Abstract
The present investigation aims to study the zooplankton composition, diversity along with physicochemical profile in a chosen pond at Medak district from December, 2010 to November, 2012. The study revealed the occurrence of 80 zooplankton species including 60 rotifers, 18 cladocerans and 02 copepods. Zooplankton density fluctuated between 119 and 26,463/L, diversity H′ = 0.89–2.68, species richness 5–21 and dominance 18.6–74.1 % over the 2 years study period. Rotifers were more predominant than other zooplankton communities, especially family Brachionidae and Lecanidae. High density of the overall zooplankton community was due to more rotifer population and the numerical dominance of the species Brachionus angularis, B. calyciflorus, B. caudatus, Keratella tropica, Filinia terminalis and Epiphanies mucronata. It was observed that the zooplankton density significantly correlates with pH values of the pond. Physicochemical profile of the pond shows tropical climate, hard water and alkaline in nature. Chloride content was found to be high may be due to the anthropogenic pressure and influx of sewage. The high content of phosphate and nitrate reveals that the pond is enriched with nutrients. This has significant correlation with zooplankton dominance. The present findings clearly indicates the eutrophication of the pond.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Smaller water bodies like ponds which are an important component of the landscape, are seriously threatened by climate change, eutrophication and their combined effects (Moss et al. 2011). It is often neglected and not sufficiently studied (Cereghino et al. 2008). In terms of services, ponds offer sustainable solutions to key issues of water management and climate change such as nutrient retention, rainfall interception, or carbon sequestration (Cereghino et al. 2013). Ponds are biodiversity hotspots both in terms of species composition and biological traits, and have a significant role to play in the provision of ecosystem services (EPCN 2008). It constitutes an important component of urban and rural landscapes. They provide ecosystem services including habitats for wildlife, livestock, fish production and recreational amenities (Jeffries 2005). Ponds are subjected to anthropogenic pressure even though these ecosystems have obvious ecological functions (Hansson et al. 2005) and recognized as social and economic uses (Chapman et al. 2001). In light of recent economic development, a major challenge is to understand the turnover of pond communities in relation to changes in local–regional environments and pond habitat characteristics (EPCN 2008).
Zooplankton has a fundamental role in energy flow and nutrient cycling in aquatic ecosystem.Its fast growth rates can provide meaningful and quantifiable indicators of ecological change in short as well as long timescales (Schindler 1987; Paerl et al. 2003). Therefore zooplankton community can help to understand the shifts in the trophic status, environmental changes and water quality of an aquatic ecosystem (Blancher 1984; Pinto-Coelho et al. 2005; Ferdous and Muktadir 2009). This has been recommended as regional bioindicators of eutrophication. Also, there is an increasing demand by environmental monitoring programs for bioindicators of water quality (Andronikova 1996; Sousa et al. 2008). Zooplankton communities in small ponds are subjected to extreme fluctuations, the causes of which are not adequately understood (Patil and Gouder 1985). The abundance, diversity of zooplankton community is usually considered to be good indicator of environmental changes and largely regulated by the resource base and tend to increase with the trophic status (Canfield and Jones 1996; Sharma et al. 2008). Very few reports are available on variation of zooplankton community in the aquatic ecosystems of Telangana (Seenayya 1971; Rao 1972; Ahsan 1982; Reddy 1984; Chandrasekhar and Kodarkar 1994, 1995, 2008; Chandrasekhar 2004, 2007; Ranjan and Reedy 2007). Zooplankton community structure is shaped primarily by the physical and chemical environment (Blancher 1984).
Bandam Kommu pond lies on the Deccan plateau of the Indian subcontinent, Medak district, Telangana, India. Recent rapid urbanisation and industrial development are degrading and depleting the water quality and aquatic habitats. This pond is situated very close to the urban city of Hyderabad and thus subjected to severe anthropogenic and industrial pressure. The purpose of the investigation was to evaluate the species composition and diversity of zooplankton communities inhabiting the littoral zones in relation to physicochemical profile of the pond.
