Abstract
The availability of agents targeting the vascular endothelial growth factor or mammalian target of rapamycin [mTOR] pathways has provided new treatment options for patients with metastatic renal cell carcinoma (RCC). Based on the results of pivotal randomized clinical trials, specific recommendations have been established for management of these patients in first- and second-line settings. However, certain subgroups of patients may be excluded or under-represented in clinical trials, including patients with poor performance status, brain metastases, and cardiac or renal comorbidities, elderly patients, and those with non-clear cell histology. For these subpopulations, management recommendations have emerged from expanded access programs (EAPs), small phase II studies, retrospective analysis of clinical data, and expert opinion. This paper describes recommendations from an expert panel for the treatment of metastatic RCC in these subpopulations. The efficacy of targeted agents appears to be inferior in these patient subgroups relative to the general RCC population. Tyrosine kinase inhibitors (TKIs) and mTOR inhibitors can be administered safely to elderly patients and those with poor performance status, although dose and schedule modifications are often needed, and close monitoring and management of adverse events is essential. In addition to local surgical treatment and radiotherapy for brain metastases, systemic treatment with a TKI should be offered as part of multidisciplinary care.
While there are currently no data from randomized trials, sunitinib has the greatest body of evidence, and it should be considered the first choice in patients with a good prognosis. Patients with an acute cardiac event within the previous 6 months, New York Heart Association grade III heart failure, or uncontrolled high blood pressure should not be treated with TKIs. In patients with mild or moderate renal failure, there are no contraindications to TKI treatment. TKIs can be administered to patients undergoing dialysis, but other, less nephrotoxic agents and other alternatives should always be considered.
In managing RCC among patients with non-clear cell histology, sunitinib seems to be more effective than everolimus for the papillary subtype, but there are no clear data to guide treatment for other subtypes. In conclusion, individualized treatment approaches are needed to manage RCC in subpopulations that are underrepresented in registration clinical trials.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Renal cell carcinoma (RCC) is among the most common adult malignancies in the United States, ranking fifth in men and eighth in women, with approximately 64,000 new cases diagnosed per year. Despite the success of local treatments for patients with early disease, up to one third of patients either present with or develop metastatic disease. For the most part, RCC is notoriously resistant to conventional chemotherapy and radiation therapy as curative treatment [1, 2], although stereotactic radiotherapy has an important role in the treatment of brain metastases and in symptom palliation in advanced disease [3].
An improved understanding of RCC biology has led to major advances in the treatment of patients with metastatic disease [1]. Previously, immunotherapeutic agents such as interleukin-2 (IL-2) and interferon-α (IFN-α) represented the standard approach, but yielded a clinical benefit in only a small subset of patients [4]. Mechanistically, new agents in RCC broadly target angiogenesis, and mainly the vascular endothelial growth factor (VEGF) axis as the key mediator in new blood vessel formation. One of the most common genetic alterations in RCC is mutation in the von Hippel-Lindau (VHL) gene that regulates cell response to hypoxia. In conditions in which this gene is altered, the hypoxia-inducible factor 1-alpha (HIF1α) is not properly degraded, leading to sustained angiogenesis mediated by VEGF production. Evidence accumulated over the past 10 years suggests that HIF2, rather than HIF1, is the key driver of renal cancer progression [5]. In vitro and cell line xenograft studies suggest that HIF2 is both necessary and sufficient for the growth of transformed VHL−/− RCC cell lines and for much of the pathology that has been described in genetically engineered mouse models in which VHL has been inactivated in specific tissue.
New drugs in RCC either block VEGF receptor tyrosine kinase activation (tyrosine kinase inhibitors [TKIs]) or bind to VEGF itself (monoclonal antibodies), preventing its activation of the VEGF receptor. The inhibitors of the mammalian target of rapamycin (mTOR) block the production of VEGF by RCC cells through inhibition of pathways involved in the translation of this growth factor [6].
It should be noted, however, that these biological characteristics are unique to the clear cell carcinoma subtype and, as discussed below, are different in non-clear cell subtypes, which harbor mutations in other genes. However, many of these genetic defects also lead to sustained production of VEGF and pathological angiogenesis, thus explaining why angiogenesis inhibitors also exhibit activity in these patients [6].
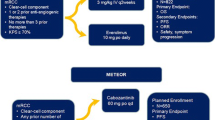
Over the last decade, the incorporation of new drugs targeting critical biological pathways in RCC has changed the natural history of the disease. These include TKIs, inhibitors of angiogenesis, and mTOR inhibitors. Table 1 lists the targeted agents available in Europe, their approved indications, and their recommended place in therapy according to European and US guidelines [7–15]. Multiple options can be offered to the treatment-naïve patient with metastatic RCC (mRCC), but the vast majority of patients will receive VEGF-directed agents up front. For patients with poor prognosis, consensus opinion supports temsirolimus as standard treatment, as this is the only agent that has been tested in these patients in a prospective clinical trial [16]. However, emerging evidence from expanded access programs (EAPs) have shown a benefit for sunitinib in patients with poor prognostic indicators such as older age, poor performance status, or brain metastases [17]. Figure 1 summarizes the current management algorithm [16, 18–20].
Management algorithm in metastatic RCC [2]
The availability of new drugs has dramatically changed the management of RCC, and the treating physician now has an armamentarium of drugs with related mechanisms of action and unique toxicity profiles from which to select specific treatment for a given patient.
