Abstract
Introduction
Neuroendocrine neoplasm of GIT (gastrointestinal tract) and pancreas is heterogenous with variable clinical features and disease outcomes. Despite multiple attempts of risk stratification by grading and staging, some have unpredictable clinical courses. Well-differentiated grade 3 neuroendocrine tumour (G3NET) is a recent subcategory introduced in the 2019 WHO classification based on morphology, molecular profile and prognosis distinguishing it from neuroendocrine carcinoma(NEC). This study aimed at describing the spectrum of NENs encountered in a tertiary centre with focus on reclassifying previously reported G3 tumours into G3 NET and NEC and comparing the survival between them.
Methodology
This is an 8-year retrospective study of all gastro-entero-pancreatic neuroendocrine neoplasms reclassified according to the 2019 WHO classification based on morphology and Ki-67 index with analysis of the survival rates between the categories. Minimum follow-up period was 20 months.
Results
Eighty-six patients with NENs of gastro-entero-pancreas were included, with median age group of 40–60 years (age range 9 to 79 years) and male:female ratio of 1.7:1. The tumour grade correlated with the TNM staging and most of the syndromic NETs were low grade. Eleven percent of the tumours were reclassified as well-differentiated G3NETs. The survival of G3 NETs was higher than NEC.
Conclusion
Grading of NEN is vital for therapeutic decisions and for prognostication. Currently, morphology is the key to recognise the well-differentiated G3 NETs, but can be subject to interobserver variability. Molecular surrogates may play a role in accurately identifying these entities, the validity of which is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine neoplasms of the gastrointestinal tract (GIT) and pancreas are heterogenous tumours with highly variable clinicopathological features. Despite WHO grading and risk stratification, many patients with GIT/pancreatic NENs have unpredictable clinical courses [1]. The grading of NET has been conventionally based on the proliferation index as assessed by Ki67 and mitotic index and categorised as grade 1 (G1), grade 2 (G2) and grade 3 (G3). The classification/grading and terminologies of gastro-entero-pancreatic neuroendocrine neoplasms (GEPNEN) have evolved over the last two decades and continue to do so [1]. WHO 2019 introduced “G3 well-differentiated NET”(G3 WD NET), separating it from the other grades of NET and neuroendocrine carcinoma (NEC) in view of the differences in the genomics and with the intent to triage patients for appropriate therapy and prognostication [2]. Although the NETs and NECs share the common neuroendocrine differentiation evidenced by immunoexpression of INSM1, Synpatophysin and Chromogranin A, it was found that some of the well-differentiated NETs can be high grade (G3) and the survival time of these patients with well-differentiated G3 NETs was better than that of NECs but worse than that of well-differentiated G2 tumours [3]. Additionally, various studies over the recent past have identified distinct molecular pathways for NET and neuroendocrine carcinomas [4,5,6,7]. There is data to support that patients with G3 well-differentiated NETs showed a poor response to platinum-based chemotherapy as opposed to NEC [8], but paradoxically survived longer than NECs. Therefore, it is clinically relevant to distinguish NETs from NECs within the high-grade category. Morphological assessment is crucial in this distinction, which is not without interobserver variability and has not been adequately validated by multicentric large studies [9]. The role of the routine application of molecular markers in adequately distinguishing these entities is yet to be clearly elucidated.
The aim of this study was to describe the clinicopathological spectrum of gastroenteropancreatic neuroendocrine neoplasm (GEPNEN) and to reclassify the high-grade GEPNENs as per the 5th Edition ( 2019) of the WHO classification of Digestive System Tumours, distinguishing them from NECs and compare the patient survival between the categories.
