Abstract
The fish assemblage structure response to rapid degradation of Enhalus acoroides seagrass beds due to overgrazing by green sea turtles (Chelonia mydas) was investigated at Iriomote Island, southern Japan, by visual censusing of fish species in several microhabitats in and around the beds (i.e., dense seagrass bed, grazed bed, their boundaries, and adjacent sandy area). Fish assemblage structure differed among the seagrass microhabitats, both species and individual numbers being higher in microhabitats with seagrass compared to overgrazed beds and unvegetated sandy areas, together with different species composition. In the dense seagrass beds and boundary areas (the border area between dense seagrass beds and grazed areas), seagrass-associated fishes such as the rabbit fish Siganus fuscescens and cardinal fish Ostorhinchus ishigakiensis were abundant and comparable with those in other seagrass beds in the area. In addition, the fish assemblages in those microhabitats varied seasonally, fish abundance being greater in summer due to higher levels of recruitment. In the grazed bed, benthic gobies, such as Ctenogobiops crocineus and Cryptocentrus caeruleomaculatus, were dominant (similarly so in unvegetated sandy areas), and seasonal variations in such assemblages were relatively low compared to those in dense seagrass microhabitats. The decrease in the abundance and diversity of seagrass-associated fish in the grazed areas could be explained partly by the shortage of vegetation (< 20 cm in leaf length) for shelter and feeding throughout the year. The results suggested that the degradation of seagrass beds due to overgrazing by green turtles has a deleterious effect on fish assemblages, and that future conservation and management strategies for seagrass beds should consider both their associated fish communities and green turtles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seagrass beds typically support higher numbers of fish species and individuals than open sandy areas, serving as nursery habitats for many fish species, including several commercially important (Edger and Shaw 1995; Nagelkerken et al. 2000; Dorenbosch et al. 2004; Nakamura and Sano 2004b). Such increased diversity is considered the result of seagrass beds being rich in invertebrates and therefore good feeding grounds for fishes (Heck et al. 2003; Nakamura and Sano 2005). In addition, seagrass habitats provide juvenile fishes with shelter against predators (Heck et al. 2003; Nakamura and Sano 2004a). Consequently, seagrass beds are considered to be essential for maintaining high levels of biodiversity in coastal ecosystems and local fisheries.
However, seagrass beds are rapidly declining around the world due to factors such as anthropogenic impact and climate change (Orth et al. 2006; Waycott et al. 2009). The loss of such beds results in a decrease in nursery and feeding grounds of seagrass-associated fishes, typically resulting in a significant decrease in numbers of fish species and individuals (Hughes et al. 2002; Nakamura 2010). Furthermore, seagrass degradation may also result in a decrease in the fish biodiversity of surrounding ecosystems, such as coral reefs, because some of them utilize seagrass beds as nurseries (Nagelkerken et al. 2002).
Recently, extensive seagrass degradation has become apparent around the islands of the Yaeyama archipelago in the Ryukyu Islands, southern Japan (Takeyama et al. 2014). Tape seagrass Enhalus acoroides, widely distributed throughout Indo-Pacific subtropical and tropical regions (Green and Short 2003), is widespread around the Yaeyama archipelago, the northern limit of its distribution (Toma 1999). However, a rapid decline in tape seagrass beds has been observed around Iriomote Island since 2013 (Takeyama et al. 2014). One of the most likely reasons for the observed decline has been excessive feeding by the green sea turtle Chelonia mydas. Although green turtles have been severely overhunted in the past, their populations have recovered rapidly in some regions (Chaloupka et al. 2008). However, an overabundance of green turtles, being primarily herbivorous and consuming large quantities of seagrass (Thayer et al. 1984), sometimes results in severe seagrass degradation (Fourqurean et al. 2010; Christianen et al. 2014). After a decline in local green turtle harvesting in the Yaeyama archipelago, which includes many green turtle spawning sites (Abe et al. 2004), the turtle population has increased (Kameda et al. 2017), resulting in severe overgrazing of the dense seagrass beds in Funauki and Amitori Bays on Iriomote Island, and their almost complete loss in 2013 (Takeyama et al. 2014).