In analyzing the evidence from which to select a specific treatment, two types of scientific data can be considered. One is the results of clinical trials, either pivotal or non-pivotal studies, and the other is clinical practice and smaller studies such as retrospective analysis of case series from center experiences. Prospective clinical trials have an inherent selection bias, as they enroll only patients with very specific predetermined characteristics such as good Eastern Cooperative Oncology Group (ECOG) performance status 0–1, clear cell carcinoma histology, and absence of comorbidities. These unbiased studies provide information regarding the efficacy of a given drug as well as sequencing strategies. However, there is a substantial subset of patients for whom no clinical trial information is available. These include patients with ECOG ≥2, non-clear cell carcinoma histology, or brain metastases, and those with associated comorbidities. The information available for treating these patients comes from post-approval studies (including EAPs and small observational studies) and routine clinical practice [14].
The aim of this manuscript is to provide a comprehensive review of available information for the first-line treatment of metastatic RCC in patient segments underrepresented in clinical trials, as well as to provide treatment recommendations for these patients. The review focuses on elderly patients, patients with ECOG 2, and those with cardiac and renal comorbidities or tumors with histology other than clear cell carcinoma.
2 Methodology
This paper is a summary of an expert review panel meeting. The expert panel comprised oncologists from Spain who had been involved in the field of RCC for more than 5 years and who had treated at least 15 new RCC patients per year. Based on a list of topics, a bibliographic search was first undertaken in the MEDLINE database (PubMed, from January 2007 to January 2015) to identify any evidence regarding phase II and III prospective studies, EAPs, or retrospective observational studies for first-line treatment of RCC in the following subgroups: elderly patients (>65 or 70 years old) and patients with brain metastases, ECOG ≥ 2, heart failure or heart comorbidities, renal failure, or non-clear cell carcinoma. In addition, relevant data from major oncology congresses were evaluated on a case-by-case basis. Each expert in the panel was assigned one topic to review and prepared a summary of the main findings.
These conclusions were then evaluated in four regional face-to-face working meetings with another 20 medical oncologists. Subsequently, the original group of experts met face-to-face on two separate occasions to discuss the topics, summarize the main conclusions, and make management recommendations. The agreed output from these meetings was used to generate the current manuscript. The draft manuscript was reviewed by the expert panel to generate the current final manuscript.
3 Elderly Patients
The median age at diagnosis of RCC is 64 years, although approximately 50 % of patients are older than 65 and 25 % are older than 75 years at presentation. Functional status, however, is more relevant than chronological age. Some elderly patients are healthy, without significant chronic disease, and are independently able to perform daily activities, whereas others present with comorbid conditions and are dependent on caregivers for one or more routine activities. Elderly patients often have underlying comorbidities such as renal failure (25 %), congestive heart failure (10 %), or chronic atrial fibrillation (30 %). Up to 40 % of patients have high blood pressure (BP) [19, 21, 22].
On average, the median age of patients included in registration trials was 61 years, with a broad range across studies. Subset analysis by age group in the registration trials of sunitinib, sorafenib, and pazopanib showed statistically significant benefits, independent of age [19, 21, 22]. For example, the hazard ratios for progression-free survival (PFS) in the pivotal studies of sunitinib and pazopanib were similar in patients >65 years and those ≤65 years of age (sunitinib: 0.42 for older patients and 0.40 for younger patients; pazopanib: 0.52 and 0.42, respectively) [19, 21, 22]. The direct comparison between sunitinib and pazopanib in the COMPARZ trial showed higher efficacy for sunitinib in patients older than 65 years, although the difference was not statistically significant [22].
Table 2 summarizes some of the available data with regard to treatment efficacy for sunitinib, sorafenib, and pazopanib in elderly patients [19, 23–30]. The available data from the EAPs of sunitinib and sorafenib showed that efficacy was similar in patients older than 65 years (sunitinib) and 70 years (sorafenib) compared to younger patients [23, 25, 30, 31].
Evidence from retrospective cohort studies shows similar efficacy for TKIs in elderly patients. Results from the retrospective study by Hutson et al. in 202 patients older than 70 years who received first-line sunitinib showed no differences with regard to overall survival (25.6 vs. 23.6 months in patients <70 years) or PFS (11 vs. 9.9 months in patients <70 years) [26]. Analysis by age in groups of patients resistant to cytokines showed comparable results. However, there was a trend towards higher toxicity in patients older than 70 years [26]. Small retrospective studies in which the mean age of patients was between 61 and 66 years also showed a higher tendency for grade 3 and 4 toxicities in the elderly population [27, 28].
Likewise, for sorafenib, an analysis of six randomized trials and two EAP trials totaling 4684 patients showed a similar toxicity profile for patients older than 75 years, but these patients had a higher incidence of grade 3 and 4 toxicities (Table 2) [32].
In a small retrospective study comparing sunitinib with sorafenib among 15 elderly patients, no statistically significant differences with regard to dose adjustments were observed [33]. There was a trend towards more frequent skin toxicity in patients who received sorafenib, while subjects treated with sunitinib were more likely to develop hematological toxicities. Regarding management of toxicities in elderly patients, data suggest that an adapted dose (a reduction in dose and/or scheduled dose compared to the standard regimen) results in less need for dose reduction (41.9 % for the adapted regimen vs. 66.7 % for the standard regimen), with similar PFS and overall survival (OS) [34]. These data are consistent with the results from the RAINBOW study and a sunitinib titration study. The RAINBOW study tested an adapted dose and schedule of sunitinib in patients with RCC, showing that a 2/1 schedule resulted in fewer toxicity events than the conventional 4/2 schedule [35]. Similar results obtained in other trials provide overall support to the notion that a modified schedule is less toxic and equally effective [36]. These approaches are particularly interesting for elderly patients more prone to developing side effects with treatment.
3.1 Conclusions
Phase III studies show no differences in either efficacy or toxicity in subjects older and younger than 65 years who meet the eligibility criteria (fit elderly patients). Retrospective studies, however, suggest that there is greater toxicity in patients over 70 years of age.