Materials and Methods
Study Area
Bandam Kommu pond is located at Medak district, Telangana, India (17°28′47″N and 78°47′36″E). The area is about 2 km2and shallow in nature. Littoral margin of the pond is covered with abundant macrophytic vegetations like water lily—Nymphaea alba, Amphibious amphibium—Polygonum amphibium, Bulrush—Typha latifolia, Red weed—Polygonum persicaria, water fern—Azolla filiculoides, Rigid horn wort—Ceratophyllum demersum, Duck weed—Lemna minor and Rootless duck weed—Wolfo iaarrhiza. The study was carried out on monthly basis from December 2010 to November 2012 in the pond.
Zooplankton Collection and Enumeration
Both qualitative and quantitative collections were made from littoral surface of the water column at different stations. Qualitative collections were done by towing surface water column, quantitative samples were collected by filtering 50 L of water through zooplankton net made of bolting silk (No. 25), 62 µm mesh size. The samples were transferred to clean plastic containers of 100 ml capacity and preserved in 4 % neutralized formaldehyde solution and containers were labelled. Identification of zooplankton species was done by using regional level literature (Michael and Sharma 1988; Sharma 1992; Ranga Reddy 1994; Segers 1995; Dhanapathi 2000; Sharma and Sharma 2008) under light microscope (Carl Zeiss 10 × 25x). Sedgwick-Rafter cell method was applied to estimate zooplankton quanititatively and the results were expressed in Ind. L−1(Welch 1948).
Physicochemical Parameters
The ambient and subsurface water temperature, electrical conductivity, pH, total dissolved solids were recorded in the field with the help of digital electronic testers (Orlab). Water samples were collected in clean plastic containers (1 L) and brought to the laboratory for chemical analysis. Dissolved oxygen content was estimated through Winkler’s method. Total hardness, total alkalinity, calcium, chloride, phosphate, nitrate and nitrite were analysed by using Orlab water quality kits prepared by following standard methods (APHA 1985).
Statistical Analysis
Standard statistical methods like Shannon diversity index H′ for species diversity; hill numbers index for species richness and abundance; Pielou index for evenness; Berger–Parker dominance index for dominance were applied (Hayek and Buzas 1997) and their working equations by using Biodiversity pro software. Principal component analysis was made by using Jolliffe (2002) method by using XLSTAT software.
where Pi = proportion of the number of individuals of species to the total number of individuals (Pi = ni/N) n = total number of species, N = total number of individuals
Hmax′ = is the Shannon maximum diversity index, S = the total number of species in the sample. Hill Numbers, H0 = S (species richness), H1 = exp H′ exponential of Shannon diversity Indices (abundance) Berger–Parker Dominance index d = Nmax/N Nmax = the number of individuals in the most abundant species, N = the total number of individuals in the sample.
Results
Composition
Eighty species of zooplankton comprising 60 species of rotifers, 18 species of cladocerans and two species of copepods were recorded in Bandam Kommu pond (Table 1). It is found that the rotifers are the most dominant component in the zooplankton community components, especially genus Lecane and Brachionus were the most common elements. In Cladocera, families Chydoridae and Daphniidae have more number of species. Copepoda had only two species which belongs to Diaptomidae and Cyclopoidae. Brachionus forficula, Trichotria tetractis, Macrochaetus sericus, Lecane hornemanni, L. simonneae, L. pyriformis, Scaridium sp., Conochilus sp., Hexarthra sp., Testudinella patina, Daphnia lumholtzi, Pseudochydorus globosus occurred only in the samples collected during 2010–2011, and species like Epiphanies clavulata, Brachionus bidentata,B. plicatilis, Lecane arcula, Karualona karua and Kurzia longirostris are recorded during 2011–2012.
Density
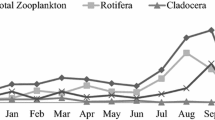
The overall zooplankton density varied between 119 and 2646/L throughout the study period (Table 2). High density was mainly because of rotifer population in June 2011(26,463/L) and 2012 (4354/L) and October 2012 (1534/L). The cladocerans fluctuated between 34 and 1088/L, high in May 2012 (1003/L) and October 2012 (1088/L). Similarly, copepod ranges between 1 and 1072/L. The high density of copepods was recorded in May, 2012 (1072/L), due to abundance of Mesocyclops leuckarti (Figs. 1and 2). The correlation analysis between physicochemical and biological parameters (Table 4) reveals that the pH and phosphate moderately correlates with overall zooplankton (r = 0.5049 and 0.4428) and rotifer density (r = 0.4894 and 0.4576). Total zooplankton density significantly correlates with rotifer density (r = 0.995) and cladocerans had moderate correlation with copepod and overall species richness (r = 0.587 and 0.499 respectively).
