Abstract
Defining the appropriate sequencing of therapies for metastatic renal cell carcinoma (mRCC) has become increasingly complex in recent years given the approval of multiple targeted therapies. These targeted therapies fall into 2 broad mechanistic categories: (1) inhibitors of the mammalian target of rapamycin (mTOR), and (2) vascular endothelial growth factor (VEGF)-directed agents. In the current manuscript, data from relevant trials are reviewed to provide a context in which to use these agents across the first- and second-line setting. Strategies to incorporate promising agents currently in late stage development for mRCC are also described.
Opinion statement
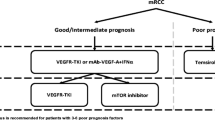
Currently, there is no consensus as to the optimal sequence of therapies for patients with metastatic renal cell carcinoma (mRCC). While interleukin-2 (IL-2) and temsirolimus are potential considerations for selected patients in the first-line setting, the majority of patients in this setting are likely candidates for vascular endothelial growth factor (VEGF)-directed therapies. Specifically, these therapies include sunitinib, pazopanib, and bevacizumab/interferon-α. Using the comparative data discussed herein, the relative merits of each should be discussed. In the second-line setting (following VEGF-directed therapy), axitinib, and everolimus are supported by phase III data. There is no data directly comparing the 2 agents—however, studies reviewed in the current manuscript (comparing VEGF- and mammalian target of rapamycin [mTOR]-directed approaches in the second-line setting) can potentially be used to inform clinical decision making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastatic renal cell carcinoma (mRCC) was previously characterized by a barren treatment landscape. Immunotherapeutic agents such as interleukin-2 (IL-2) and interferon-α (IFN-α) represented the standard approach, but yielded clinical benefit in only a small subset of patients [1, 2]. Over the past decade, these agents have been largely supplanted by 2 classes of targeted therapy, agents that inhibit either (1) vascular endothelial growth factor (VEGF)-mediated signaling, or (2) the mammalian target of rapamycin (mTOR) [3] (See Tables 1 and 2). Within these categories, a total of 7 agents have been approved. Given the significant mechanistic overlap of these agents, a challenge that has been recently encountered is defining the optimal sequence of agents.
A practical approach is to utilize targeted agents in a manner akin to how they were evaluated in studies leading to regulatory approval. For example, sunitinib was compared with IFN-α in a pivotal phase III trial conducted in treatment-naïve patients [4•]. Thus, current guidelines support use of sunitinib for patients with mRCC that have not received prior therapy [5•]. However, other agents can be utilized in the same disease space. The pivotal trials evaluating pazopanib and bevacizumab with IFN-α have also included treatment-naïve patients and have similarly shown significant improvements in clinical outcome [6, 7•, 8•]. To date, no reports have suggested superiority of one agent over the other, making it challenging for the clinician to choose amongst them.
A similar dilemma exists in the second-line setting. Everolimus, an mTOR inhibitor, was compared with placebo in the phase III RECORD-1 study in patients that had received prior sunitinib and/or sorafenib [9•]. Based on this data, everolimus was incorporated into guidelines for management of patients after failure of initial VEGF-directed therapy. Soon after this dataset emerged, a separate trial evaluating axitinib (a second generation VEGF-tyrosine kinase inhibitor [VEGF-TKI]) was reported, also providing data in patients with prior VEGF-directed therapy exposure [10•]. As no comparative data exists for axitinib and everolimus, there is equipoise regarding the optimal second-line therapy.
Multiple other review manuscripts have provided a critical evaluation of pivotal trials that have led to regulatory approval of targeted therapies for mRCC. In the current manuscript, we will instead critically evaluate those studies that provide insight into the most effective sequencing of targeted therapies for mRCC.
Comparative data in the first-line setting
Multiple options can be offered to the treatment-naïve patient with mRCC, but the vast majority of patients will receive VEGF-directed agents upfront. Sunitinib, pazopanib, and bevacizumab/IFN-α all carry category 1 (ie, unanimous) recommendations from the National Comprehensive Cancer Network (NCCN) for use in the first-line setting [5•]. The phase III COMPARZ trial allows for a direct comparison of sunitinib and pazopanib [11••]. The study randomized 1110 patients with clear cell mRCC to receive either sunitinib or pazopanib at conventional doses. The study was designed to demonstrate non-inferiority of pazopanib compared with sunitinib with respect to progression-free survival (PFS). The demographic composition of this trial was similar to other pivotal studies in mRCC—median age was 61, and the majority of patients were male (73 %) and had prior nephrectomy (83 %).