Previous studies have shown that numerous fish species (including scarids, mullids, gobiids and labrids) utilize the seagrass habitats around the archipelago, the latter two families being dominant in both species and individual numbers (Nakamura and Sano 2004b). In addition, the juveniles of some coral reef fishes, such as the five-lined cardinalfish Cheilodipterus quinquelineatus, pacific yellowtail emperor Lethrinus atkinsoni and thumbprint emperor Lethrinus harak, all use seagrass beds for protection (Nakamura et al. 2012). Consequently, the fragmentation and degradation of seagrass habitats may lead to a decrease in formerly numerous fish species, as many depend on seagrass to some degree. However, relatively few studies have examined the response of fish assemblages to the decrease and degradation of seagrass beds, although the effects on fishes of complete loss of seagrass beds have been reported previously (Nakamura 2010). When green turtles graze exclusively on seagrass beds, the change in the habitat structure conferred by seagrass shoots is considerable (Lal et al. 2010; Kelkar et al. 2013), which may negatively impact seagrass fishes. However, such habitat fragmentation may also provide a variety of microhabitats of differing structural complexity, e.g., seagrass-remaining areas, grazed beds, and their boundaries. The responses of fishes to seagrass bed fragmentation are often complex and species specific, with macro-habitat use patterns and dependence on seagrass beds differing among fish species (Horinouchi 2007). Accordingly, the effects on fish assemblages of seagrass degradation by turtle grazing should be assessed by comparing fish distribution among grazed/unglazed seagrass microhabitats, so as to establish effective conservation and management strategies, which contribute to the high biodiversity of coastal ecosystems and local fisheries.
In the present study, we investigated the fish assemblage structure (i.e., numbers of species, individuals and species composition) in E. acoroides seagrass beds, which have been suffering fragmentation and decline due to overgrazing by green turtles, in northwestern Iriomote Island, Japan. Specifically, we compared fish assemblages among several microhabitats (dense seagrass beds, grazed beds, their boundaries and sandy areas) found in and around fragmented seagrass habitats, to clarify the response of each fish species to the grazing of seagrass by green turtles.
Materials and methods
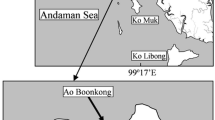
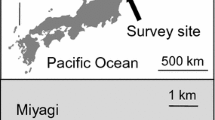
Study site. The study was conducted in Enhalus acoroides seagrass beds at Hoshidate, northwestern Iriomote Island, Ryukyu Islands, Japan. The Yonada River drains into the inner area of the bay, which measures approximately 0.9 km across and opens to coral reefs offshore (Fig. 1). Ungrazed seagrass beds (> 50 cm in leaf length) concentrated near the center of the bay covered a total area of ca. 10 ha in 2013, a 25% reduction more or less of the area covered in 1974 (Takeyama et al. 2014). One of the reasons for the reduction in the area of the beds is considered to be overgrazing by green turtles, since most leaves on the outer edges of the beds were extremely short (< 20 cm in leaf length) with diagonally cut tips, characteristic of turtle grazing.
Map of the study site at Iriomote Island, Ryukyu Islands, Japan. a Dense seagrass bed, b boundary area, c grazed bed, d sandy area. Hatched area indicates seagrass meadow consisting predominantly of Enhalus acoroides (Takeyama et al. 2014)
Four types of microhabitats within the present seagrass beds were identified: (1) areas of flourishing E. acoroides growth (hereafter referred to as “dense seagrass beds”), (2) areas where evidence of grazing by green turtles was clearly apparent (hereafter referred to as “grazed beds”), (3) boundary areas between dense seagrass beds and grazed beds (“boundary areas”), and (4) areas of sand flats near the seagrass beds ( “sandy areas”) (Figs. 1, 2). Each microhabitat occurred in depths between 1.5 m and 2.0 m at high tide. The following surveys were conducted in each microhabitat at monthly intervals from April 2017 to March 2018.
