Opinion statement
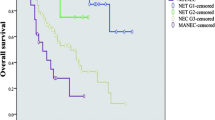
In the 2019 WHO guidelines, the classification of gastro-entero-pancreatic neuroendocrine neoplasms (GEP NEN) has changed from one being based on Ki-67 proliferation index alone to one that also includes tumor differentiation. Consequently, GEP NENs are now classified as well-differentiated neuroendocrine tumor (NET), NET G1 (Ki-67 <3%), NET G2 (Ki-67 3–20%) and NET G3 (Ki-67 >20%), and poorly differentiated neuroendocrine carcinoma (NEC) (Ki-67 >20%). It has been suggested that NET G3 should be treated as NET G2 with respect to surgery, while surgical management of NEC should be expanded from local disease to also include patients with advanced disease where curative surgery is possible. High grade mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN) have a neuroendocrine and a non-neuroendocrine component mostly with a poor prognosis. All studies evaluating the effect of surgery in NEC and MiNEN are observational and hold a risk of selection bias, which may overestimate the beneficial effect of surgery. Further, only a few studies on the effect of surgery in MiNEN exist. This review aims to summarize the data on the outcome of surgery in patients with GEP NET G3, GEP NEC and high grade MiNEN. The current evidence suggests that patients with NEN G3 and localized disease and NEN G3 patients with metastatic disease where curative surgery can be achieved may benefit from surgery. In patients with MiNEN, it is currently not possible to evaluate on the potential beneficial effect of surgery due to the low number of studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The classification of gastro-entero-pancreatic neuroendocrine neoplasms (GEP NEN) has changed with the 2019 WHO classification [1••]. GEP NENs are no longer classified only from the Ki-67 proliferation index but also classified according to cell type, tumor differentiation and molecular genetic markers. NENs are classified as well-differentiated neuroendocrine tumors (NET): NET G1 (Ki-67 <3%), NET G2 (Ki-67 3–20%), NET G3 (Ki-67 >20%) and poorly differentiated neuroendocrine carcinomas (NEC), all with Ki-67 proliferation index >20% [1••].
NET G3 constitutes 15–20% of the NEN G3s and is primarily found in the pancreas, while NECs are distributed among the esophagus, stomach, pancreas, colon and rectum. NECs have an invasive behavior and are characterized by early metastases [2].
The mixed neuroendocrine and non-neuroendocrine neoplasms (MiNEN) are defined as tumors with a neuroendocrine and a non-neuroendocrine component such as an adenocarcinoma, squamous-cell carcinoma or acinar-cell carcinoma, of which each component consists of at least 30% [3, 4]. The classification of MiNEN is now based on the differentiation of the components; thus, MiNENs are divided into low grade MiNEN, intermediate grade MiNEN and high grade MiNEN [3].
After the revision of the WHO guidelines in 2019, a consensus paper on GEP NEN was published by the European Neuroendocrine Tumor Society (ENETS) [1, 5]. The paper states that principles for surgery in patients with NET G3 should follow the principles for surgery in patients with NET G2 [5]. This statement is not only based on studies reporting the effect of surgery, but also on studies reporting the response to systemic therapy [6, 7]. With respect to surgery in patients with NEC, the recommendation by the North American Society for NeuroEndocrine tumors (NANETS) has changed from a recommendation in 2010 against surgery in patients with metastatic disease to surgery in all patients with both localized and regional disease and even selected patients with metastatic disease if curative resection can be achieved [5, 8].
We aimed to summarize the current literature on the outcome of surgery in patients with GEP NET G3, GEP NEC and high grade MiNEN.