Efficacy is similar across the different TKIs, and is independent of age, with sunitinib having a greater amount of available evidence than other TKIs, and what seems to be a tendency towards higher efficacy. Further studies are needed to confirm these results.
3.2 Recommendations
Standard management for elderly patients with advanced RCC should follow the same criteria applied to the general population, considering classic factors such as prognostic groups and histological subtypes, among others. Because these patients are more prone to toxicity events, close follow-up is required. Alternative dose and schedule regimens with similar efficacy but less toxicity may be considered.
4 ECOG 2
Poor performance status is associated with poor prognosis in clinical trials, both in the cytokine era and with modern therapeutics [37].
Furthermore, low performance status is one of the most common reasons for ineligibility for clinical trials. For example, in the study by Heng et al., poor performance status was the single most frequent ineligibility factor, affecting 13 % of the 2210 patients with RCC screened for inclusion [38]. In addition, compared to the study-eligible population, these patients had worse PFS and OS [38, 39].
Because patients with ECOG 2 are generally not enrolled in clinical trials, treatment recommendations are based on data from EAPs [23, 25]. These studies show a worse outcome in these patients for both sunitinib and sorafenib, with decreased PFS, OS, and disease control. The updated results of the sunitinib EAP revealed a median PFS of 3.9 months for patients with ECOG 2 (600 patients) compared to 9.5 months for the entire study population [25]. Likewise, in the sorafenib EAP, the disease control rate was substantially lower (51.9 %) in ECOG 2 patients compared with patients with performance status of 0 (80 %). These patients also showed a higher incidence of adverse events—specifically, hand-foot skin reactions of any grade, diarrhea, and hypertension [23].
4.1 Conclusions
Patients with ECOG ≥2 have a worse prognosis and are under-represented in clinical trials. In addition, the efficacy of available treatments is lower in these patients, and toxicity may be increased.
4.2 Recommendations
Treatment of patients with ECOG ≥2 should be individualized and should take into account other prognostic factors in order to establish the best therapeutic option. Symptom management and reduction of potential toxicities should be the first priority of treatment. Reduced dose regimens as well as different dosing schedules could be considered.
5 Brain Metastases
According to a recent study, brain metastases occur in approximately 8 % of patients with RCC, with only 24 % of those having exclusively brain metastases [40]. In general, these patients are excluded from enrollment in clinical trials. For this reason, data regarding the efficacy and safety of drugs in this setting come from EAPs.
Preclinical results have shown that these drugs can pass through the blood-brain barrier (BBB) [41], and so there have been reports of brain metastases from RCC that have responded to sunitinib [42, 43]. A retrospective study of patients treated from 2008 to 2010 with either cytokines or TKIs showed that the median time to development of brain metastasis was 1.6 months longer for patients treated with a TKI than those who received cytokines, thus suggesting, at least indirectly, a protective effect [44]. Indeed, in prospective studies, the incidence of new brain metastases has been shown to be lower in patients treated with sorafenib compared to placebo, although the evidence is limited [45].
Data for sorafenib and sunitinib in patients with brain metastases is presented in Table 3.
With sorafenib, there are data in a cohort of 70 patients with brain metastases, most of whom had not undergone a nephrectomy, which showed a lower response rate than expected for the general RCC population [30]. With regard to sunitinib, the EAP included 213 patients with brain metastases among a total of 3464 patients. In the trial, 12 % of the patients achieved a partial response, 52 % had stable disease at 3 months, and 64 % demonstrated clinical benefit [45]. While this was not a comparative trial, the results were worse than for the general RCC population. However, there were no differences in adverse events between patients with and without brain metastases. In particular, the incidence of bleeding was very rare in these patients [45]. With regard to pazopanib, the evidence is limited to single case reports [46].
In a retrospective study including 65 RCC patients with brain metastases, results showed that the combination of systemic therapy and local treatment (radiotherapy) did not appear to increase the risk of cerebral hemorrhage [47]. In this study, a multimodal approach was not associated with an excess of neurological adverse events. The study also found that clear cell histology, favorable Memorial Sloan Kettering Cancer Center (MSKCC) risk status, and solitary brain metastasis were associated with more favorable OS [47]. This corresponds with findings from the International Metastatic Renal Cell Carcinoma Database Consortium, which collected data from 705 patients, 15 % of whom had brain metastases, among which 12 % had a good prognosis according to the Heng criteria [48]. Most of these patients had received systemic treatment with sunitinib (n = 77) and some form of local treatment such as whole-brain radiotherapy (81 %). In multivariate analysis, patients with poor performance status (Karnofsky < 80 %), time from diagnosis to TKI treatment of less than 1 year, and more than four brain lesions showed worse survival [48].
5.1 Conclusions
Available evidence suggests that brain metastases predict a worse prognosis.
The current data suggest that TKIs diffuse through the BBB. Results from the substantial number of patients treated in EAPs show that sunitinib (12 % response rate) and sorafenib (4 % response rate) are effective in this setting. In particular, sunitinib has been shown to delay the onset of brain metastases, and could be considered the preferred systemic treatment for these patients.
With regard to toxicity, the overall toxicity pattern in patients with brain metastases is similar to that for other patients. As with most cancer types, local treatment is also critical for control of brain metastases.
5.2 Recommendations
Brain metastases should not be cause to withhold or delay treatment for a patient with RCC. Surgical resection and/or radiosurgery contribute to prolonged survival. As most patients harbor life-threatening extracranial metastases, systemic treatment may theoretically play a role in the management of these patients, although more data are needed to confirm the clinical impact of TKI therapy.