Physical parameters. Water temperature was measured at 10 min intervals using a small data logger (HOBO U20-001-04-TI, Onset Ltd., America) in dense seagrass beds (N24°23′46.40′′, E123°45′06.50′′) from May 2017 to March 2018. The logger was attached to a rope and positioned 10 cm above the substrate using a buoy. Monthly mean values were calculated from the data to show seasonal variations in water temperature. Shoot densities of E. acroides were determined by counting all of the shoots that had become established within a 1 × 1 m quadrat in each microhabitat in October 2017 (except the sandy area) (a total of five replicates separated from each other by at least 10 m). Leaf lengths were determined from five shoots randomly selected from each quadrat randomly established in each microhabitat at monthly intervals from May 2017 to March 2018, the longest leaf being measured to the nearest 1 cm. In the boundary areas, quadrats were established around the outer edge of the seagrass beds, a total of five shoots being selected randomly from inside and outside the seagrass outer edge.
Sampling design. The fish assemblage structures were determined from visual censuses conducted in each microhabitat at monthly intervals from April 2017 to March 2018. For each census, five 20 × 2 m linear transects were randomly positioned (parallel to the shore and separated from each other by at least 10 m). All of the fishes observed within the transects over a 20 min period (i.e., 1.0 m on either side of the center line transect) by an observer using goggles and a snorkel were counted. In the boundary areas, transects were established along the outer edge of the densely vegetated beds, fishes occurring within 1.0 m on either side of the seagrass edge being recorded. To reduce the possibility of a double count, we made an effort to maintain a swimming speed of about 1.0 m−1, with a balance between swimming slow enough to identify fish species and record fish numbers and fast enough to reduce the possibility of a double count of the same fish, following to the previous study (Horinouchi et al. 2005). Each census was conducted at high tide between 09:00 and 17:00. Individual fishes were identified to species level following Okamura and Amaoka (1997), Seno et al. (2004) and Nakabo (2013).
Fish species were classified into the following types by observation of their swimming, hovering, or resting behavior, following Park and Kwak (2018): (1) pelagic, fish species that swim over the seagrasses; (2) semi-benthic, fish species that swim and hover between the seagrass plants; (3) benthic, fish species that rest on the vegetated and unvegetated substrata.
Data analysis. During the study period, water temperature in the seagrass bed was lowest in February and highest in August (Fig. 3). Given the water temperature patterns at the study site, data were pooled as follows for each season: winter, January–March (21.5–23.6 °C); spring, April–June (27.3–29.3 °C); summer, July–September (30.2–30.6 °C); autumn, October–December (22.8–28.3 °C).
The mean numbers of fish species and individuals (/40 m2) were compared among microhabitats and seasons using a two-way ANOVA. When significant differences were observed (p < 0.05), post hoc Tukey–Kramer tests were applied. Because the first-order interactions were frequently significant, one-way ANOVAs and Tukey–Kramer tests were performed to compare the values among microhabitats in each season, and among seasons in each microhabitat. The same analyses were performed to analyze leaf length, the species and individual numbers of pelagic, semi-benthic and benthic fishes. Mean shoot densities were compared among microhabitats using a one-way ANOVA.
The degree of similarity in fish assemblages between each microhabitat and each season was estimated using the Bray–Curtis similarity coefficient, based on the number of individuals of each species. The resultant similarity matrix was subjected to cluster analysis using the group-average method. Species contributing to significant variation between the groups were identified using the SIMPER (similarity percentage) subroutine. All data were [log(x + 1)] transformed prior to analyses. Large shoals of Spratelloides delicatulus were exclude from the above analyses, the occasional occurrence of the species in seagrass habitats likely distorting the results.