Diversity
During the study period, it is found that zooplankton diversity varied between H′ = 0.893–2.683 (Table 2), the diversity was more in 2011–2012 (1.9 ± 0.3) than 2010–2011 (1.6 ± 0.5). The high diversity was in November, December, 2011 and October, 2012; less in September, 2011 (Fig. 3). It had significant correlation with abundance (r = 0.914), species richness (r = 0.779) and evenness (r = 0.660) (Table 4). Evenness varied between J′ = 0.47–0.961 (Table 2), less in December, 2010 and September, 2011 and high in February and March, 2011 (Fig. 4). This had moderate correlation with abundance of zooplankton (r = 0.503). The richness of the species varied between 5 and 21 (Table 2), high number of 21 species recorded in November, 2011 and 20 species in October, 2012 (Fig. 5). Similarly the abundance ranged between 11.4–69, high in November, 2011 and October, 2012, which was 69.2 and 55.2 % respectively (Fig. 6). Dominance of zooplankton of the pond varied between 16.3–80.1 % and the values are reciprocal to the abundance (Fig. 7). Nitrate, nitrite and ammonia contents correlated with dominance (r = 0.5385, 0.3792, 0.4415 respectively). The present investigation over 2 years showed that the high density, less diversity, low species richness, less abundance and more dominance during 2010–2011. The diversity, evenness and abundance decreased, whereas the dominance increased.
Physicochemical Parameters
The physicochemical profile of the pond was given in Table 3. The atmospheric temperature ranges from 25 to 33 °C and surface water temperature varied between 19 and 25 °C (Fig. 8). Significant correlation between atmospheric and surface water temperature (r = 0.7930) was observed. The pH of the pond ranges between 7.5 and 8.9 (Fig. 9). Electrical conductivity was 0.9–4.8 mS (Fig. 10), this had significant correlation with total dissolved solids (r = 0.9000) and chloride (r = 0.9082), moderate correlation with total hardness (r = 0.6532) and magnesium (r = 0.6756). The dissolved oxygen content fluctuated between 0.8 and 12.35 mg/L, low in October, 2011 and high 12.35 mg/L in August, 2012 (Fig. 9). Dissolved oxygen has moderate correlation with phosphate content (r = 0.5557). Total dissolved solids ranged between 690 and 2000 ppm, which was less in the initial months and gradually increased to 2000 ppm from February to May, 2012 (Fig. 12). It has significant correlation with total hardness (r = 0.7949) and chloride (r = 0.9263). Total hardness ranged between 189 and 355 mg/L (Fig. 11) and significantly correlated with chloride (r = 0.7046) and Magnesium (r = 0.9816). Alkalinity ranges between 204 to 331 mg/L (Fig. 11). Similarly, the ionic contents such as chloride, calcium and magnesium ranged between 182–445, 28.4–47.4 and 34.7–77.5 mg/L respectively (Figs. 12 and 13). Nutrient content of the pond such as total phosphate, nitrate, nitrite and ammonia ranged between 0.1–0.4, 0.3–1.2, 1–40, and 1–3.7 mg/L respectively (Figs. 14, 15 and 16). Nitrate has significant correlation with nitrite (r = 0.7473). Principal component analysis (PAC) of bio-physicochemical parameters shows the variability 24.66 % (p value < 0.0005) and among the physiochemical parameters the variability was 37.16 % (p value < 0.05 %) and among biological parameters variability was 42.4 % (p value < 0.05). The scattered interrelationship and their correlation are given in Figs. 17, 18 and 19.
Discussion
The zooplankton composition of the Bandham Kommu pond was higher compared to the other ponds of this region (Ahsan 1982; Arshaduddin and Khan 1991; Karuthapandi et al. 2012). The study reveals that among the various zooplankton communities, rotifer component was more dominat, especially genus Brachionus and Lecane. The presence of Brachionus and Lecane is a general tropical character of the ponds (Sharma 1987, 1991, 1996; Segers 1996; Sharma and Naik 1996; Kiran et al. 2007). The family Chydoridae and Daphniidae of the Cladocera have more number of species than other families, whereas copepoda represented only two species Tropodiaptomus orientalis and Mesocyclops leuckarti. The species Tropodiaptomus orientalis is being reported for the first time from Telangana Table 4.