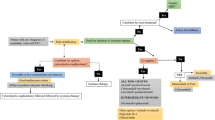
Systemic treatment with a TKI should be offered to these patients as part of multidisciplinary care. While there are currently no data from randomized trials, sunitinib has the greatest body of evidence, and it should be considered the first choice in patients with a good prognosis. Local treatment is of paramount importance, particularly for patients with solitary lesions. Figure 2 depicts a proposed algorithm to manage patients with RCC and brain metastases.
6 Heart Disease
Cardiac toxicity is one of the most common toxicities in current treatments for patients with RCC. This side effect is related to the predominant mechanism of action of these drugs, which is based on angiogenesis inhibition. Table 4 summarizes the target toxicity and the putative mechanism of action implicated in side effects observed with angiogenesis inhibitors [49].
The most common side effect is arterial hypertension; grade 3 events have been reported in 8 %, 4 %, and 8 % of patients treated with sunitinib, sorafenib, and pazopanib, respectively [50].
Other, less common toxicities include left ventricular dysfunction, congestive heart failure, elevated troponin and creatine phosphokinase levels, and changes in electrocardiogram [49]. In recent updates from the SWITCH and COMPARZ trials, the overall occurrence of cardiac events was similar among the drugs tested [22, 51]. The SWITCH study assessed the sequential treatment of mRCC patients with sunitinib followed by sorafenib, or vice versa, and found no difference in the incidence of cardiac adverse events. Likewise, in the pazopanib versus sunitinib comparative COMPARZ study, the incidence of myocardial infarction or ischemia was 2 % for pazopanib and 4 % for sunitinib (not significantly different) [22].
One important issue, at least for sunitinib, is that the rate of cardiac events is similar after any administered cycle, suggesting that the risk for this toxicity is not cumulative; on the other hand, continuous surveillance is needed [52].
6.1 Conclusions
Cardiac toxicity is a class effect for TKIs. The spectrum of potential cardiac events associated with TKIs and other angiogenesis inhibitors is broad. Elevated BP is probably the most common effect and, if not controlled, may lead to more severe cardiac damage. Proper diagnosis and management are critical.
6.2 Recommendations
From a practical perspective, it is important to achieve adequate BP control before starting RCC treatment and to monitor BP and cardiac function throughout the treatment course, following current management guidelines for these events [53].
For patients with left ventricular ejection fraction (LVEF) <50 % or severe cardiac problems, there are no data supporting the administration of a TKI. In patients with LVEF ≥50 %, treatment can be administered on an individual basis with close monitoring (LVEF assessment prior to each cycle) and in consultation with a cardiologist. This recommendation is based on the low frequency of cardiac toxicity as well as its reversibility.
Patients with an acute cardiac event within the previous six months, New York Heart Association grade III heart failure, or uncontrolled high BP should not be treated with TKIs.
7 Renal Failure
Approximately one third of patients with metastatic RCC present with renal failure, defined as plasma creatinine levels >1.9 mg/dL or creatinine clearance <30 mL/min. The presence of renal failure is associated with elevated creatinine levels (55–70 %), proteinuria (11 %), hyponatremia, hypophosphatemia, and altered electrolyte levels [2]. However, neither the package insert nor the clinical experiences limits the use of targeted agents in patients with renal failure. It is worth mentioning that sunitinib, sorafenib, pazopanib, and temsirolimus are mainly metabolized in the liver [20, 54–58].
Patients with chronic renal failure have a 40- to 100-fold increased risk of needing dialysis than the general population [59]. Evidence is scarce on the use of new targeted therapies in RCC patients on dialysis. A retrospective report of six patients treated with sunitinib showed similar efficacy and toxicity profiles among patients undergoing dialysis and those with normal renal function [60]. The package insert for sunitinib notes that the drug and its active metabolite are not cleared by hemodialysis in patients with end-stage renal failure; however, systemic exposure in these patients was found to be 47 % and 31 % lower for sunitinib and its active metabolite, respectively, when compared to subjects with normal renal function [7]. The report by Izzedine on six patients, however, suggests that classic administration of 50 mg/day for 4 weeks is well tolerated in dialysis patients, reaching plasma concentrations and showing a pharmacokinetic profile similar to that seen in patients with normal renal function [59]. Treatment of RCC with sorafenib in patients undergoing dialysis is limited to the report by Maroto et al. of two patients who tolerated the treatment well, presenting only with high BP as a major adverse event [61].
7.1 Conclusions
Although all TKIs can reduce renal function in patients with already impaired function, these agents can be safely used in patients with mRCC.
Evidence about the use of TKIs in patients undergoing dialysis is limited; however, the results suggest that the efficacy and tolerability profiles of TKIs do not differ between patients with normal renal function and those on dialysis.
7.2 Recommendations
In patients with mild or moderate renal failure, there are no contraindications to administration of a TKI. However, in patients with severe renal failure, there is a risk of higher toxicity as well as the need for dialysis, and treatment should be highly individualized in these patients.
TKIs can be administered to patients undergoing dialysis, but other, less nephrotoxic agents and other alternatives should always be considered.
8 Non-Clear Cell Histology
Up to 25 % of patients with RCC have non-clear cell histology. This spectrum includes type 1 (5 %) and type 2 (10 %) papillary tumor, chromophobe (5 %), and oncocytoma (5 %) subtypes [62].
These tumors are characterized by mutations in genes such as c-MET and fumarate hydratase (FH) that are involved in HIF1α and mTOR signalling. Thus, from a mechanistic perspective, targeting non-clear cell RCC with TKIs and mTOR inhibitors fits within the described pathological pathway of the disease.
The only agent that has been tested in pivotal clinical trials in patients with non-clear cell histology is temsirolimus, and that study demonstrated PFS and OS of 7 and 11.6 months, respectively, in this patient subset [63]. PFS and OS in patients with non-clear cell histology in this study were not significantly different from those with clear cell histology [63]. With regard to everolimus, the phase II RAPTOR study in patients with papillary histology aimed to determine the proportion of patients with progression-free status at 6 months. The investigators reported 6-month PFS of 56.8 % and median PFS of 7.8 months, while the centralized review indicated PFS of 34.1 % and median PFS of 3.9 months. The median survival was 20 months [64].
