. Late relapses may also still occur. It is also possible that radioimmunotherapy may be more effective in eliminating CD20 positive lymphocytes.
Another cooperative group study testing radioimmunotherapy was ECOG E3402, which enrolled patients with either stage I/IE disease with one IPI risk factors to get R-CHOP followed by ibritumomab tiuxetan if in CR [20•]. Patients were restaged with a CT scan after R-CHOP2. Patients in CR received 2 additional cycles; those in PR or CRu received 4 additional cycles. At end of R-CHOP, a PET scan was performed and centrally reviewed; those with a PR or functional CR proceeded to ibritumomab tiuxetan within 12 weeks of the last RCHOP. Patients were restaged 12-weeks post-ibritumomab; PET-negative patients were observed without maintenance; PET-positive patients received 30 Gy IFRT and then were restaged. Out of 53 patients considered eligible, 42 % (22/53) patients were stage I/IE; 58 % (31/53) were stage II/IIE. Fifty-seven percent (30/53) received 6 cycles of R-CHOP; 40 % (21/53) received 4 cycles; and 2 patients received 1 and 2 cycles, respectively. After R-CHOP, 79 % (42/53) were in functional CR and 19 % (10/53) PR; 1 was unevaluable. Of the 53 eligible patients who completed R-CHOP therapy 91 % (48/53) proceeded to ibritumomab tiuxetan. After ibritumomab, 87 % (46/53; 95 % CI: 75–95) were in CR/CRu and 89 % (47/53; 95 % CI:77–96 %) were in functional CR. Only one patient proceeded to IFRT. Five-year PFS was 78 % (95 % CI 66–92 %) and 5-year OS was 94 % (95 % CI, 88–100 %). Among 52 responders, 84 % (95 % CI, 73–96 %) remained in remission at 5 years. The median for PFS and OS was not reached. This study’s results are similar to those from S0313, where PFS was slightly higher and OS slightly lower at 5 years. This trial almost entirely avoided radiation therapy. But it has to be noted that 34 % of the patients had sm-IPI of 0 and would not have been eligible for S0313 due to excellent prognosis, and 57 % ended up receiving R-CHOP6, which would be considered full dose therapy for advanced stage disease.
PET/CT scan-directed therapy
PET/CT scans are important tools in staging lymphoma, and preponderance of studies have shown that they are prognostic at interim restaging in DLBCL. As mentioned above LYSA/GOELAMS 02-03 trial and ECOG E3402 have used PET/CT scans to direct additional therapy after initial R-CHOP treatment, but in a graduated fashion which also directed treatment based on definitions of CR and PR, and in the former case used IPI as well. British Columbia Cancer Agency treatment paradigm for limited stage DLBCL incorporated PET/CT response after 3 cycles of R-CHOP to direct further therapy, without additional conditions [21]. Patients with a negative PET/CT scan received one additional cycle of R-CHOP for a total of 4 cycles, whereas patients with a positive PET scan result completed therapy with IFRT. Results were analyzed after the initial 134 patients were treated. Median age of the patients was 64 years; 43 % had stage II disease and most patients (80 %) had at least one negative risk factor according to the stage-modified IPI. After 3 cycles of R-CHOP, 103 (77 %) of the 134 patients had a negative PET scan result, 30 (22 %) of the 134 patients had a positive PET scan result, and one patient had an indeterminate PET scan. With a median follow-up of 30 months, the 3-year estimated time to progression for the patients with a negative PET scan result was 92 % compared with 60 % for the patients with a positive PET scan result. The 3-year OS was 96 and 83 % for the patients with a negative PET scan result and those with a positive PET scan result, respectively. So far, there have been no excess relapses in PET/CT negative patients, but longer follow-up is needed.
Current intergroup study, S1001 melds the British Columbia Cancer Agency approach with SWOG S0313. Patients with all limited stage DLBCL regardless of sm-IPI are restaged after RCHOP3, and those with negative interim PET/CT receive one additional cycle of RCHOP, while patients with positive interim PET/CT receive 36 Gy of IFRT followed by ibritumomab tiuxetan. The study recently completed accrual with 159 patients enrolled, but will require additional follow-up since its primary endpoint is 5-year PFS, in order to better capture later relapse.
Biologic factors
As shown by many clinical trials mentioned above, limited stage DLBCL has propensity towards continued late relapses, as opposed to advanced stage disease, where there is significant leveling off at 2 years and a survival plateau at 5 years. Similarly, studies of patients relapsing beyond 5 years showed that 63 % were early stage [22]. It is also known that limited stage disease seems to be more of germinal center B-cell (GCB) subtype than advanced stage disease [18, 23]. The question of prevalence of MYC and BCL gene translocation (“double hit”) or protein overexpression (“double protein” lymphoma) in limited stage disease has not been answered yet, but will be studied in prospective fashion. Of note, the only patient in S0313 to relapse before proceeding with radiation had double hit lymphoma.