The high density of zooplankton was due to numerical abundance of rotifer population. This was due to the occurrence of Brachionus angularis, B. calyciflorus, B. caudatus, Keratella tropica, Filinia sp. and Epiphanies mucronata. Laal and Karthikeyan (1993) found that these species in clean and polluted waters; B. angularis, B. calyciflorus were abundant in high chloride waters. The pH values of the pond water are moderately correlated with zooplankton and rotifer density. Singh et al. (2002) reported that high rotifer population dominance was due to hypertrophical conditions of the pond and may be due to high temperature and low level of water. Several earlier studies noted that the genus Brachionus was characteristic of hard water. Brachionus calyciflorus, B. caudatus, Filinia sp. have been considered as indicators of eutrophicated ponds (Saksena 1987; Mudgal et al. 1989; Sharma and Dudani 1992). Numerical variations in rotifers may apparently be influenced by water quality (Kiran et al. 2007). The study found that the abundance of cladoceran was mainly because of Macrothrix spinosa, Karualona karua, Moino micrura. Whereas the copepod abundance due to Mesocyclops leuckarti. Goswami et al. (2007) also made similar observations from a pond at East Kolkata. Yadav et al. (2003) recorded high rotifer peak in summer which corroborates with the present study. Rotifers are one of the prominent groups among the zooplankton of any water body irrespective of its trophic status. This may be due to the less specialized feeding, parthenogenetic reproduction and high fecundity (Sampaio et al. 2002). Predominant species may have more functional importance than total species numbers in the zooplankton community (Ferrara et al. 2002).
The high diversity, species richness and abundance coincided with the months November, 2011 and October, 2012. The overall diversity of the present pond is less than two. It indicates the poor water quality (Bhat et al. 2014). An increase in dominance, decrease in diversity, species richness and abundance might be due to high variation in physicochemical features. The low abundance and diversity found in this study might be due to unfavourable conditions. Plafkin et al. (1989) reported that a community dominated by relatively fewer species indicates environmental stress. A scale of pollution in terms of species diversity shows Bandam Kommu pond is moderately polluted and rarely falls under heavy pollution (Staub et al. 1970; Mishra et al. 2010). The dominance of the zooplankton moderately correlating with nutrient contents of the Bandam Kommu pond was noticed. Sorf et al. (2015) reported that the zooplankton community respond to the combined effects of nutrients. Wani and Subla (1995) noted that the decrease in diversity and increase in density of rotifer may be attributed to high nutrient contents. This statement is true with the present findings. Chattopadhyay and Barik (2009) reported that rotifer population was more abundant than other net zooplankton groups, because of their ability to withstand and survive in varying limnological conditions prevailing in different seasons.
The physicochemical profile of the pond viz. alkalinity, pH, and high electrical conductivity during perishing of macrophytic vegetation, and receding of the water level. Mustapha and Omotosho (2002) reported the pH ranges of 6–9 supports large communities of organisms. Mozumder et al. (2014) observed the similar variation of pH in fish pond of Manikganj. Total dissolved solid was high may be due to more ionic contents, decreasing water level and presence of various pollutants in the pond. This was in decreasing trend when the water level increased during the monsoon. The dissolved oxygen widely fluctuating and was less in the second year of the study period. The presence of high dissolved oxygen content is an indication of healthy system in a water body (Basu et al. 2010). The low value of dissolved oxygen may be because of biological oxygen demands. Patil and Gouder (1985) reported that considerable reduction in dissolved oxygen concomitant with rise in conductivity and the drop in pH strongly suggesting the higher levels of dissolved salts and less photosynthetic activity. Total hardness, alkalinity is high due to variation in the ionic content and changes in the climatic condition. Zooplankton community increase with rise in alkalinity of water was noticed by Rajashekar et al. (2010). According to Yeole et al. (2008) the water bodies with alkalinity value above 100 mg/L were nutrient rich and rotifers utilize nutrients more rapidly to build their population. Similar observation was also made by Jeelani et al. (2005) in Dal lake, Kashmir. Similar wide variation of temperature, pH, total dissolved solids, dissolved oxygen and total alkalinity were observed in a freshwater pond in Calcutta by Dattta et al. (1987). Chloride content was high because of anthropogenic pressure and sewage from the nearby pharma and soft drinking industries. Similarly, Sharma and Dudani (1992) noted the high chloride content in a eutrophicated pond in Bihar due to pollution and sewage influx. Most of the rotifer species occuring in the pond indicates the alkaline pH, conductivity, alkalinity and chloride tolerant (Kuezynski 1987). The nutrient enrichment might be due to the high content of the phosphates and nitrates. Robin et al. (2014) reported that nutrient rich freshwater ecosystems were generally considered as having low ecological quality and less biodiversity. Mirza et al. (2014) noted that the high electrical conductivity, total dissolved solid, chloride and presence of nitrate, nitrite may be due to sewage and anthropogenic pressure. They also reported the low dissolved oxygen because of less solubility due to pollution.