A retrospective analysis was conducted in 85 patients with non-clear cell or sarcomatoid histology who received mTOR inhibitors (temsirolimus: n = 59; everolimus: n = 27) at the MSKCC between 2007 and 2013 [65]. Of the 82 patients assessable for response, four of the 23 with sarcomatoid histology (17 %) and 14 of the 59 with non-clear cell histology (24 %) demonstrated a partial response or stable disease for ≥6 months. Median PFS was 2.9 months across the whole cohort, 2.8 months in those with non-clear cell disease, and 3.5 months in those with sarcomatoid histology. Median OS in these three cohorts was 8.7, 9.1, and 8.2 months, respectively [65].
The EAP for sunitinib evaluated 437 patients with RCC and non-clear cell histology, and showed a clinical benefit of 68 %, with PFS of 7.8 months and OS of 13.4 months [25]. In the phase II SUPAP trial, patients with untreated type 1 and 2 papillary renal cell cancer received sunitinib as a single agent following a standard regimen. The results demonstrated median PFS and OS of 6.6 and 17.5 months, respectively, for patients with type 1 tumors, and 5.5 and 12.4 months, respectively, in patients with type 2 tumors [66]. Similarly, a recent retrospective study in 63 patients receiving sunitinib for RCC showed PFS of approximately 7 months in those with papillary histology (88 % of patients enrolled) [67].
The sorafenib pivotal trials included only patients with clear cell histology [23, 30]. The data for other subtypes come from the EAP. In the US program, a total of 107 patients with papillary cancers and 20 with chromophobe tumors were enrolled. This study showed a low partial response rate of 3 % and 5 %, respectively, but a high disease control rate of greater than 80 % [30]. In the European study, the overall PFS for these patients was 6.6 months, with a 78 % disease control rate at 12 weeks [23].
Three head-to-head comparative studies have investigated sunitinib versus everolimus in patients with non-clear cell RCC. The RECORD-3 study included a subgroup of 76 patients with non-clear cell tumors who were randomized to everolimus or sunitinib. PFS was 7.2 months for sunitinib versus 5 months for everolimus [68]. More recently, Tannir et al. presented a study comparing everolimus and sunitinib in the first-line setting for patients with non-clear cell histology of any type. The results showed that sunitinib resulted in longer PFS (6.1 months) and OS (16.1 months) compared to everolimus (4.1 and 14.1 months, respectively), although these differences were not statistically significant [69]. In addition, the final clinical results of a randomized phase II trial of everolimus versus sunitinib in patients with metastatic non-clear renal cell carcinoma (ASPEN trial) were presented at ASCO 2015 [70]. The ASPEN study was an international randomized trial of patients with metastatic RCC of papillary, chromophobe, or unclassified histology. Patients were randomized 1:1 to everolimus or sunitinib until progression, and the primary endpoint was radiographic PFS (rPFS). One hundred and eight patients were enrolled, and the results showed prolonged rPFS with sunitinib relative to everolimus in these patients (8.3 months vs. 5.6 months, HR 1.41; p = 0.16, assuming a two-sided type I error rate of 0.20) [70].
Finally, in a recent phase II study presented by McKay et al., patients with sarcomatoid RCC were treated with sunitinib and gemcitabine. OS was 10 months and the stable disease rate was 38 % (15 patients). Interestingly, increased sarcomatoid histology (>10 %) correlated with an improved clinical benefit [71]. Table 5 shows the results of studies by subtype.
8.1 Conclusions
RCC of non-clear cell histology is a heterogeneous and biologically distinct group of neoplasms. The current armamentarium for treating RCC provides a large repertoire of therapeutic options, but the response of different histological subtypes to individual agents may vary, and further research is urgently needed. Most treatment decisions are based on data from the EAPs of sunitinib, sorafenib, everolimus, and temsirolimus.
The recent data from the SUPAP, ESPN, RECORD-3, and ASPEN studies suggest that sunitinib is more effective than mTOR inhibitors in this setting [64, 68, 70, 72]. For sarcomatoid histology, the combination of chemotherapy and sunitinib could represent a new treatment option.
Overall, however, the results in these patient subsets are worse than in those with clear cell RCC, indicating that further research is needed. Data indicate that c-MET expression is higher in papillary and sarcomatoid tumors than in clear cell RCC [73], suggesting that c-MET inhibitors may have a future role in the management of RCC with non-clear cell histology.
8.2 Recommendations
For management of RCC in patients with non-clear cell histology, sunitinib appears to be more effective than everolimus for the papillary subtype. Indeed, there are no clear data with regard to the preferred agent for these patients. Subjects with a high sarcomatoid component should also be offered conventional chemotherapy, but in combination with a TKI.
9 Final Conclusions
With the introduction of TKIs and mTOR inhibitors, the treatment of advanced RCC has changed dramatically. Based on registration trials and approved indications, clear recommendations have been established for managing a substantial proportion of these patients. Some groups, however, including elderly patients and patients with ECOG 2, brain metastases, cardiac and renal comorbidities, and histology other than clear cell carcinoma, are either not included or are poorly represented in pivotal trials.