Conclusion
Current treatment paradigms in limited stage DLBCL allow either R-CHOP for 3–4 cycles closely followed by 30–40 Gy of radiation therapy, or R-CHOP × 6 cycles with omission of radiation by majority of lymphoma specialists, particularly in the North America. Success of PET/CT-guided strategies is still uncertain, but PET/CT scans are clearly an important diagnostic tool that will continue to be used. There is a continuous rate of relapse in limited stage DLBCL that belies its generally favorable outcome. Thus, studies should be dedicated to limited stage DLBCL that take into account its biology.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–76.
Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339:21–6.
Miller TP, Leblanc M, Spier CM, et al. CHOP alone compared to CHOP plus radiotherapy for early aggressive non-Hodgkin’s lymphoma: update of the Southwest Oncology Group (SWOG) randomized trial. Blood. 2001;98:724a.
Stephens DM, Li H, Leblanc M, et al. Continued risk of relapse independent of treatment modality in limited stage diffuse large B-cell lymphoma: final and long-term analysis of SWOG Study S8736. J Clin Oncol. 2016. doi:10.1200/JCO.2015.65.4582. Final results of Landmark study SWOG 8736, with comparison to rituximab containing S0014, which show continued late relapses in both arms of the study, as well as illustrate similar rates of secondary malignancies.
Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22:3032–8.
Ballonoff A, Rusthoven KE, Schwer A, McCammon R, Kavanagh B, Bassetti M, Newman F, Rabinovitch R: Outcomes and effect of radiotherapy in patients with stage I or II diffuse large B-cell lymphoma: a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys. 2008:72:1465–1471.
Reyes F, Lepage E, Ganem G, et al. ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med. 2005;352:1197–205.
Bonnet C, Fillet G, Mounier N, et al. CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2007;25:787–92.
Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26:2258–63.
Persky DO, Miller TP. Localized large cell lymphoma: is there any need for radiation therapy? Curr Opin Oncol. 2009;21(5):401–6.
Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91.
Recher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378:1858–67.
Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–22.
Lamy T, Damaj G, Gyan E, et al. R-CHOP with or without radiotherapy in non-bulky limited-stage diffuse large B cell lymphoma (DLBCL): preliminary results of the prospective randomized phase III 02-03 trial from the LYSA/GOELAMS. Blood. 2014;124: Abstract 393. First randomized phase III trial in rituximab era dedicated to limited stage DLBCL, which showed excellent outcomes in both RT and observation arms questioning the need for radiation.
Phan J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28:4170–6.
Terada Y, Take H, Shibayama H, et al. Short cycle of immunochemotherapy followed by radiation therapy compared with prolonged cycles of immunochemotherapy for localized DLBCL: the Osaka Lymphoma Study Group (OLSG) retrospective analysis. Blood. 2012;120:Abstract 1628.
Vargo JA, Gill BS, Balasubramani GK, Beriwal S. Treatment selection and survival outcomes in early-stage diffuse large B-cell lymphoma: do we still need consolidative radiotherapy? J Clin Oncol. 2015;33(32):3710–7. Database study suggesting that the outcomes for radiation containing treatment are better, even though the use of radiation is declining
Kumar A, Lunning MA, Zhang Z, et al. Excellent outcomes and lack of prognostic impact of cell of origin for localized diffuse large B-cell lymphoma in the rituximab era. Br J Haematol. 2015;171(5):776–83. Single center study showing excellent outcomes in limited stage DLBCL regardless of treatment strategy and cell of origin designation as based on immunohistochemistry, with majority of patients being of germinal center B-cell origin
Persky DO, Miller TP, Unger JM, et al. Ibritumomab consolidation after 3 cycles of CHOP plus radiotherapy in high-risk limited-stage aggressive B-cell lymphoma: SWOG S0313. Blood. 2015;125:236–41. Long-term results of using radioimmunotherapy instead of “naked” anti-CD20 antibody showing similar, if not slightly better PFS at 7 years
Witzig TE, Hong F, Micallef IN, et al. A phase II trial of RCHOP followed by radioimmunotherapy for early stage (stages I/II) diffuse large B-cell non-Hodgkin lymphoma: ECOG3402. Br J Haematol. 2015;170:679–86. Phase II study showing excellent outcomes with RCHOP followed by radioimmunotherapy, almost completely omitting radiation
Sehn LH, Savage KJ, Hoskins P, et al. Treatment of limited stage DLBCL can be effectively tailored using a PET-based approach. Ann Oncol. 2011;22:Abstract 028.
Larouche JF, Berger F, Chassagne-Clement C, et al. Lymphoma recurrence 5 years or later following diffuse large B-cell lymphoma: clinical characteristics and outcome. J Clin Oncol. 2010;28:2094–100.
Roberts RA, Rimsza LM, Staudt L, et al. Gene expression differences between low and high stage diffuse large B cell lymphoma (DLBCL). Blood. 2006;108(11):Abstract 809.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Daniel O. Persky has received research funding through a grant from Spectrum Pharmaceuticals, Inc.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Lymphoma
Rights and permissions
About this article
Cite this article
Kumar, A., Sundararajan, S., Puvvada, S. et al. Limited Stage Aggressive Non-Hodgkin Lymphoma: What Is Optimal Therapy?. Curr. Treat. Options in Oncol. 17, 45 (2016). https://doi.org/10.1007/s11864-016-0424-2
Published:
DOI: https://doi.org/10.1007/s11864-016-0424-2