The present study in the Bandam Kommu pond shows decreasing diversity index, high density with dominant species indicates the moderate pollution of the pond. This was evidenced with nutrient enrichments, low dissolved oxygen, more electrical conductivity and chloride contents. The high density and dominance of species like Brachionus angularis, B. calyciflorus, B. caudatus, Keratella tropica, Filinia terminalis and Epiphanies mucronata were indicators of polluted aquatic environment. The investigation recommends that the zooplankton composition, density and diversity along with the physicochemical features would be helpful tool for assessing the status of the water body.
References
Ahsan, M. 1982. Ecology of freshwater Zooplankton. Ph.D thesis, vi+150. Hyderabad: Osmania University.
Andronikova, I.N. 1996. Zooplankton characteristics in monitoring of lake Ladoga. Hydrobiologia 322: 173–177.
APHA, AWPC, WWCP. 1985. Standard methods for the examination of water and waste water, 16th ed, xlix+ 1268. Washington D.C: American Public Health Association.
Arshaduddin, M.D., and M.A. Khan. 1991. Rotifer Fauna of some seasonal ponds of Osmania University Campus, Hyderabad (AP), India. Indian Journal of Microbial Ecology 2: 29–40.
Basu, M., N. Roy, and A. Barik. 2010. Seasonal abundance of net zooplankton correlated with physico-chemical parameters in a freshwater ecosystem. International Journal of Lakes and Rivers 3(1): 67–77.
Bhat, N.A., A. Wanganeo, and R. Raina. 2014. The composition and diversity of net zooplankton species in a tropical water body (Bhoj Wetland) of Bhopal, India. International Journal of Biodiversity and Conservation 6(5): 373–381.
Blancher, C.E. 1984. Zooplankton-trophic relationship in some north and central Florida lakes. Hydrobiologia 109: 251–263.
Canfield, T.J., and J.R. Jones. 1996. Zooplankton abundance, biomass, and size-distribution in selected mid western water bodies and relation with trophic state. Journal of Freshwater Ecology 11: 171–181.
Cereghino, R., J. Biggs, B. Oertli, S. Declerck, and S. Declerck. 2008. The ecology of European ponds: Defining the characteristics of a neglected freshwater habitat. Hydrobiologia 597: 1–6.
Cereghino, R., D. Boix, H.M. Cauchie, K. Martens, and B. Oertli. 2013. The ecological role of ponds in a changing world. Hydrobiologia 723: 1–6.
Chandrasekhar, S.V.A. 2004. A study on the Cladoceran Fauna of Hyderabad and its environs, Andhra Pradesh. Records of the Zoological Survey of India 102(1–2): 155–167.
Chandrasekhar, S.V.A. 2007. The rotifer fauna of Himayatsagar and Osmansagar along with other rotifers known from Hyderabad and neighborhood, Andhra Pradesh, India. Records of the Zoological Survey of India 107(3): 105–108.
Chandrasekhar, S.V.A., and M.S. Kodarkar. 1994. Biodiversity of zooplankton from Saroornagar lake, Hyderabad, A.P. Journal of Aquatic Biology 9(1–2): 30–33.
Chandrasekhar, S.V.A., and M.S. Kodarkar. 1995. Studies on Brachionus from Saroornagar lake, Hyderabad. Journal of Aquatic Biology 10(1&2): 48–52.