Results
The mean shoot density of Enhalus acoroides was 174.6 ± 12.6 shoots/m2 (dense seagrass beds), 110.2 ± 11.1 shoots/m2 (boundary areas), and 96.4 ± 14.6 shoots/m2 (grazed beds), being significantly higher in the dense seagrass beds than in the boundary areas and grazed beds (Table 1).
Two-way ANOVAs revealed significant differences in the mean leaf length of E. acorides among microhabitats and seasons, with an interaction among the latter (Table 1; Fig. 4). The mean leaf length in the dense seagrass beds was significantly greater than those in the boundary areas and grazed beds in all seasons. Additionally, in all microhabitats, except the sandy areas, mean leaf length was significantly greater in spring and summer compared with winter.
The fish surveys resulted in a total of 4,311 individuals, representing 17 families and 52 species, being observed over the course of the study period (Table 2). Of these, 2,639 individuals, representing 29 species (14 families) were observed in the dense seagrass beds, and 993 individuals, representing 31 species (16 families) in the boundary areas. A total of 412 individuals, representing 19 species (9 families), were observed in the grazed beds, and 267 individuals, representing 13 species (4 families), in the sandy areas.
The dominant species in the dense seagrass bed were Siganus fuscescens (a total of 1,428 individuals, 54.1% of total in the dense seagrass bed during the study period), Ostorhinchus ishigakiensis (500 individuals, 21.0%), Cheilodipterus quinquelineatus (173 individuals, 6.6%), Stethojulis strigiventer (167 individuals, 6.3%) and Lethrinus harak (111 individuals, 5.6%). In the boundary area, Si. fuscescens (247 individuals, 24.9% of total in the boundary area), Ch. quinquelineatus (174 individuals, 17.5%), L. harak (154 individuals, 16.1%), Cryptocentrus caeruleomaculatus (84 individuals, 8.5%) and Cheilio inermis (80 individuals, 5.0%) were most abundant. The dominant species in the grazed bed were Ctenogobiops crocineus (178 individuals, 43.2% of total in the grazed bed), Cr. caeruleomaculatus (140 individuals, 34.0%), Ch. quinquelineatus (37 individuals, 9.0%), L. harak (14 individuals, 3.4%) and Amblygobius phalaena (11 individuals, 2.7%), whereas Oplopomus oplopomus (113 individuals, 42.3% of total in the sandy area), Cr. caeruleomaculatus (83 individuals, 31.1%), Cryptocentrus singapurensis (29 individuals, 10.9%), Ct. crocineus (10 individuals, 3.7%) and Valenciennea longipinnis (8 individuals, 2.6%) were most abundant in the sandy area.
The mean numbers of both species and individuals differed significantly among microhabitats and seasons, an interaction among microhabitats and seasons also being evident (Table 3; Fig. 5). In most (all) seasons, the mean number of species (and individuals) was significantly higher in the dense seagrass beds than in the grazed beds and sandy areas, as well as highest in summer in each microhabitat.
Table 1 Results of a one-way ANOVA comparing differences in shoot density among microhabitats, and a two-way ANOVA comparing differences in leaf length of Enhalus acoroides among microhabitats and seasons. Mean numbers (and standard deviation) of (a) species and (b) individuals per transect (40 m2, n = 5) for each microhabitat use group in each microhabitat at Hoshidate from April 2017 to March 2018. Black bars indicate pelagic species, gray bars indicate semi-benthic species, and white bars indicate benthic species
The observed fishes included 3 pelagic (2 families), 26 semi-benthic (12 families) and 23 benthic (3 families) individuals (Table 2). The numbers of species and individuals of semi-benthic and benthic fishes, but not pelagic fishes, differed significantly among microhabitats and seasons (Tables 4 and 5; Fig. 5). The mean numbers of species and individuals of semi-benthic fishes were significantly greater in the dense seagrass beds and/or boundary areas compared to grazed beds and/or sandy areas, and in most microhabitats, higher in summer than in other seasons. The mean number of benthic fish species was highest in dense seagrass beds and that of individuals, greatest in boundary areas. Those of both species and individuals were highest in summer (Tables 4 and 5).