GEP NEN G3
Localized and regional disease
The studies on NET G3 and NEC are presented in Table 1. Overall, hazard ratio (HR) for mortality was below one suggesting a beneficial effect of surgery. Median progression-free survival (PFS) was between six and 42 months, while median overall survival (OS) was between nine and 152 months. The studies diverge according to differences in localization of primary tumor, tumor stage and the distribution of patients with either NET G3 or NEC [10, 12, 23, 25]. Early studies have addressed the poor prognosis of NEN G3s [26, 27] leading to a recommendation against surgery in the NANETS 2010 guidelines [8, 26, 27]. However, after the publication of two large studies [10, 12], both ENETS and NANETS now recommend surgery of tumors with Ki-67 proliferation index >20% [5, 28]. A study based on the Surveillance, Epidemiology and End Results (SEER) database included 335 patients with non-metastatic NET G1-G3 and NEC [12] followed with respect to disease-specific survival (DSS). Median survival was higher in patients undergoing surgery (153 vs. 71 months), while non-surgical management was a poor prognostic factor associated with reduced DSS (HR 4.5) in patients with NET G1–3 and NEC [12]. The authors recommended surgery in patients with localized and regional disease. This recommendation was supported by a more recent European analysis of 60 patients (28% NET G3, 72% NEC) from eight centers [10]. The study found that recurrence-free survival (RFS) after radical surgery was 14 months, and the 2-year overall survival (OS) was 65%, but the effect of surgery was better in patients with NET G3 compared to NEC (HR for survival 4.2) [10].
Metastatic disease
The ENETS guidelines from 2016 did not recommend surgery in NEN G3 patients with metastatic disease. The first publication to challenge this opinion was a study from 2017 where 32 patients with metastatic disease (24 with NEC, two with NET G3 and six which could not be determined) underwent resection of the primary tumor and/or radio frequency ablation of liver metastases [9]. The 5-year survival rate was 43%, and four patients were disease-free after 5 years. Recently, data from two multicenter studies have supported the feasibility of surgery in selected patients with metastatic disease [11, 13]. The largest study included recent data from the Nordic NEC study group on 154 patients with NET G3 or NEC diagnosed from 2007 to 2015 and aimed to investigate outcomes after tumor surgery in patients with localized, regional and metastatic disease [13]. R0 resection in patients with localized and regional disease was associated with a 5-year OS and median OS of 42% and 39 months respectively. In metastatic disease, the median OS in patients with R0 resection vs. an R1 or R2 resection was 32 and 11 months, respectively. The results of these studies support the recommendation of surgery in selected patients with metastatic disease as stated in the ENETS conference paper from 2019 [5, 9, 11, 13].
NEN G3 according to organ
Esophagus and stomach
Only a few studies report the outcome after surgery in patients with NEN G3 from esophagus or stomach, and results are conflicting [14,15,16]. The largest study is from the SEER database of 12,878 patients with GEP NEC and GEP MiNEN from 1975 to 2016 which includes 198 patients with esophageal NEC and 1011 patients with gastric NEC [14]. In the whole database, 11% had stage I disease, 7% had stage II disease, 9% had stage III disease and 18% had disseminated disease, while stage was unknown in 55% of patients [14]. Surgery had no prognostic impact in patients with esophageal NEC, while no surgical treatment was a poor prognostic factor in patients with gastric NEC with respect to OS (HR 3.2). The beneficial effect of surgery in gastric NEC was also reported in a small study including 12 patients with gastric NEN G3 and a larger study which pooled gastric NEN G3 with NET G1 and G2 [15, 16]. In the small study, 94% of patients with gastric NEN G3 had lymph node metastases, while distant metastases were found in 13% [16]. With regard to MiNEN, 85% had lymph node metastases, and 30% had distant metastases [16]. In the larger study lymph node metastases were found in all patients with esophageal tumors and in 96% of patients with gastric tumors [15].