Methodology
This is a retrospective study done at a tertiary health care centre in South India. All gastro-entero-pancreatic neuroendocrine neoplasms (G1, G2, G3) diagnosed between 2012 to 2019 were included in the study. Resection specimens of gastrointestinal and pancreatic NEN/NEC and mucosal/trucut biopsies of high-grade NETs were included. Cases with unavailable tissue blocks/scanty tissue in blocks were excluded from the study. All cases were reviewed by 2 pathologists (DA, RRP) blinded to the clinical history and previous diagnosis, and reclassified based on the 2019 WHO classification and grading of neuroendocrine neoplasms of the GI tract and hepatopancreatic organs, paying due attention to the morphological differentiation, mitosis and Ki-67% index. Patient demography, syndromic association, treatment history and follow-up details were obtained from the hospital patient records and cancer registry.
Well-differentiated neuroendocrine tumours with mitoses < 2/2mm2, Ki-67 index ≤ 3%, were classified as grade 1 (G1), those with mitoses 2–20/2mm2, Ki-67 index 3 to 20%, were classified as grade 2 (G2) NET and those with mitosis > 20/2mm2, Ki-67 index of > 20%, were classified as grade 3 (G3) NET, while poorly differentiated high-grade neuroendocrine tumours with Ki-67% > 20% were categorised as neuroendocrine carcinoma (NEC). If there was a discordance between the mitotic count and Ki 67 index, the higher value was taken for categorisation. Necrosis was not included in the grading of the tumours. Tumours showing > 30% neuroendocrine component and > 30% discreet adenocarcinoma/non-neuroendocrine component were termed mixed neuroendocrine–non-neuroendocrine neoplasms (MiNEN). The diagnosis of syndromic/functional tumours was made clinically. IHC for peptide hormones and bioamines (gastrin, insulin, glucogon) was not performed. All patients were followed up till May 2022, with a median follow-up period of 35 months (range 20–114 months).
Statistical analysis
T test was used to determine the association between continuous variables. Chi-square test was used for the association between categorical variables. The date of histopathological diagnosis was considered the entry point for determining the DFS and OS. The date of disease progression and date of disease-related death or date of the last follow-up was the end-point for DFS and OS, respectively. Kaplan–Meier method was used to calculate the DFS and OS for groups and log-rank test was applied to know the strength of association between the DFS and OS with each of the parameters. Results were analysed using SPSS version 27.
Results
A total of 86 patients with NENs of GIT and pancreas were included in the study. The male:female ratio of 1.7:1 and the majority (45%) belonged to the 40–60-year group (age range 9 to 79 years). The pancreas was the most common site of involvement (34%) followed by the small intestine (29%), the gastric including the GE junction (16%), the large intestine (11%), the appendix (8%) and the retroperitoneum (2%). Of 86 cases, 31 (36%), 32 (37%) and 23 (27%) belonged to grade 1, grade 2 and grade 3/NEC respectively as per the 2010 WHO classification. Of the 23 grade 3 tumours, 9 (39%) were reclassified as grade 3 NET and 14 (61%) were NEC, 8 of which were small cell type and 6 large cell NEC.
On comparing the anatomical primary sites of the tumours, it was noted that grade 3 NET and NEC predominated in the pancreas and stomach, while the appendix harboured the grade 1 tumours (Table 1).
Grades of NENs significantly correlated with the TNM staging (p < 0.05). The majority of grade 1 tumours were of the lower stage (stage I/II), while the majority of grade 2 and grade 3 tumours belonged to the higher stage (stage III/IV) (p 0.002). Though metastasis occurred in all grades, higher rates of metastasis were seen in WD G3 NETs and NEC (Table 2). Seventeen patients (19%) had functioning tumours and were low grade. Insulinomas were the most common followed by gastrinomas associated with Zollinger–Ellison syndrome. Four patients with functional NETs were part of MEN 1 syndrome, and three with tumours in the pancreas and stomach. Syndromic and functional tumours were more commonly grade 1 (53%) and grade 2 (41%) as compared to grade 3 tumours (5%) (p = 0.000).