Ultimately, the study met this endpoint, demonstrating that the PFS associated with pazopanib was noninferior to that of sunitinib (HR 1.05; 95 % CI 0.90–1.22) [11••]. A recent update suggests that survival was also balanced with pazopanib as compared with sunitinib (HR 0.92, 95 % CI 0.79–1.06; P = 0.24) [12••]. Importantly, COMPARZ establishes a new benchmark in OS for patients with mRCC, with the highest median survival reported in a phase III clinical trial in RCC to date (29.1 months with sunitinib vs 28.3 months with pazopanib). Other efficacy endpoints were balanced in the study; for instance, response rate was 33 % with pazopanib compared with 29 % with sunitinib (P = 0.12).
Several differences were noted with respect to toxicity. Hand-foot syndrome, stomatitis, dyspepsia, fatigue, and hematologic abnormalities (leucopenia, thrombocytopenia, and anemia) were more prominent with sunitinib, while weight loss, alopecia, and liver function test abnormalities were more prominent with pazopanib. As the perception of each of these toxicities vary from patient to patient, it is challenging to perceive a clear victor between the 2 agents on the basis of this criterion alone. Several quality of life (QOL) studies were also reported in COMPARZ. Out of a total of 14 standard metrics, 11 favored pazopanib. With efficacy endpoints being more or less balanced, these QOL data may seem to inherently favor use of pazopanib. This interpretation is somewhat controversial, as the QOL measures were made at day 28 of therapy with sunitinib and pazopanib during the first 9 cycles of therapy (and at day 42 for subsequent cycles). With the conventional schedule for sunitinib (28 days on and 14 days off), toxicity tends to peak at day 28 and recover thereafter. In contrast, the dosing of pazopanib (and the resultant toxicity profile) tends to remain consistent through the duration of the 42 day cycle.
Ultimately, COMPARZ leaves the practicing oncologist with no definitive guidance regarding optimal first-line therapy. However, these data can be used to inform conversations with patients regarding the relative merits (ie, differing toxicity) with sunitinib and pazopanib.
Comparative data in the second-line setting
As noted, the phase III AXIS and RECORD-1 trials establish the role of axitinib and everolimus, respectively, in patients that have received prior VEGF-directed therapy. However, to date, there is no randomized data that pits the 2 agents head-to-head. The phase III INTORSECT trial was intended to potentially address the role of whether mTOR inhibition or VEGF inhibition might represent a superior approach in the second-line setting [13••]. In this study, 512 patients with mRCC that had only received prior therapy with sunitinib were randomized to receive temsirolimus or sorafenib at standard doses. The study was powered to detect an improvement in PFS with temsirolimus, projecting a PFS of 5.3 months with temsirolimus and 4.0 months with sorafenib.
Ultimately, no significant differences in PFS were observed (4.3 months for temsirolimus vs 3.9 months for sorafenib; P = 0.19). No significant difference in PFS was observed based on pre-specified stratification factors, including duration of prior sunitinib therapy (≤ 180 days or >180 days), Memorial Sloan-Kettering Cancer Center (MSKCC) risk group, tumor histology (clear cell or non-clear cell), and presence or absence of prior nephrectomy. However, an intriguing finding was made with respect to OS, a secondary endpoint in the study. Specifically, OS with sorafenib was 16.6 months compared with 12.3 months with temsirolimus (HR 1.31, 95 % CI 1.05–1.63; P = 0.01). In many presentations of this data, attention has been drawn to a subset analysis of OS based on duration of prior therapy with sunitinib. In the subset of patients with ≤ 180 days of sunitinib therapy, the HR for OS was 1.30 (95 % CI 0.94–1.81) favoring sorafenib. The HR for OS in patients with > 180 days of prior sunitinib was 1.37 (95 % CI 1.04–1.80) favoring sorafenib. Although the latter 95%CI crosses the threshold of 1.0, it is unclear that the absolute differences in HR (ie, 1.30 vs 1.37) are meaningful.