Pancreas
The prognostic impact of surgery in patients with GEP NEN G3 has best been described in pancreatic NEN [18,19,20,21, 29]. The NANETS guidelines from 2020 do not recommend surgical treatment of patients with pancreatic NEC as the generally poor prognosis does not seem to be improved by surgery [30]. This opinion, however, was not supported by the Nordic NEC study [29] including 76 patients with NET G3, 39 with NEC and four with non-classified tumors from 10 Nordic university hospitals [29]. The patients were included over a 14-year period and were followed with respect to survival. Resection of the primary tumor and subsequent metastatic surgery were associated with a significantly higher 3-year survival rate of 69% compared to 45% for patients with resection of the primary tumor and chemotherapy at recurrence and 17% for patients only treated with chemotherapy [29]. Therefore, the authors recommended surgery to selected patients with metastatic disease.
The results from the Nordic study were supported by several other studies in patients with NET G3 but not in pancreatic NEC [18,19,20,21]. The effect of surgery on median OS varied from 4 months [19] to 33 months [21]. The difference in OS could be explained by differences in the cohorts as the long OS was found in a NET G3 cohort as opposed to the short OS which was reported in a mixed NET G3 and NEC cohort [19, 21].
Small intestine
In the small intestine NEN G3s are extremely rare, and if they occur NET G3s are the most common, while poorly differentiated NECs are rare [31]. The NANETS guidelines recommend resection of localized disease in NET G3 [31], but the evidence with respect to surgery is limited. For NEC, a SEER database study included 249 patients with small intestinal NEC and showed no surgery to be a negative prognostic factor with respect to mortality (HR 1.5) [14].
Colon and rectum
Although several large studies have been performed on colon and rectum [14, 22,23,24,25], there are only few studies published after the new WHO guidelines in 2019. Two large American retrospective studies investigated the effect of surgery [14, 23]. One of them including 1208 patients with colorectal NEC from the National Cancer Database showed a beneficial effect of surgery (HR 0.5) with a median OS of 9 months [23]. The results were supported in a SEER database study including 798 patients with colonic NEC and 1376 patients with rectal NEC [14]. A non-surgical approach was a negative prognostic factor with respect to OS in both the colon (HR 0.39) and rectum NEC (HR 0.26) [14]. These results were in contrast to an earlier retrospective study in 126 patients with NEC from a single center [25]. These patients had a median OS of 13.2 months [25]. The discrepancy between the studies may reflect differences in the proportions of patients with metastatic disease [23, 25]. The effect of surgery may differ in small-cell NECs compared to non-small-cell NECs [24]. In another SEER database study including 1367 patients, surgery was a prognostic factor if tumor was a localized non-small cell NET G3 or NEC, as median OS was 21 months in patients undergoing surgery compared to 6 months in the no surgery group. This beneficial effect of surgery could not be found in patients with small-cell NET G3 and NEC [24].
Interpretation of studies
Based on the 2019 WHO classification, there is a need for studies considering the distinction between NET G3 and NEC. Because the classification of tumors with a Ki-67 proliferation index >20% as either NET G3 or NEC is new, the previous literature is difficult to interpret according to the 2019 classification, as many studies included both patients with NET G3s and NECs [12, 16,17,18, 24, 29, 32]. However, in one study the cohort was divided into two groups (well-differentiated and poorly differentiated) using features mainly based on morphology with criteria comparable to those of the current classification. This allows differentiation between NET G3 and NEC [10]. The differences in cohorts with respect to distribution of NET G3 and NEC patients contribute to differences in observed beneficial effects of surgery as surgery is associated with longer OS in NET G3 compared to NEC [19, 21]. Another challenge is studies mixing NET G3 and NEC with NET G1 and NET G2, which makes the interpretation difficult. This approach may overestimate a potential beneficial effect of surgery due to an improved prognosis in patients with NET G1 and G2 [20, 33,34,35].
Management of NET G3 and NEC
Our proposal for management of NET G3 and NEC is illustrated in Fig. 1. The use of adjuvant therapy is not recommended in NET G3 in the recent Nordic guidelines, while patients with NEC are recommended adjuvant therapy with 4–6 cycles of Cis-/Carboplatin and Etoposide [36]. Currently, neoadjuvant therapy is not recommended in guidelines [5, 36].