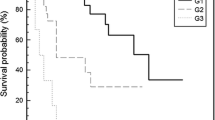
Ten out of 14 patients with NEC received chemotherapy (cisplatin + etoposide), 5 of whom developed recurrence and 3 patients died of the disease. Among 4 patients who did not receive chemotherapy, 3 of them died within 2 months of surgery prior to initiating chemotherapy and one patient was lost to follow-up. Six patients with G3 NET received chemotherapy (cisplatin + etoposide), 4 of whom had a recurrence of the disease and two succumbed. Two patients received octreotide and are alive with no recurrence. One patient was lost to follow-up. Among G2 tumours, 6 patients with an advanced stage of the disease received chemotherapy (cisplatin + etoposide), two of them had a recurrence of the disease and none died. Seven received octreotide, three of them had a recurrence and died of the disease. The rest were regularly followed up, of whom 3 had a recurrence and one died of the disease. No adjuvant therapy was given to patients with G1 tumours. None of the G1 patients had recurrence or death due to disease. Grade 1 and grade 2 tumours had better disease-free survival (p = 0.001) and overall survival compared to grade 3 tumours (p = 0.002), shown in Table 3.
Well-differentiated G3 NETS vs NEC
Twenty-three grade 3 NENs were reclassified as per the 2019 WHO classification, with 9 (39%) reclassified as well-differentiated G3 NET and 14 (41%) as NEC (Figs. 1 and 2). Among 14 NECs, 7 were mixed neuroendocrine–non-neuroendocrine neoplasms (MiNEN–adenocarcinoma–NEC). The average Ki-67% among G3 NET and NEC was 51% and 61% respectively (p value = 0.26) (inset Figs. 1 and 2).
Lymph node and distant metastases were higher among NEC compared to well-differentiated G3 NET (Table 4). The overall survival (OS) among G3 NET was higher compared to NEC (Table 4).
Discussion
NETs are a heterogenous group of neoplasms that can arise in any epithelial organs of the body with the highest occurrences in GIT and pancreas (55%) followed by the respiratory tract (25%) [10, 11]. The pancreas alone accounts for 7% of all neuroendocrine tumours. NETs account for 2% of GI malignancy [11]. The incidence of NETs is on the rise owing to improved diagnostic modalities and screening tests.
The small intestine has been described as the most frequent site (38%), followed by the rectum (34%), colon (16%), stomach (11%) and unknown sites [1%] [1]. In our study, the pancreas was the commonest followed by the small intestine. Although female predominance has been commonly described for GI NETs [12, 13], our study and Amarapurkar et al. [12] had male predominance. Equal gender distribution was observed among pancreatic NENs as described in the literature [2, 12].
NETs commonly express somatostatin receptors and secrete neurosecretory hormones, but only about 40% of them are functional. Serum chromogranin A is the biomarker used to assess the bulk of the disease and response to therapy in both functional and non-functional tumours [7]. Syndromic association and familial clustering are noted in about 20% of NETs [13], of which MEN 1,2 and neurofibromatosis 1 are the most common [7]. Contrary to sporadic tumours, hereditary NETs present earlier (3rd decade vs 6th decade), and are multicentric tumours with high secretory activity [14]. In our series, 19% of tumours were secretory, and 4 (21%) were associated with MEN1 syndrome. Akin to the literature, the average age of diagnosis among syndromic NETs was 32 years as opposed to 53 years among sporadic tumours. A higher prevalence of lower-grade NENs in syndromic/functional tumours reported by other authors [15] was also observed in this study. Functional/syndromic NENs have a higher risk of liver metastasis, despite the lower grade but with better survival than non-functional NETS [9, 15].