As one might anticipate, toxicities varied greatly between the 2 treatment arms. Patients receiving sorafenib had a higher incidence of diarrhea, hand-foot syndrome, and hypertension, while patients receiving temsirolimus had a higher incidence of stomatitis, cough, dyspnea, hyperglycemia, and hypercholesterolemia. Notably, the cumulative grades of grade 3/4 toxicity were similar across study arms.
There are several challenges in applying data from INTORSECT to second-line decision making. First, the trial does not examine what are perhaps the most relevant agents in this setting—axitinib has been shown to be superior to sorafenib in the AXIS trial, and RECORD-1 establishes everolimus (not temsirolimus) as a second-line option. Second, while much as been made of the OS advantage with sorafenib in INTORSECT, it is unclear whether the protocol-based therapies truly drove this survival advantage, or whether it may have been an effect of subsequent therapies. Market research suggests that a large proportion of patients who receive second-line treatment (nearly half) will go on to receive third-line therapy [14]. Unfortunately, use of poststudy treatments was poorly captured in INTORSECT, with only 6 % of patients reporting treatment within 1 month of protocol discontinuation.
Sequencing trials
Several studies have taken the approach of comparing a sequence of therapies across 2 lines. In the phase III SWITCH trial, a total of 365 patients in Germany, Austria, and the Netherlands with no prior therapy for mRCC were randomized in a 1:1 fashion to receive either sorafenib or sunitinib at conventional doses [15]. At the time of progression or intolerable toxicity, patients received the opposing therapy ie, patients receiving sunitinib first-line were crossed over to sorafenib, and patients receiving sorafenib first-line were crossed-over to sunitinib. The primary endpoint of this trial was total PFS across first- and second-line therapy, with an aim of determining a superior PFS with the sorafenib to sunitinib sequence. Although the study did permit non-clear cell histology, the vast majority of patients were clear cell (87 %), and most had received prior nephrectomy (92 %).
A major challenge in interpreting the primary endpoint was the attrition seen between first- and second-line treatment [15]. While 182 and 183 patients were randomized to receive sorafenib and sunitinib, respectively, only 103 and 76 patients ultimately received second-line treatment with the opposing agent. Total PFS in the first line was not significantly different—12.5 months with sorafenib to sunitinib vs 14.9 months with sunitinib to sorafenib (P = 0.54). Similarly, there was no difference in OS. Although there was no significant difference in first-line PFS between the 2 arms, PFS was improved with second-line sunitinib compared with sorafenib (5.4 months vs 2.8 months; P < 0.001). Side effect profiles of the agents were consistent with previously reported experiences.
The intent of the SWITCH trial was to demonstrate whether the strategy of migrating from a less specific multi-kinase inhibitor to one with narrower specificity might yield improved outcomes. Ultimately, this strategy did not prove to be viable. Little can be made of the findings of superior PFS with sunitinib in the second-line setting—there was no randomization at this stage of the study, and there was substantial attrition subsequent to first-line therapy.
In contrast to SWITCH, the RECORD-3 trial examined the sequence of mTOR inhibitor and VEGF-TKI, and vice versa. In RECORD-3, a total of 471 patients with treatment-naïve mRCC were randomized to receive either everolimus or sunitinib in the front-line setting, followed by the opposing therapy in the second-line. The primary objective of the trial was to demonstrate noninferiority with first-line everolimus compared with sunitinib. Of 238 patients and 231 patients who initiated therapy with everolimus and sunitinib, 108 and 99 patients received protocol-specified second-line therapy.
Ultimately, the study failed to meet its primary endpoint—PFS was 7.84 months and 10.71 months with everolimus and sunitinib, respectively, (HR 1.43, 95 % CI 1.15–1.77). Improved PFS with sunitinib in the front-line was seen across multiple subsets, including patients with good-risk and poor-risk disease by MSKCC risk score. Patients with non-clear cell histology also had a nonsignificant improvement in PFS with sunitinib (5.09 vs 7.23; HR 1.54, 95 % CI 0.86–2.75). Although, median OS strongly favored the sequence of sunitinib followed by everolimus (32.03 months vs 22.41 months; HR 1.24, 95 % CI 0.94–1.64), it was statistically not significant. Toxicities incurred with sunitinib and everolimus largely mirrored previously reported experiences.