Recommendations for these patients are less well established, and have come from EAPs, small phase II studies, and clinical experience. The panel agreed that, despite the relative scarcity of available data, these patients should be considered for treatment with modern agents, since some subgroups may not experience greater toxicity or worse efficacy than patients without these characteristics. Thus, individualized patient management in the context of multidisciplinary care teams, with special attention to drug selection and dose and schedule modifications, are critical to achieving the safest and most effective results. Further research is needed to clarify how best to use new agents in treating patients with RCC who are currently under-represented in randomized controlled trials, as well as to explore novel approaches to treatment (such as the c-MET inhibitors and immune checkpoint inhibitors targeting programmed cell death 1 [PD-1] and cytotoxic leukocyte antigen 4 [CTLA-4]) in order to further increase the rate of survival in all patients with RCC.
References
Khoo VS, Pyle L (2007) Radiotherapy and supportive care. In: Eisen T, Christimas T (eds) Clinical Progress in Renal Cancer. Informa UK Ltd., Oxford
Rini BI, Campbell SC, Escudier B (2009) Renal cell carcinoma. Lancet 373(9669):1119–32. doi:10.1016/S0140-6736(09)60229-4
Blanco AI, Teh BS, Amato RJ (2011) Role of radiation therapy in the management of renal cell cancer. Cancers (Basel) 3(4):4010–23. doi:10.3390/cancers3044010
Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T (2005) Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev 1:CD001425. doi:10.1002/14651858.CD001425.pub2
Gossage L, Eisen T, Maher ER (2015) VHL, the story of a tumour suppressor gene. Nat Rev Cancer 15(1):55–64. doi:10.1038/nrc3844
Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A et al (2005) Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol 23(12):2763–71. doi:10.1200/JCO.2005.07.055
Agency EM. Sutent : EPAR - Product Information 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000687/WC500057737.pdf . Accessed May 11th, 2015.
Agency EM. Nexavar : EPAR - Product Information 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000690/WC500027704.pdf . Accessed Nov 5 2015.
Agency EM. Votrient : EPAR - Product Information 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001141/WC500094272.pdf . Accessed Nov 5 2015.
Agency EM. Torisel : EPAR - Product Information 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000799/WC500039912.pdf . Accessed Nov 5 2015.
Agency EM. Afinitor : EPAR - Product Information 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001038/WC500022814.pdf . Accessed Nov 5 2015.
Agency EM. Avastin : EPAR - Product Information 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf . Accessed Nov 5 2015.
Agency EM. Inlyta : EPAR - Product Information 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002406/WC500132188.pdf . Accessed Nov 5 2015.
Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ, Khoo V et al (2014) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 25(suppl 3):iii49–56. doi:10.1093/annonc/mdu259
Network NCC. NCCN Clinical Practice Guidelines in Oncology: Kidney Cancer. Version I 2016
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356(22):2271–81. doi:10.1056/NEJMoa066838
Sternberg CN, Calabro F, Bracarda S, Carteni G, Lo Re G, Ruggeri EM et al (2015) Safety and efficacy of sunitinib in patients from Italy with metastatic renal cell carcinoma: final results from an expanded-access trial. Oncology 88(5):273–80. doi:10.1159/000369256
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C et al (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370(9605):2103–11. doi:10.1016/S0140-6736(07)61904-7
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356(2):115–24. doi:10.1056/NEJMoa065044
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J et al (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28(6):1061–8. doi:10.1200/JCO.2009.23.9764
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M et al (2007) Sorafenib in advanced clearcell renal-cell carcinoma. N Engl J Med 356(2):125–34. doi:10.1056/NEJMoa060655
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J et al (2013) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369(8):722–31. doi:10.1056/NEJMoa1303989
Beck J, Procopio G, Bajetta E, Keilholz U, Negrier S, Szczylik C et al (2011) Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) expanded-access study: a large open-label study in diverse community settings. Ann Oncol 22(8):1812–23. doi:10.1093/annonc/mdq651
Brunello A, Basso U, Sacco C, Sava T, de Vivo R, Camerini A et al (2013) Safety and activity of sunitinib in elderly patients (≥70 years) with metastatic renal cell carcinoma: a multicenter study. Ann Oncol 24(2):336–42. doi:10.1093/annonc/mds431
Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S et al (2009) Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 10(8):757–63. doi:10.1016/S1470-2045(09)70162-7
Hutson TE, Bukowski RM, Rini BI, Gore ME, Larkin JM, Figlin RA et al (2014) Efficacy and safety of sunitinib in elderly patients with metastatic renal cell carcinoma. Br J Cancer 110(5):1125–32. doi:10.1038/bjc.2013.832
Josephs D, Hutson TE, Cowey CL, Pickering LM, Larkin JM, Gore ME et al (2011) Efficacy and toxicity of sunitinib in patients with metastatic renal cell carcinoma with severe renal impairment or on haemodialysis. BJU Int 108(8):1279–83. doi:10.1111/j.1464-410X.2010.09990.x
Kim KH, Kim HY, Kim HR, Sun JM, Lim HY, Lee HJ et al (2014) Efficacy and toxicity of sunitinib in patients with metastatic renal cell carcinoma with renal insufficiency. Eur J Cancer 50(4):746–52. doi:10.1016/j.ejca.2013.11.029