Chandrasekhar, S.V.A., and M.S. Kodarkar. 2008. Progressive deterioration of water quality of Hussainsagar Lake, Hyderabad, Andhra Pradesh, and an Assessment of its Impact on Zooplankton community in the Lake ecosystem. Proceedings of Taal 2007: The 12th World Lake Conference. 2039–2043.
Chapman, L.J., J. Balirwa, F.W.B. Bugenyi, C. Chapman, and T.L. Crisman. 2001. Wetlands of East-Africa: biodiversity, exploitation and policy perspectives. In Biodiversity in Wetlands: Assessment function and conservation, vol. 2, ed. B. Gopal, W.J. Junk, and J.A. Davis, 101–131. Leiden: Backhuys Publishers.
Chattopadhyay, C., and A. Barik. 2009. The composition and diversity of net Zooplankton species in a tropical freshwater lake. International Journal of Lakes and Rivers 2(1): 21–30.
Datta, N.C., N. Mandal, and B.K. Bandyopadhyay. 1987. Seasonal abundance of rotifers in a perennial freshwater pond Calcutta. Journal of Environmental Biology 8(1): 63–71.
Dhanapathi, M.V.S.S.S. 2000. Taxonomic notes on the rotifers, vi+ 178. Hyderabad: Indian Association of Aquatic Biologists p.
EPCN. 2008. The Pond Manifesto. http://campus.hesge.ch/epcn/projects.asp.
Ferrara, O., D. Vagaggini, and F.G. Margaritora. 2002. Zooplankton abundance and diversity in Lake Bracciano, Latium, Italy. Journal of Limnology 61(2): 169–175.
Ferdous, Z., and A.K.M. Muktadir. 2009. A Review: potentiality of Zooplankton as bioindicator. American Journal of Applied Science 6(10): 1815–1819.
Goswami, R.A., R.U. Singha, A. Aich, B. Chattopadhyay, S. Datta, and S. K. Mukhopadhyay. 2007. Spatial heterogeneity of zooplankton community in relation of physicochemical factors thriving in ponds. Proceedings of Taal2007: The 12th World Lake conference. p. 221.
Hansson, L.A., C. Bronmark, P.A. Nilsson, and K. Abjornsson. 2005. Conflicting demands on wetland ecosystem services: Nutrient retention, biodiversity or both? Freshwater Biology 50: 705–714.
Hayek, L.C., and M.A. Buzas. 1997. Surveying natural populations, 1–563. New York: Columbia University Press.
Jeelani, M., H. Kaur, and S.G. Sarwar. 2005. Population dynamics of rotifers in the Anchar lake Kashmir (India). In Ecology of Plankton, ed. Arvind Kumar, 55–60. Delhi: Daya Publishing House.
Jeffries, M. 2005. Small ponds and big landscapes: The challenge of invertebrate spatial and temporal dynamics for European pond conservation. Aquatic Conservation 15: 541–547.
Jolliffe, I.T. 2002. Principal component analysis, 2nd ed. New York: Springer.
Karuthapandi, M., B. Xavier Innocent, and S.Z. Siddiqi. 2012. Zooplankton in a temporary freshwater pond habitat, in Attapur, Hyderabad Andhra Pradesh, India. International Journal of Advanced Life Sciences 1: 22–31.
Kiran, B.R., E.T. Puttaiah, and D. Kamath. 2007. Diversity and seasonal fluctuation of zooplankton in fish pond of Bhadra fish farm, Karnataka. Zoos’ Print Journal 22(12): 2935–2936.
Kuezynski, D. 1987. The rotifer fauna of Argentine Patagonia as a potential limnological indicator. Hydrobiologia 150: 3–10.
Laal, A.K., and M. Karthikeyan. 1993. Rotifers-pollution or productivity indicator? Current Science 65(1): 874–875.
Michael, R.G., and B.K. Sharma. 1988. Indian Cladocera (Crustacea: Brachiopoda: Cladocera), xvii+ 262. Calcutta: Zoological Survey of India.
Mirza, M.A., M.A. Choudhary, M.Y. Khuhawar, and M. Arain. 2014. Seasonal and environmental pollution impact on the quality of water of river Pooch near district Kotli, Pakistan. International Journal of Engineering and Technical Research 2(11): 367–372.
Mishra, A., S.K. Chakraborty, A.K. Jaiswar, A.P. Sharma, G. Deshmukhe, and M. Mohan. 2010. Plankton diversity in Dhaura and Baigul reservoirs of Uttarakhand. Indian Journal of Fisheries 57(3): 19–27.