While the RECORD-3 trial affirms the sequence of VEGF-directed therapy to mTOR inhibitor in the transition from first- to second-line treatment, it does not provide insight as to whether this sequence is preferable to 2 sequential VEGF-directed agents. In broader terms, both RECORD-3 and SWITCH provide an important lesson related to the feasibility of sequential trials. Specifically, these studies often suffer from substantial attrition, with only approximately half of patients receiving protocol specified therapy in the second-line setting. The same observation has been made in much smaller sequential studies in mRCC. For instance, the randomized, phase II PISCES trial explored the sequence of sunitinib followed by pazopanib or the opposing sequence [16••]. The primary endpoint of the study was patient preference, and the study was conducted in a blinded fashion with 12 weeks of therapy with each agent. With a limited duration therapy, one might anticipate a greater degree of compliance with the protocol-specified second-line treatment. However, of 169 patients randomized, only 114 patients ultimately received both sunitinib and pazopanib. Although it was determined that the majority of patients preferred pazopanib therapy, the attrition in the second-line setting limits the robustness of this conclusion.
Future perspective
Of note, several other sequential and comparative trials are underway currently or have pending results. With respect to sequential trials, the phase III SWITCH-2 study employs a design similar to its predecessor (SWITCH) [17]. Specifically, 544 patients with treatment-naïve mRCC will be randomized to receive sorafenib followed by pazopanib, or the opposing sequence. The study aims to demonstrate noninferiority of total PFS with the sequence of sorafenib followed by pazopanib vs pazopanib followed by sunitinib. It is unclear how the results of this trial might impact the current treatment landscape—with options such as axitinib and everolimus, sorafenib is infrequently considered in the second-line setting.
A second study, the START trial, takes the ambitious approach of comparing 6 potential sequences of targeted therapy across first- and second-line treatment. The study is led by the MD Anderson Cancer Center, and includes permutations of pazopanib, bevacizumab, and everolimus. The study explores the primary endpoint of time to treatment failure. Certainly, many of the sequences included in this study may be employed in the clinic today (eg, pazopanib followed by everolimus). However, with only 40 patients per study arm, it is unclear what power the study will have to demonstrate a meaningful difference in effect with any specific sequence. Presumably, this power will be diminished even further by the issues of attrition cited previously.
A rather unique sequencing approach has been proposed by investigators at the Roswell Park Cancer Institute [18]. In an ongoing phase I/II study, patients with treatment-naïve mRCC will receive pazopanib from days 1–28 and bevacizumab on days 36 and 50 on a 70-day cycle. The phase II component of the study will examine PFS as a primary endpoint. Although trials directly combining VEGF-directed therapies (eg, sunitinib with bevacizumab simultaneously) have been mired by significant toxicities, alternating therapy may circumvent these issues [19].
Of all the studies cited herein, 2 comparative trials—CheckMate 025 and METOR —have the potential to drastically alter the existing sequencing debate (Fig. 1) [20, 21]. Both studies pit novel agents against everolimus in patients who have had prior exposure to VEGF-directed therapy. CheckMate 025 examines the programmed death-1 (PD-1) inhibitor nivolumab, which abrogates the interaction between PD-1 on the T-cell and PD-1 ligand on the antigen-presenting cell [22]. Through this mechanism, nivolumab prevents T-cell anergy and augments the antitumor immune response. Recently presented phase II studies demonstrate an impressive OS in association with nivolumab, ranging from 18 to 25 months in previously treated patients [23]. CheckMate 025 permits enrollment of patients who have received up to 2 prior VEGF-directed therapies, and up to 3 therapies total. If the study meets its primary endpoint (demonstrating an improvement in OS), nivolumab might certainly be sequenced ahead of everolimus. However, it will be challenging to ascertain whether a sequence of VEGF-directed therapy followed by nivolumab would be preferable to a sequence of 2 VEGF-directed agents. A careful examination of the study composition will be warranted—if the majority of patients enrolled in the trial have already received 2 VEGF-directed agents, nivolumab may be confined to the third-line setting. CheckMate 025 has completed accrual, and study results are eagerly anticipated.
The METEOR trial explores the dual MET and VEGFR2 inhibitor cabozantinib [21]. Recent reports suggest that MET, while classically associated with type I papillary RCC, may be aberrantly expressed in all RCC subtypes and may be associated with aggressive pathologic features and poor clinical outcome [24, 25]. In a phase I trial including 25 patients with heavily pretreated mRCC, a median PFS of 12.9 months was encountered. In contrast to CheckMate 025, the METEOR trial places no restriction on the number of lines of prior VEGF therapy, and examines a primary endpoint of PFS (as opposed to OS). Again, if the study is positive, cabozantinib would be placed ahead of everolimus in therapeutic sequencing—but it will remain unclear where how it would compare with VEGF-directed agents such as axitinib and PD-1 based therapies which are still in development.