A cluster analysis showed that the fish assemblages in each microhabitat and season could be divided into three major groups with a similarity level of 30% (Fig. 6). The fish assemblages in the dense seagrass beds constituted a group excluding other microhabitats (Group II). Similarly, the assemblage in the boundary areas constituted their own group, although it included the dense seagrass bed in winter (Group I). However, the assemblages in the grazed bed and sandy area were grouped together with the boundary area in winter (Group III). In Group I, which consisted mainly of assemblages in the boundary areas from spring to autumn, the semi-benthic Si. fuscescens and L. harak, and the benthic Cr. caeruleomaculatus were dominant, contributing markedly to the observed similarity from the results of the SIMPER analysis (Table 6). Group II comprised mainly the dense seagrass assemblages from spring to autumn, the semi-benthic Si. fuscescens, L. harak, and O. ishigakiensis being dominant, with the former two contributing significantly to the observed similarity. Conversely, in Group III, which comprised mostly the assemblages in the grazed bed and sandy area, the benthic Cr. caeruleomaculatus, Ct. crocineus and O. oplopomus were dominant, all contributing to the high level of similarity observed.
Discussion
The present study revealed that the fish assemblage structures differed significantly among microhabitats within seagrass beds degraded by turtle grazing, the numbers of species and individuals being greater (and having different species composition) in the seagrass-remaining microhabitat compared to those in the grazed beds and sandy areas. As found in previous studies, common species, such as Siganus fuscescens, Ostorhinchus ishigakiensis and Cheilodipterus quinquelineatus, were abundant in dense seagrass beds and boundary areas (Nakamura and Tsuchiya 2008; Nakamura 2010). Conversely, like sandy area assemblages, which are characterized by relatively low species richness and abundance, the grazed beds supported less diverse fish assemblages (comprising mainly benthic species, such as the gobiids Cryptocentrus caeruleomaculatus and Ctenogobiops crocineus).
Seagrass beds subjected to partial disturbance/degradation typically have patches where the leaves are shorter and less dense, which results in the fragmentation of large contiguous beds into smaller patches. Interestingly, the heterogeneity of such fragmented seagrass beds, which can contain habitat edges and seagrass patches that differ with respect to canopy height, density, and area of exposed substrate, can sometimes support higher levels of species diversity compared to a single contiguous seagrass bed (Horinouchi et al. 2009). Horinouchi (2009) also demonstrated horizontal gradients in the fish assemblages found in and around seagrass beds with inside sand patches, the outer and inner boarder areas between the beds and sand areas sometimes having higher levels of species diversity and/or abundance than seagrass microhabitats, due to some group-forming goby juveniles, such as Chaenogobius gulosus, Pterogobius zonoleucus and Gymnogobius heptacanthus, favoring edge-type microhabitats. In the present study, however, such species did not occur in the boundary areas, semi-benthic seagrass-associated fishes (such as Si. fuscescens and O. ishigakiensis) instead being observed in both dense seagrass beds and boundary areas, occasionally moving in schools from the former to the latter, where their abundance was less. Contrary to this, the benthic goby Cr. caeruleomaculatus, also occurring in the dense seagrass and boundary areas, was more abundant in the latter. The distribution patterns of these fish types determined the boundary area assemblages, with similar or sometimes lower levels of species richness and abundance, and different species composition compared to those of the dense seagrass bed. These findings suggest that fish assemblages can change in a seagrass microhabitat when about half of the area is grazed (i.e., the boundary area, containing both seagrass-remaining and grazed areas). In addition, the fish assemblages in such microhabitats varies seasonally, with fish abundance increasing in the summer due to high levels of fish recruitment. For example, juvenile Si. fuscescens and O. ishigakiensis were observed in June to September in seagrass bed microhabitat during this study, corroborating previous studies conducted in other seagrass beds (Nakamura et al. 2004b; Nakamura and Tsuchiya 2008).