Rautiola J, Utriainen T, Peltola K, Joensuu H, Bono P (2014) Pazopanib after sunitinib failure in patients with metastatic renal cell carcinoma. Acta Oncol 53(1):113–8. doi:10.3109/0284186X.2013.794957
Stadler WM, Figlin RA, McDermott DF, Dutcher JP, Knox JJ, Miller WH Jr et al (2010) Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer 116(5):1272–80. doi:10.1002/cncr.24864
Bukowski RM, Stadler WM, McDermott DF, Dutcher JP, Knox JJ, Miller WH Jr et al (2010) Safety and efficacy of sorafenib in elderly patients treated in the North American advanced renal cell carcinoma sorafenib expanded access program. Oncology 78(5–6):340–7. doi:10.1159/000320223
Procopio G, Bellmunt J, Dutcher J, Bracarda S, Knox J, Brueckner A et al (2013) Sorafenib tolerability in elderly patients with advanced renal cell carcinoma: results from a large pooled analysis. Br J Cancer 108(2):311–8. doi:10.1038/bjc.2012.543
Derbel Miled O, Dionne C, Terret C, Segura-Ferlay C, Flechon A, Neidhart EM et al (2013) Sorafenib and sunitinib for elderly patients with renal cell carcinoma. J Geriatr Oncol 4(3):255–61. doi:10.1016/j.jgo.2013.04.004
De Giorgi U, Scarpi E, Sacco C, Aieta M, Lo Re G, Sava T et al (2014) Standard vs. Adapted Sunitinib Regimen in Elderly Patients With Metastatic Renal Cell Cancer: Results From a Large Retrospective Analysis. Clin Genitourin Cancer 12(3):182–9. doi:10.1016/j.clgc.2013.11.005
Bracarda S, Iacovelli R, Rizzo M, Rossi M, Galli L, Procopio G et al (2014) Retrospective observational study of sunitinib administered on schedule 2/1 in patients with metastatic renal cell carcinoma (mRCC): The rainbow study. J Clin Oncol 32(suppl 4):471
Bjarnason GA, Naveen B, Winquist E, Kollmannsberger CK, Canil C, North S et al (2014) Phase II study of individualized sunitinib as first-line therapy for metastatic clear cell renal cell cancer (mRCC). Ann Oncol 25(suppl4):iv280–304
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M (2002) Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20(1):289–96
Heng DYC, Choueiri TK, Rini BI, Lee J, Yuasa T, Pal SK et al (2014) Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol 25(1):149–54. doi:10.1093/annonc/mdt492
Heng DYC, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C et al (2009) Prognostic Factors for Overall Survival in Patients With Metastatic Renal Cell Carcinoma Treated With Vascular Endothelial Growth Factor–Targeted Agents: Results From a Large. Multicenter Study J of Clin Oncol 27(34):5794–9. doi:10.1200/jco.2008.21.4809
Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh Q-D, Briganti A et al (2012) Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol 23(4):973–80. doi:10.1093/annonc/mdr362
Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH et al (2005) The Effect of Bcrp1 (Abcg2) on the In vivo pharmacokinetics and brain penetration of Imatinib Mesylate (Gleevec): Implications for the use of breast cancer resistance protein and P-Glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res 65(7):2577–82. doi:10.1158/0008-5472.can-04-2416
Helgason HH, Mallo HA, Droogendijk H, Haanen JG, van der Veldt AAM, van den Eertwegh AJ et al (2008) Brain metastases in patients with renal cell cancer receiving new targeted treatment. J Clin Oncol 26(1):152–4. doi:10.1200/jco.2007.13.5814
Medioni J, Cojocarasu O, Belcaceres J-L, Halimi P, Oudard S (2007) Complete cerebral response with sunitinib for metastatic renal cell carcinoma. Ann Oncol 18(7):1282–3. doi:10.1093/annonc/mdm275
Dudek AZ, Raza A, Chi M, Singhal M, Oberoi R, Mittapalli RK et al (2013) Brain metastases from renal cell carcinoma in the era of tyrosine kinase inhibitors. Clin Genitourin Cancer 11(2):155–60. doi:10.1016/j.clgc.2012.11.001
Gore ME, Hariharan S, Porta C, Bracarda S, Hawkins R, Bjarnason GA et al (2011) Sunitinib in metastatic renal cell carcinoma patients with brain metastases. Cancer 117(3):501–9. doi:10.1002/cncr.25452
Jacobs C, Kim DW, Straka C, Timmerman RD, Brugarolas J (2013) Prolonged survival of a patient with papillary renal cell carcinoma and brain metastases using pazopanib. J Clin Oncol 31(7):e114–7. doi:10.1200/JCO.2012.46.0501
Bastos DA, Molina AM, Hatzoglou V, Jia X, Velasco S, Patil S et al (2015) Safety and efficacy of targeted therapy for renal cell carcinoma with brain metastasis. Clin Genitourin Cancer 13(1):59–66. doi:10.1016/j.clgc.2014.06.002
Vickers MM, Al-Harbi H, Choueiri TK, Kollmannsberger C, North S, MacKenzie M et al (2013) Prognostic factors of survival for patients with metastatic renal cell carcinoma with brain metastases treated with targeted therapy: results from the international metastatic renal cell carcinoma database consortium. Clin Genitourin Cancer 11(3):311–5. doi:10.1016/j.clgc.2013.04.012
Suter TM, Ewer MS (2013) Cancer drugs and the heart: importance and management. Eur Heart J 34(15):1102–11. doi:10.1093/eurheartj/ehs181
Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ et al (2010) Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 102(9):596–604. doi:10.1093/jnci/djq091
Eichelberg C, Vervenne WL, de Santis M, von Fischer W, Goebell PJ, Lerchenmuller C et al (2015) SWITCH: A Randomised, Sequential, Open-label Study to Evaluate the Efficacy and Safety of Sorafenib-sunitinib Versus Sunitinib-sorafenib in the Treatment of Metastatic Renal Cell Cancer. Eur Urol. doi:10.1016/j.eururo.2015.04.017
Di Lorenzo G, Autorino R, Bruni G, Carteni G, Ricevuto E, Tudini M et al (2009) Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol 20(9):1535–42. doi:10.1093/annonc/mdp025
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M et al (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of hypertension (ESH) and of the european society of cardiology (ESC). Eur Heart J 34(28):2159–219. doi:10.1093/eurheartj/eht151