Conclusions
Investigators frequently describe the current therapeutic landscape of mRCC as an “embarrassment of riches”, with 7 targeted agents approved over a 5 year span. These agents have substantial mechanistic overlap. Furthermore, there is still equipoise as to the optimal sequence of therapies, despite the abundance of prospective data highlighted in this manuscript. This debate will only intensify in the coming years, with multiple promising agents (eg, nivolumab and cabozantinib) currently in late-stage development.
Two approaches can be taken moving forward. Investigators may continue to propose large sequential trials incorporating relevant agents—for instance, one might envision a trial comparing the sequence of sunitinib followed by either nivolumab or axitinib. However, these studies are destined to suffer from issues related to attrition from protocol-specified therapy in the second-line setting. Further, these studies run the risk of becoming antiquated even while they are ongoing. In the example provided (using sunitinib as front-line treatment), what if current phase III studies exploring the combination of sunitinib with vaccine therapy (eg, IMA-901 or AGS-003) are positive [26–28]? Would the effect of an immune-based therapy such as nivolumab be propagated or hindered by prior use of a vaccine? With multiple agents in evolution, a sequential trial may be low-yield.
A second approach is to develop salient biomarkers. Biomarker studies paired to the COMPARZ and RECORD-3 trials were recently presented. An extensive genome-wide association study (GWAS) was performed in patients enrolled in COMPARZ [29]. Certain genetic variations were linked to both toxicity and efficacy, but unfortunately, there appeared to be no variations that distinguished the effect of sunitinib and pazopanib. In RECORD-3, an extensive assessment of serum biomarkers was performed [30]. A panel of 3 biomarkers (AXL, ITAC, and KIM-1) was found to be prognostic for benefit with sunitinib and everolimus, but there was no specific biomarker (or panel) that was found to discern superior activity with sunitinib or pazopanib. Although predictive biomarkers remain elusive in these preliminary efforts, there is no doubt that this represents the “Holy Grail” in sequencing of therapies in mRCC. A decade from now, in addition to an expanded list of agents approved for mRCC, one must hope for relevant biomarkers to facilitate the optimal sequence of therapy.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–96.
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–96.
Pal SK, Kortylewski M, Yu H, Figlin RA. Breaking through a plateau in renal cell carcinoma therapeutics: development and incorporation of biomarkers. Mol Cancer Ther. 2010;9:3115–25.
Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib vs interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. This study led to approval of sunitinib as first line therapy for clear cell renal cancer.
National Comprehensive Cancer Network Clinical Practice Guidelines: Renal Cell Carcinoma. Available at http://www.nccn.org. Accessed 29 Mar 2014. This provides the consensus guidelines of national and international experts for treatment of renal cancer.
Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer A, Escudier B. Randomized phase IIIB trial of temsirolimus and bevacizumab vs interferon and bevacizumab in metastatic renal cell carcinoma: results from INTORACT. Ann Oncol. 2012;23. [Abstract LBA21_PR].
Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28:2144–50. This trial led to approval of bevacizumab as a first-line option for kidney cancer.
Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. This study led to the approval of pazopanib as first line therapy for renal cell carcinoma.
Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. This trial led to the approval of everolimus as second line option of renal cancer progressed on VEGF TKi's.
Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib vs sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. This trial led to the approval of axitinib as second-line option for renal cancer progressed on prior VEGF TKi's and also concluded that axitinib is better than sorafenib.
Motzer RJ, Hutson TE, Cella D, et al. Pazopanib vs sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31. Landmark trial showing no difference between 2 similar therapies as first-line agents for renal cancer though one better tolerated than other.
Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib vs sunitinib. N Engl J Med. 2014;370:1769–70. This is the comparative study which showed similar survival but different tolerability on 2 first-line agents.
Hutson TE, Escudier B, Esteban E, et al. Randomized Phase III trial of temsirolimus vs sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2013;32:760–7. This trial demonstrated that sequential VEGF therapies may portend longer overall survival.