On the other hand, the fish assemblages in the areas of completely grazed seagrass differed significantly to that in dense seagrass beds, with lower fish species richness and abundance. The grazed bed assemblages were dominated mostly by benthic gobies, such as Cr. caeruleomaculatus and Ct. crocineus, similar to the assemblages associated with the sandy areas. Moreover, seasonal variations in the grazed bed assemblage were relatively small as no summer recruitment of seagrass-associated fish was found. The decline in species and individual numbers of seagrass-associated fishes in the grazed bed may be attributed to decreases in the canopy height (< 20 cm) and shoot density compared to those in dense seagrass beds, since a reduction of seagrass structure typically has an adverse effect on seagrass fishes (Hughes et al. 2002). Gullstorm et al. (2008) reported that seagrass coverage and canopy height have a marked effect on the species richness of juveniles in seagrass beds. One of the primary factors responsible for the reduced fish species diversity in grazed beds may be a reduction in the shelter provided by seagrasses. Nakamura and Sano (2004a), who undertook tethering experiments to evaluate the predation risk for two juvenile fish species, demonstrated that the presence of seagrass cover had a positive effect on the survival of juvenile Stethojulis strigiventer and O. ishigakiensis. For fishes that depend on seagrass for protection from predators, the grazed areas, with their extremely low canopies and densities, may be unsuitable habitats. However, some benthic gobies, such as Cr. caeruleomaculatus and Ct. crocineus, were abundant in grazed areas. These bottom-dwelling gobies may not necessarily utilize seagrass beds for shelter, due to their adaptation to unvegetated areas where they often utilize the burrows of alpheid shrimps for protection. Similar significant changes in fish assemblages have been observed in areas around Iriomote Island, where seagrass beds have been completely lost (Nakamura 2010). The overall findings suggest, therefore, that declining seagrass areas due to turtle grazing negatively impact seagrass fish assemblages, to a similar extent as complete seagrass loss, even though low shoot height/densities remain in the grazed areas.
In addition to the sheltering effects of seagrass meadows, food availability can influence fish distribution patterns. The density of invertebrates, such as gammaridean amphipods and harpacticoid copepods, is higher in seagrass beds than on unvegetated substrata (Ansari et al. 1991; Nakamura and Sano 2005), such small crustaceans being important food items for seagrass fishes (Nakamura et al. 2003). Similar prey organisms associated with seagrass may decrease in grazed beds, leading to a decrease in seagrass fishes. However, data on prey abundance were not obtained during the present study, and further study is awaited.
The present study revealed that the degradation of seagrass beds due to overgrazing by green turtles had a negative effect on seagrass fish assemblages, with seagrass-associated fish species in the newly grazed beds declining and being replaced by bottom-dwelling gobies. In the event that such degradation of seagrass habitat progresses further in the Yaeyama archipelago, most of the seagrass-associated fish species are likely to be significantly reduced or lost, with a shift in the fish assemblage structure toward that encountered in areas with sandy/muddy substrates. Importantly, habitat alteration may lead to declines in the overall fish diversity and fisheries resources in areas where green turtles have become abundant. Accordingly, monitoring of areas of seagrass beds as well as the number of green turtles, a previously protected species, should be continued to design and optimize local fisheries management strategies and conservation policies for the region.