The grading and staging of GEPNENs have been continuously evolving over a few decades. The European Neuroendocrine Tumor Society (ENETS) in 2006 first proposed a separate grading system for pancreatic neuroendocrine neoplasm based on mitotic rate and Ki-67 proliferative index [16] which was later adopted in the World Health Organization 2010 classification [16]. WHO 2010 grading of NENs of GIT and pancreas has been long debated for not being optimal in terms of prognosis [1, 17]. A revolutionary study by Basturk et al. [3] on high-grade pancreatic NETs found that some pancreatic NETs showed discordance in the mitotic rate and Ki-67%, prompting them to recognise the subcategory of well-differentiated high-grade (G3) pancreatic NETs with Ki 67 > 20%, the latter showing better survival than that of poorly differentiated tumours/NEC. Sorbye et al. [8] showed, among the WHO 2010 G3 tumours, WD NETs with Ki-67 < 55% did not respond well to platinum-based chemotherapy as opposed to poorly differentiated tumours with a higher Ki67 index. Many other investigators also supported the existence of a well-differentiated neuroendocrine tumour with a Ki-67 index > 20% which behaved differently from the neuroendocrine carcinoma [4]. Genomic profiling data of the high-grade category has revealed that NETs and NECs are not related. Frequent mutations in MEN1, DAXX and ATRX were observed in NETs including G3 NETS, particularly in the pancreas, but were not seen in NECs, the latter instead showing TP53 and RB1 inactivation mutations [4]. The mounting evidence prompted the subclassification of the original G3 group into well-differentiated pancreatic neuroendocrine tumours G3 (pNETs G3) and poorly differentiated pancreatic neuroendocrine carcinomas (pNECs) in 2017, which now extends to all NETs in GI tract and hepatobiliary organs as reflected in the 2019 WHO classification of digestive system tumours [7]. Additionally, the purpose of the newly added WD G3 NET category also was to improve the prediction of clinical outcomes and to determine better therapeutic strategies [17].
The well-differentiated G3 NETs display heterogeneity as seen in the G1and G2NETs with low-grade and high-grade areas in the same tumour at presentation or between sites (primary vs metastases). The terminology of NEC is reserved for poorly differentiated high-grade epithelial neoplasms displaying neuroendocrine differentiation and are not subject to conventional grading (Fig. 2). NECs are histologically divided into small and large cell variants or rarely can be mixed [18]. NECs do not arise in association with NET, but can arise from non-neuroendocrine precursors [18]. In our study, 39% of G3NENSs were reclassified as G3 NETs based on morphology and Ki 67 index, comprising 10% of the study population (Figs. 1 and 2). G3 NETs constituted 7% of the cohort in another study [19]. The estimated prevalence of G3 NET among all GEPNEN is 5.6 to 8% [19].
The current TNM staging of GEPNETs fails to incorporate the histological grade and functional status of the tumour [9]. In our study, majority of grade 1 tumours had lower stage (stage I/II) and grade 2 and 3 tumours were advanced stage (stage III/IV) with statistical significance (Table 3). We found no significant difference in the average Ki-67% between the G3 NET and NEC (51% vs 61%). Basturk et al. [3] found that the discordant tumours (G3NET) had average Ki-67 of 40% compared to 70% in NEC. Across many studies, the mean Ki-67% for G3 NET was found to be around 30–40% [3, 19,20,21]. Lithgow et al. [21] found that though the median Ki-67% among G3 NET was 30%, 15% of them had Ki-67% > 55% [21]. Though WHO 2019 described the Ki-67% between 20 and 55% for G3 NETS, a uniform upper cut-off for Ki-67% for differentiating G3 NETs from NEC is not defined by WHO or other studies [20]. In our study, lymph node/distant metastasis among G3 NETs was lower compared to NEC (Table 4).