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M et al (2009) Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 27(20):3312–8. doi:10.1200/JCO.2008.19.5511
Hudes GR, Carducci MA, Choueiri TK, Esper P, Jonasch E, Kumar R et al (2011) NCCN Task Force report: optimizing treatment of advanced renal cell carcinoma with molecular targeted therapy. J Natl Compr Canc Netw 9(suppl 1):S1–29
Kelly RJ, Billemont B, Rixe O (2009) Renal toxicity of targeted therapies. Target Oncol 4(2):121–33. doi:10.1007/s11523-009-0109-x
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S et al (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27(22):3584–90. doi:10.1200/JCO.2008.20.1293
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE et al (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378(9807):1931–9. doi:10.1016/S0140-6736(11)61613-9
Izzedine H, Etienne-Grimaldi MC, Renée N, Vignot S, Milano G (2009) Pharmacokinetics of sunitinib in hemodialysis. Ann Oncol 20(1):190–2. doi:10.1093/annonc/mdn626
Park S, Lee J, Park SH, Park JO, Kang WK, Park YS et al (2010) Treatment of hemodialyzed patients with sunitinib in renal cell carcinoma. Chemotherapy 56(6):485–91. doi:10.1159/000321033
Maroto Rey P, Villavicencio H (2008) Sorafenib: tolerance in patients on chronic hemodialysis. Oncology 74(3–4):245–6
López-Beltrán A, Scarpelli M, Montironi R, Kirkali Z (2006) 2004 WHO classification of the renal tumors of the adults. Eur Urol 49(5):798–805. doi:10.1016/j.eururo.2005.11.035
Dutcher JP, de Souza P, McDermott D, Figlin RA, Berkenblit A, Thiele A et al (2009) Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol 26(2):202–9. doi:10.1007/s12032-009-9177-0
Escudier BE, Bracarda S, Maroto-Rey JP, Szczylik C, Nathan PD, Negrier S et al (2014) Open-label, phase II raptor study of everolimus (EVE) for papillary mRCC: Efficacy in type 1 and type 2 histology. J Clin Oncol 32(suppl 4):410
Voss MH, Bastos DA, Karlo CA, Ajeti A, Hakimi AA, Feldman DR et al (2014) Treatment outcome with mTOR inhibitors for metastatic renal cell carcinoma with non-clear and sarcomatoid histologies. Ann Oncol 25(3):663–8. doi:10.1093/annonc/mdt578
Ravaud A, Oudard S, de Fromont M, Chevreau C, Gravis G, Zanetta S et al (2015) First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: a phase II study (SUPAP) by the French Genitourinary Group (GETUG) dagger. Ann Oncol. doi:10.1093/annonc/mdv149
Yildiz I, Ekenel M, Akman T, Kocar M, Uysal M, Kanitez M et al (2014) Sunitinib for patients with metastatic non-clear cell renal cell carcinoma: a multicenter retrospective turkish oncology group trial. Anticancer Res 34(8):4329–34
Motzer RJ, Barrios CH, Kim TM, Falcon S, Cosgriff T, Harker WG et al (2014) Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line Everolimus in patients with metastatic renal cell Carcinoma. J Clin Oncol. doi:10.1200/jco.2013.54.6911
Tannir NM, Jonasch E, Altinmakas E, Ng CS, Qiao W, Tamboli P et al (2014) Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (The ESPN Trial): A multicenter randomized phase 2 trial. J Clin Oncol 32(5s):4505
Armstrong AJ, Broderick S, Eisen T, Stadler WM RJJ, García JA et al (2015) Final clinical results of a randomized phase II international trial of everolimus vs. sunitinib in patients with metastatic non-clear cell renal cell carcinoma (ASPEN). J Clin Oncol 33(suppl):4507
McKay RR, Choueiri TK, Werner L, Atkins MB, Olivier KM, Song J et al (2015) A phase II trial of sunitinib and gemcitabine in sarcomatoid and/or poor-risk patients with metastatic renal cell carcinoma. J Clin Oncol 33(suppl 7):408
Tannir NM, Plimack E, Ng C, Tamboli P, Bekele NB, Xiao L et al (2012) A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol 62(6):1013–9. doi:10.1016/j.eururo.2012.06.043
Gibney GT, Aziz SA, Camp RL, Conrad P, Schwartz BE, Chen CR et al (2013) c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol 24(2):343–9. doi:10.1093/annonc/mds463
Acknowledgements
The authors thank Sofía Perea and Soledad Santos from Springer Healthcare Communications for their help in preparing the first draft of this manuscript. We thank Catherine Rees, who provided assistance with post-submission revisions on behalf of Springer Healthcare Communications; this assistance was funded by Pfizer. Pfizer Spain were given the opportunity to comment on the first draft of the manuscript, but all decisions about its content were made by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The scientific meetings and medical writing services were funded by Pfizer Spain.
Conflict of Interest
Javier Puente and Enrique Gallardo have received consulting fees or honoraria from Pfizer and Novartis. Enrique Gallardo and Cristina Suárez have received support from Pfizer for travel to meetings, manuscript preparation, or other purposes. Pablo Maroto has received fees for participation in advisory boards for Pfizer, Bayer, Glaxo, and Novartis. José Ángel Arranz has received fees for participating in advisory boards for Pfizer and Novartis, and has received support from Astellas for travel to meetings. Cristina Suárez has also received payment from Pfizer for writing or reviewing the manuscript and for lectures, and has been provided with writing assistance, medicines, equipment or administrative support; she has also received payment from Janssen for lectures. Xavier García del Muro, Álvaro Pinto, Nuria Láinez, Emilio Esteban, María José Méndez, and Enrique Grande declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Puente, J., García del Muro, X., Pinto, Á. et al. Expert Recommendations for First-Line Management of Metastatic Renal Cell Carcinoma in Special Subpopulations. Targ Oncol 11, 129–141 (2016). https://doi.org/10.1007/s11523-015-0408-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-015-0408-3