References
Abe O, Shibuno T, Takada Y, Hashimoto K, Tanizaki S, Ishii H, Funakura Y, Sano K, Okamura Y (2004) Nesting populations of sea turtle in Ishigaki Island, Okinawa. Proc 4th SEASTAR2000 Workshop 2004:40–43
Ansari ZA, Rivonker CU, Ramani P, Parulekar AH (1991) Seagrass habitat complexity and macroinvertebrate abundance in Lakshadweep coral reef lagoons, Arabian Sea. Coral Reefs 10:127–131
Chaloupka M, Bjorndal KA, Balazs GH, Bolten AB, Ehrhart LM, Limpus CJ, Suganuma H, Troëng S, Yamaguchi M (2008) Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Glob Ecol Biogeogr 17:297–304
Christianen MJA, Herman PMJ, Bouma TJ, Lamers LPM, Van Katwijk MM, van der Heide T, Mumby PJ, Silliman BR, Engelhard SL, van de Kerk M, Kiswara W, van de Koppel J (2014) Habitat collapse due to overgrazing threatens turtle conservation in marine protected areas. Proc R Soc B 281:20132890
Dorenbosch M, van Riel MC, Nagelkerken I, van der Velde G (2004) The relationship of reef fish densities to the proximity of mangrove and seagrass nurseries. Estuar Coast Shelf Sci 60:37–48
Edgar GJ, Shaw C (1995) The production and trophic ecology of shallow-water fish assemblages in southern Australia. I. Species richness, size–structure and production of fishes in Western Port Victoria. J Exp Mar Biol Ecol 194:53–81
Fourqurean JW, Manuel S, Coates KA, Kenworthy WJ, Smith SR (2010) Effects of excluding sea turtle herbivores from a seagrass bed: overgrazing may have led to loss of seagrass meadows in Bermuda. Mar Ecol Prog Ser 419:223–232
Green EP, Short FT (2003) World Atlas of Seagrasses. Prepared by the UNEP World Conservation Monitoring Centre. University of California, Berkeley
Gullstrom M, Bodin M, Nilsson PG, Ohman MC (2008) Seagrass structural complexity and landscape configuration as determinants of tropical fish assemblage composition. Mar Ecol Prog Ser 363:241–255
Heck KL Jr, Hays G, Orth RJ (2003) Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar Ecol Prog Ser 253:123–136
Horinouchi M (2007) Review of the effects of within-patch scale structural complexity on seagrass fishes. J Exp Mar Biol Ecol 350:111–129
Horinouchi M (2009) Horizontal gradient in fish assemblage structures in and around a seagrass habitat: some implications for seagrass habitat conservation. Ichthyol Res 56:109–125
Horinouchi M, Nakamura Y, Sano M (2005) Comparative analysis of visual censuses using different width strip-transects for a fish assemblage in a seagrass bed. Estuar Coast Shelf Sci 65: 53–60
Horinouchi M, Tongnunui P, Nanjo K, Nakamura Y, Sano M, Ogawa H (2009) Differences in fish assemblage structures between fragmented and continuous seagrass beds in Trang, southern Thailand. Fish Sci 75:1409–1416
Hughes JE, Deegan LA, Wyda JC, Weaver MJ, Wright A (2002) The effects of eelgrass habitat loss on estuarine fish communities of southern new England. Estuaries 25:235–249
Kameda K, Wakatsuki M, Kuroyanagi K, Iwase F, Shima T, Kondo T, Asai Y, Kotera Y, Takase M, Kamezaki N (2017) Change in population structure, growth and mortality rate of juvenile green turtle (Chelonia mydas) after the decline of the sea turtle fishery in Yaeyama Islands, Ryukyu Archipelago. Mar Biol 164:164–143
Kelkar N, Arthur R, Marba N, Alcoverro T (2013) Green turtle herbivory dominates the fate of seagrass primary production in the Lakshadweep islands (Indian Ocean). Mar Ecol Prog Ser 485:235–243
Lal A, Arthur R, Marbà N, Lill AWT, Alcoverro T (2010) Implications of conserving an ecosystem modifier: Increasing green turtle (Chelonia mydas) densities substantially alters seagrass meadows. Biol Conserv, 143:2730–2738