Moss, B., S. Kosten, M. Meerhoff, R. Battarbee, E. Jeppesen, N. Mazzeo, K. Havens, G. Lacerot, Z. Liu, L. de Meester, H. Paerl, and M. Scheffer. 2011. Allied attack: climate change and eutrophication. Inland Waters 1: 101–105.
Mozumder, P. K., M. N. Naser and A. T. A. Ahmed. 2014. Abundance of zooplankton and physicochemical parameters of a ployculture fish pond of Manikganj, Bangladesh. Bangladesh Journal of Zoology 42(1): 67–76.
Mudgal, S., S.C. Arya, V. Vyas, and P.Shrivastava. 1989. Rotifer fauna of two Freshwater fish ponds in Bhopal. Environmental Strands of Bioscience: 223–226.
Mustapha, M.K., and J.S. Omotosho. 2002. An ecological study of a temporary pond, in Ilorin, Kwara State,Nigeria. Bioscience Research Communications 14(2): 165–174.
Paerl, H.W., J. Dyble, P.H. Moisander, R.T. Noble, M.F. Piehler, J.L. Pinckney, T.F. Steppe, L. Twomey, and L.M. Valdes. 2003. Microbial indicators of ecosystem change: current applications to Eutrophication studies. FEMS Microbiology Ecology 46: 233–240.
Patil, C.S., and B.Y.M. Gouder. 1985. Ecological study of freshwater Zooplankton of a subtropical pond (Karnataka State, India). International Review der gesamten Hydrobiologie und Hydrographie 70(2): 259–267.
Pinto-Coelho, R., A. Pinel, G. Methot, and K.E. Havens. 2005. Crustacean zooplankton in lake and reservoirs of temperate and tropical regions: variation with trophic status. Canadian Journal of Fisheries and Aquatic Sciences 62: 348–361.
Plafkin, J.L., M.T. Barber, K.D. Poter, S.K. Gross, and R.M. Highes. 1989. Rapid bioassessment protocol for use in streams and rivers for benthic macro invertebrates and fish, EPA/444/4-89/001. Washingaton DC: Office of Water Regulation and Standard U.S Environmental Protection Agency.
Rajashekar, M., K. Vijayakumar, and Z. Paerveen. 2010. Seasonal variation of zooplankton community in freshwater reservoir, Gulbarga district, Karnataka, South India. International Journal of Systems Biology 2(1): 6–11.
Ranjan, J and K. S. Reedy. 2007. Conservation of urban lakes in Hyderabad Development Area, Andhra Pradesh. Proceedings of Taal2007: The 12th World Lake conference. pp 266.
Ranga Reddy, Y. 1994. Copepoda: Calanoida: Diaptomidae. In Guides to the identification of microinvertebrates of the continental waters of the World, ed. H.J.F. Dumont, Viii+ 220. Netherlands: SPB Academic Publishing bv.
Rao, V.S. 1972. An ecological study of three freshwater ponds of Hyderabad, India II. The environment. Hydrobiologia 39(3): 351–372.
Reddy, S.N. 1984. The limnological study of the Himayatsagar and Hussainsagar Lake. Ph.D. thesis, Hyderabad: Osmania University.
Robin, J., A. Wezel, G. Borneette, F. Arthaud, S. Angelibert, V. Rosset, and B. Oertli. 2014. Biodiversity in eutrophicated shallow lakes: determination of tipping points and tools for monitoring. Hydrobiologia 723: 63–75.
Saksena, D.N. 1987. Rotifers as indicators of water quality. Acta of Hydrochemistry and Hydrobiology 15: 481–485.
Sampaio, E.V., O. Rocha, T. Matsumura-Tundisi, and J.G. Tundisi. 2002. Composition and abundance of zooplankton in the limonitic zone of seven reservoirs of the Paranapanema River, Brazil. Brazilian Journal of Biology 62: 325–545.
Schindler, D.W. 1987. Detecting ecosystem responses to anthropogenic stress. Canadian Journal of Fisheries and Aquatic Sciences 44(supp. 1): 6–25.
Seenayya, G. 1971. Ecological studies in the plankton of the certain freshwater ponds of Hyderabad, India. Physico-chemical complexes. Hydrobiologia 37: 7–31.