Pal SK, Malangone E, Bhurke S, et al. Real-world treatment patterns in third-line patients with metastatic renal cell carcinoma (mRCC): the changing landscape in the United States. ASCO Meet Abstr. 2013;31:390.
Michel MS, Vervenne W, de Santis M, et al. SWITCH: a randomized sequential open-label study to evaluate efficacy and safety of sorafenib (SO)/sunitinib (SU) vs SU/SO in the treatment of metastatic renal cell cancer (mRCC). ASCO Meet Abstr. 2014;32:393.
Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol. 2014;32:1412–8. Landmark crossover study which showed patient preference can help decide therapy between equally efficacious agents.
NCT01613846: phase III randomized sequential open-label study to evaluate the efficacy and safety of sorafenib followed by pazopanib versus pazopanib followed by sorafenib in the treatment of advanced / metastatic renal cell carcinoma. Available at http://www.clinicaltrials.gov. Accessed 12 Jul 2014.
NCT01684397: a phase I/ II trial of pazopanib alternating with bevacizumab in treatment-naive metastatic clear cell renal cell carcinoma patients. Available at http://www.clinicaltrials.gov.
Rini BI, Garcia JA, Cooney MM, et al. Toxicity of sunitinib plus bevacizumab in renal cell carcinoma. J Clin Oncol. 2010;28:e284–5.
NCT01668784: study of nivolumab (BMS-936558) vs. everolimus in pre-treated advanced or metastatic clear-cell renal cell carcinoma (CheckMate 025) Available at http://www.clinicaltrials.gov. Accessed 20 Feb 2014.
NCT01865747: a study of cabozantinib (XL184) vs everolimus in subjects with metastatic renal cell carcinoma (METEOR). Available at http://www.clinicaltrials.gov. Accessed 20 Feb 2014.
Pal SK, Hu A, Chang M, Figlin RA. Programmed death-1 inhibition in renal cell carcinoma: clinical insights and future directions. Clin Adv Hematol Oncol. 2014;12:90–9.
Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma (mRCC): results of a randomized, dose-ranging phase II trial. ASCO Meeting Abstracts. 2014;32.
Gibney GT, Aziz SA, Camp RL, et al. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol. 2013;24:343–9.
Albiges L, Guegan J, Le Formal A, et al. MET is a potential target across all papillary renal cell carcinomas. Result from a large molecular study of pRCC with CGHa and matching gene expression array. Clin Cancer Res. 2014;20:3411–21.
NCT01582672: phase 3 trial of autologous dendritic cell immunotherapy (AGS-003) plus standard treatment of advanced renal cell carcinoma (RCC) (ADAPT). Available at http://www.clinicaltrials.gov. Accessed 15 Dec 2013.
Amin A, Dudek A, Logan T, et al. Prolonged survival with personalized immunotherapy (AGS-003) in combination with sunitinib in unfavorable risk metastatic RCC (mRCC). ASCO Meet Abstr. 2013;31:357.
NCT01265901: a randomized, controlled phase III study investigating IMA901 multipeptide cancer vaccine in patients receiving sunitinib as first-line therapy for advanced/metastatic renal cell carcinoma. Available at http://clinicaltrials.gov/ct2/show/NCT01265901?term=IMA-901&rank=2. Accessed 22 Oct 2012.
Johnson T, Xu C-f, Choueiri TK, et al. Genome-wide association study (GWAS) of efficacy and safety endpoints in pazopanib- or sunitinib-treated patients with renal cell carcinoma (RCC). ASCO. Meet Abstr. 2014;32:4503.
Voss MH, Chen D, Marker M, et al. Identification and validation of predictive biomarkers (BM) for everolimus (EVE) in metastatic renal cell carcinoma: analysis of 442 patients on RECORD-3. ASCO Meet Abstr. 2014;32:4531.
Compliance with Ethics Guidelines
Conflict of Interest
Sumanta K. Pal received consulting fees from Novartis and Pfizer. Neeraj Agarwal and Parminder Singh declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Genitourinary Cancers
Rights and permissions
About this article
Cite this article
Singh, P., Agarwal, N. & Pal, S.K. Sequencing Systemic Therapies for Metastatic Kidney Cancer. Curr. Treat. Options in Oncol. 16, 3 (2015). https://doi.org/10.1007/s11864-014-0316-2
Published:
DOI: https://doi.org/10.1007/s11864-014-0316-2