Yang et al. [17] showed that pancreatic G3 NETs had better survival than NEC but worse outcome than G1 and G2 tumours [17]. In our study, G3 NET had significantly worse OS and DFS compared to G1/G2 NETs (Table 3), but better survival compared to NEC (30 months vs 20 months). Rindi et al. [19] found that there was a statistically significant difference in the OS between the G3NET and NEC, but not for event-free survival. As G3 NETs do not respond to platinum-based chemotherapy, it is clinically relevant to distinguish G3 NET from NECs [19]. The WHO 2019 classification emphasizes on morphology along with proliferation index (Ki67) to distinguish G3 NET and NEC. This involves interobserver variability and has not been validated by multicentric large series studies [9, 19]. Although the histological difference between the G3NET and NEC is apparent, it can be challenging at times. In a study by Tang et al. [4], to reclassify G3 tumours into G3 NET and NEC by 3 pathologists, 61% of cases had an uncertain diagnosis with discrepancies [4]. Loss of nuclear expression for DAXX and ATRX in G3 NETs and loss of Rb and mutant p53 in NEC [5, 6] observed in gene sequencing studies suggest the role of reliable molecular markers to categorically differentiate the high-grade neoplasms (NET vs NEC). A few newer molecular signatures and markers are also on the horizon such as loss of nuclear forkhead box (FOX), M1 expression in NETs G3 and high expression in NECs which have been observed [22]. The cell adhesion molecule L1 may be a potential marker for poorly differentiated pancreatic NECs. Cavalcanti et al. [23] studied PD-L1 expressed in GEPNENs, and showed increased PD-L1 positivity rate and signal intensity with increasing grades.
Conclusion
Neuroendocrine tumours of GIT and pancreas are heterogenous and are being increasingly recognised due to the advances in diagnostic modalities. Syndromic/functional neuroendocrine tumours present earlier and are associated with a higher frequency of metastasis, despite lower grade. Recently introduced G3 NETs show better survival than NEC and accurate identification is vital for therapeutic decisions and prognostication. Currently, morphology along with proliferation index is the key to distinguishing well-differentiated G3 NETs from NECs. Molecular surrogates may play a role in accurately identifying these entities and the validity of its use in routine practice is warranted.
References
Kim JY, Hong SM (2016) Recent updates on neuroendocrine tumors from the gastrointestinal and pancreatobiliary tracts. Arch Pathol Lab Med 140:437–448. https://doi.org/10.5858/arpa.2015-0314-RA
Lokuhetty D, White V, Watanabe R, Cree I (2018) WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer
Basturk O, Yang Z, Tang LH et al (2015) The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 39:683–690. https://doi.org/10.1097/pas.0000000000000408
Tang LH, Basturk O, Sue JJ, Klimstra DS (2016) A practical approach to the classification of WHO grade 3 (G3) well differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC) of the pancreas. Am J Surg Pathol 40:1192. https://doi.org/10.1097/pas.0000000000000662
Park JK, Paik WH, Lee K et al (2017) DAXX/ATRX and MEN1 genes are strong prognostic markers in pancreatic neuroendocrine tumors. Oncotarget 8:49796. https://doi.org/10.18632/oncotarget.17964
Ali AS, Grönberg M, Federspiel B et al (2017) Expression of p53 protein in high-grade gastroenteropancreatic neuroendocrine carcinoma. PLoS ONE 12:e0187667. https://doi.org/10.1371/journal.pone.0187667
Zhang MY, Du He SZ (2020) Pancreatic neuroendocrine tumors G3 and pancreatic neuroendocrine carcinomas: differences in basic biology and treatment. World J Gastrointest Oncol 12:705. https://doi.org/10.4251/wjgo.v12.i7.705
Sorbye H, Strosberg J, Baudin E et al (2014) Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 120:2814–2823. https://doi.org/10.1002/cncr.28721