Segers, H. 1995. Rotifera 2. The Lecanidae (Monogononta). In Guides to the identification of the microinvertebrates of the continental waters of the World 6, ed. T. Nogrady, and H.J. Dumont. The Hague: SPB Academic.
Segers, H. 1996. The biogeography of littoral Lecane Rotifera. Hydrobiologia 446(447): 233–246.
Sharma, B.K. 1987. Rotifera: Eurotatoria: Monogononta (freshwater), Fauna of Orissa: State fauna series, part 1, 321–340. Calcutta: Zoological Survey of India.
Sharma, B.K. 1991. Rotifera. Animal resources of india (Protozoa to Mammalia), 69–88. Calcutta: State of the Art Report Zoological Survey of India.
Sharma, B.K. 1992. Freshwater rotifers (Rotifera: Eurotatoria): Fauna of West Bengal. State Fauna Series, vol. 3, 1–121. Calcutta: Zoological Survey of India.
Sharma, B.K. 1996. Biodiversity of freshwater Rotifera in India—A status report. Proceedings of the Zoological Society Calcutta 49(2): 73–85.
Sharma, S., and B.K. Sharma. 2008. Zooplankton diversity in floodplain lakes of Assam. Recodes of the Zoological Survey of India (Occ. Paper) 290: 1–370.
Sharma, B.K., and L.P. Naik. 1996. Results on planktonic rotifers in the Narmada rivier (Madhya Pradesh, Central India). In Perspectives in tropical limnology, ed. F. Schiemer, and K.K. Boland, 189–198. Amsterdam: SPB Academic Publishing bv.
Sharma, B.K., and V.K. Dudani. 1992. Rotifers from some tropical ponds in Bihar: species composition, similarities and tropic indicators. Journal of Indian Institute of Science 72: 121–130.
Sharma, V., M.S. Sharma, H. Malara, R. Sharma and B.S. Bafheal. 2008. Trophic status and Zooplankton diversity of lake Jaisamand in relation to physicochemical characteristics. Proceedings of Taal2007: The 12th World Lake conference: 490–495.
Singh, S.P., D. Pathak, and R. Singh. 2002. Hyderobiological studies of two ponds of Satna (M.P), India. Ecology and Environmental Conservation 8: 289–292.
Sorf, M., T.A. Davidson, S. Brucet, R.F. Menezes, M. Sondergaard, T.L. Lauridsen, F. Landkildehus, L. Liboriussen, and E. Jeppesen. 2015. Zooplankton response to climate warming: A mesocosm experiment at contrasting temperatures and nutrient levels. Hydrobiologia 742: 185–203.
Sousa, W., J.L. Attayde, E.D.S. Rocha, and E.M.E. Anna. 2008. The response of zooplankton assemblages to variations in the water quality of four man-made lakes in semi-arid northeastern Brazil. Journal of Plankton Research 30(6): 699–708.
Staub, R., Hofstaler, and I.J. Hass. 1970. The effect of industrial effluents of Memphis and Shelby country on primary plankton production. Biosciences 20: 905–912.
Wani, I.A., and I.A. Subla. 1995. Changes in rotifer abundance and composition in two lakes in the Kashmir Vally (Himalayas). Journal of Indian Institute of Science 75: 699–705.
Welch, P.S. 1948. Limnological methods, 1–381. New York: MC Graw Hill. Co.
Yadav, G., P. Pundhir, and K.S. Rana. 2003. Population dynamics of rotifer fauna at FatehpurSikri pond Agra. Pollution Research 22(4): 541–542.
Yeole, S. M., G. T. Kedar, and G. P. Patil. 2008. Rotifer Biodiversity of Yedshi Lake, Maharashtra. Proceedings of Taal 2007: The 12th World Lake Conference. pp 477–479.
Acknowledgments
We are grateful to Dr. K. Venkataraman, Director, Zoological Survey of India, Kolkata for providing facilities and constant encouragements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karuthapandi, M., Rao, D.V. & Xavier Innocent, B. Zooplankton Composition, Diversity and Physicochemical Features of Bandam Kommu Pond, Medak District, Telangana, India. Proc Zool Soc 69, 189–204 (2016). https://doi.org/10.1007/s12595-015-0142-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12595-015-0142-y