Nagelkerken I, Roberts CM, van der Velde G, Dorenbosch M, van Riel MC, Cocheret de la Morinière E, Nienhuis PH (2002) How important are mangroves and seagrass beds for coral-reef fish? The nursery hypothesis tested on an island scale. Mar Ecol Prog Ser 244:299–305
Nagelkerken I, van der Velde G, Gorissen MW, Meijer GJ, van’t Hof T, den Hartog C (2000) Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar Coast Shelf Sci 51:31–44
Nakabo T (ed) (2013) Fishes of Japan with pictorial keys to the species, third edition. Tokai University press, Hadano
Nakamura Y (2010) Patterns in fish response to seagrass bed loss at the southern Ryukyu Islands, Japan. Mar Biol 157:2397–2406
Nakamura Y, Hirota K, Shibuno T, Watanabe Y (2012) Variability in nursery function of tropical seagrass beds during fish ontogeny: timing of ontogenetic habitat shift. Mar Biol 159:1305–1315
Nakamura Y, Horinouchi M, Nakai T, Sano M (2003) Food habits of fishes in a seagrass bed on a fringing coral reef at Iriomote Island, southern Japan. Ichthyol Res 50:15–22
Nakamura Y, Sano M (2004a) Is there really lower predation risk for juvenile fishes in a seagrass bed compared with an adjacent coral area? Bull Mar Sci 74:477–482
Nakamura Y, Sano M (2004b) Overlaps in habitat use of fishes between a seagrass bed and adjacent coral and sand areas at Amitori Bay, Iriomote Island, Japan: Importance of the seagrass bed as juvenile habitat. Fish Sci 70:788–803
Nakamura Y, Sano M (2005) Comparison of invertebrate abundance in a seagrass bed and adjacent coral and sand areas at Amitori Bay, Iriomote Island, Japan. Fish Sci 71:543–550
Nakamura Y, Tsuchiya M (2008) Spatial and temporal patterns of seagrass habitat use by fishes at the Ryukyu Islands, Japan. Estuar Coast Shelf Sci 76:345–356
Okamura O, Amaoka K (1997) Sea Fishes of Japan. Yama-kei, Tokyo
Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL Jr, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S, Short FT, Waycott M, Williams SL (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996
Park JM, Kwak SN (2018) Seagrass fish assemblages in the Namhae Island, Korea: The influences of seagrass vegetation and biomass. J Sea Res 139:41–49
Senou H, Suzuki T, Shibukawa K, Yano K (2004) A photographic guide to the gobioid fishes of Japan. Heibonsha, Tokyo
Takeyama K, Kohno H, Kuramochi T, Iwasaki A, Murakami T, Kimura K, Ukai A, Nakase K (2014) Distribution and growth condition of Enhalus Acroides in Iriomote Island. Jour Japan Civil Engine Ser B3 70 (2):I_1068–I_1073
Thayer GW, Bjorndal KA, Ogden JC, Williams SL, Zieman JC (1984) Role of larger herbivores in seagrass communities. Estuaries 7:351–376
Toma T (1999) Seagrass from the Ryukyu Islands - I, Species and distribution. Biol Mag Okinawa 37:75–92
Waycott, M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Short FT, Williams SL (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Nat Acad Sci USA 106:12377–12381
Acknowledgments
We are grateful to K. Sakihara for assistance with fieldwork. Constructive comments on the manuscript from Graham Hardy and two anonymous reviewers were much appreciated. This study was supported by a grant for Study and Education from the Okinawa Regional Research Center, Tokai University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Inoue, H., Mizutani, A., Nanjo, K. et al. Fish assemblage structure response to seagrass bed degradation due to overgrazing by the green sea turtle Chelonia mydas at Iriomote Island, southern Japan. Ichthyol Res 68, 111–125 (2021). https://doi.org/10.1007/s10228-020-00775-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-020-00775-1