Wang H, Ding D, Qin T et al (2022) Prognostic validity of the American joint committee on cancer eighth edition staging system for well-differentiated pancreatic neuroendocrine tumors. HPB 24:681–690. https://doi.org/10.1016/j.hpb.2021.10.017
Ahmed M (2020) Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 12(8):791. https://doi.org/10.4251/2Fwjgo.v12.i8.791
Parra-Medina R, Moreno-Lucero P, Jimenez-Moreno J et al (2019) Neuroendocrine neoplasms of gastrointestinal tract and secondary primary synchronous tumors: a systematic review of case reports. Casualty or causality? PLoS One 14:e0216647. https://doi.org/10.1371/journal.pone.0216647
Amarapurkar DN, Juneja MP, Patel ND et al (2010) A retrospective clinico-pathological analysis of neuroendocrine tumors of the gastrointestinal tract. Trop Gastroenterol 31:101–104
Oronsky B, Ma PC, Morgensztern D, Carter CA (2017) Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia 19:991–1002. https://doi.org/10.1016/j.neo.2017.09.002
Faggiano A, Ramundo V, Circelli L, Colao A (2013) Hereditary neuroendocrine tumor syndromes. Hot Topics in endocrine and endocrine-related diseases, InTech 8:1–26. https://doi.org/10.5772/53841
Conemans EB, Brosens LA, Raicu-Ionita GM et al (2017) Prognostic value of WHO grade in pancreatic neuro-endocrine tumors in multiple endocrine neoplasia type 1: results from the DutchMEN1 Study Group. Pancreatology 17:766–772. https://doi.org/10.1016/j.pan.2017.07.196
Cavalcanti MS, Gönen M, Klimstra DS (2016) The ENETS/WHO grading system for neuroendocrine neoplasms of the gastroenteropancreatic system: a review of the current state, limitations and proposals for modifications. Int J Endocr Oncol 3:203–219. https://doi.org/10.2217/ije-2016-0006
Yang M, Zeng L, Ke NW et al (2020) World Health Organization grading classification for pancreatic neuroendocrine neoplasms: a comprehensive analysis from a large Chinese institution. BMC Cancer 1–11. https://doi.org/10.1186/s12885-020-07356-5
Rindi G, Klimstra DS, Abedi-Ardekani B et al (2018) A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 31:1770–1786. https://doi.org/10.1038/s41379-018-0110-y
Rindi G, Klersy C, Albarello L et al (2018) Competitive testing of the WHO 2010 versus the WHO 2017 grading of pancreatic neuroendocrine neoplasms: data from a large international cohort study. Neuroendocrinology 107:375–386. https://doi.org/10.1159/000494355
Shi H, Chen L, Meng L et al (2020) The role of Ki-67 index cut-off of 55% and differentiation in redefining NET and NEC for grade 3 gastrointestinal neuroendocrine neoplasms. J Gastroint Surg 24:2302–2305. https://doi.org/10.1007/s11605-020-04651-1
Lithgow K, Venkataraman H, Hughes S et al (2021) Well-differentiated gastroenteropancreatic G3 NET: findings from a large single centre cohort. Sci Rep 11:1–8. https://doi.org/10.1038/s41598-021-97247-x
Briest F, Berg E, Grass I et al (2015) FOXM1: a novel drug target in gastroenteropancreatic neuroendocrine tumors. Oncotarget 6:8185. https://doi.org/10.18632/oncotarget.3600
Cavalcanti E, Armentano R, Valentini AM et al (2017) Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis 8:e3004–e3004. https://doi.org/10.1038/cddis.2017.401
Author information
Authors and Affiliations
Contributions
Conceptualization: Divya Achutha Ail, Roopa Rachel Paulose. Data preparation/acquisition: Divay Achutha Ail. Methodology: Divya Achutha Ail, Roopa Rachel Paulose. Formal analysis and investigation: Divya Achutha Ail, Roopa Rachel Paulose. Writing—original draft preparation: Divya Achutha Ail. Writing—review and editing: Roopa Rachel Paulose. Supervision: Roopa Rachel Paulose.
Corresponding author
Ethics declarations
Ethical approval
Compliant.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ail, D.A., Paulose, R.R. A relook at gastroenteropancreatic neuroendocrine tumours as per 2019 WHO classification—A tertiary centre experience. Ir J Med Sci 192, 2065–2070 (2023). https://doi.org/10.1007/s11845-022-03217-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-03217-1