GEP MiNEN
MiNENs consist of a neuroendocrine and a non-neuroendocrine component where each component contributes with a minimum of 30% of the total component [3]. This 30% threshold, although arbitrary, is based on the assumption that the lesser component is unlikely to influence the biological behavior of the neoplasm. However, this may be questioned as a study including 88 patients with gastric MiNEN found a poor prognosis in patients with a NEC component of 10% [3, 37]. The study concluded that the definition of MiNEN needs future verification and revision. The prognosis and malignant potential of MiNEN are determined by the most aggressive component [38, 39]. Most MiNENs are found in the esophagus and the esophageal/gastric junction and in the colon and rectum [40, 41]. The prognosis depends on the localization, as MiNENs with a pancreatico-duodenal origin have a poorer prognosis compared to lower and upper gastro-intestinal MiNENs [41].
The effect of surgery in MiNEN is shown in Table 2. HR for mortality was below one indicating a prognostic effect of surgery. Median PFS was between 8 and 32 months, and median OS was between 11 and 92 months. Based on the current literature, there are several challenges when MiNEN studies are evaluated. Studies with MiNEN published before 2017 are characterized by lack of grading [16, 23, 41]. Some studies only report pooled data on MiNEN and NEC [13, 15]. The prognosis of MiNEN compared to a “pure” NEC differs in different organs being worse in the esophagus, small intestine, appendix and stomach but slightly better in the pancreas, gall bladder, colon and rectum [14, 25, 32, 37, 42, 45,46,47,48,49]. Finally, the results of surgery have not been studied exclusively, but in combination with other treatments which makes evaluation of the surgical treatment difficult [41]. In 50 patients with GEP MiNEN, we have previously shown that the median OS was 46 months in patients with localized disease vs. 7 months in patients with metastatic disease, and no surgery was associated with higher mortality (HR 3.4) [50]. Our data suggest that it is important to consider surgery in patients with localized MiNEN.
MiNEN according to organ
Esophagus and stomach
Esophageal MiNENs normally develop in the lower third of the esophagus [38, 51]. In the stomach, MiNENs account for 7% of gastric NENs and 25% of all gastric NECs [32, 37, 45]. A SEER database study of 41 patients with gastric MiNEN found no prognostic effect of surgery, but no data on tumor stage were reported [14]. This is in contrast to a previous single-center study including 20 patients of whom 90% had regional or metastatic disease, [16]. Here, an R0 resection was associated with an improvement in OS. The difference in outcome, however, may be explained by the small number of patients included in the studies or unequal tumor stages.
Appendix
Appendicular MiNEN is associated with a poor prognosis, but the literature is sparse [43, 44, 52]. Recent data of 315 patients found that no surgery was associated with a poor prognosis, HR 2.5 [44].
Pancreas
The indication for surgery in localized MiNEN is unclear. The effect of surgery in localized MiNEN was presented in two retrospective studies [47, 53]. However, the material was heterogeneous in both studies as they included possible acinar carcinomas and NENs [47, 53]. Currently, it is unknown if surgery should be recommended in pancreatic MiNEN.
Small intestine, colon and rectum
Contrary to the colorectal form, MiNENs in the small intestine are rare [25, 38, 39]. In a SEER database study, surgery had no impact on prognosis in small intestinal MiNEN [14]. There was no effect of surgery in patients with colonic MiNEN, while a non-surgical approach was associated with a poorer prognosis in rectal MiNEN (HR 4.1) [14]. However, the study neither reported tumor stage, performance status (PS) nor MiNEN grade [14].
Interpretation of studies
The evidence of surgery in MiNEN is scarce. Only few studies on the effect of surgery in patients with MiNEN have been performed [14, 16, 41, 44, 50, 53] which makes it impossible to evaluate the potential beneficial effect of surgery in this group of patients. There is a need for studies investigating the effect of surgery according to localization of primary tumor and disease stage.
Management of high grade MiNEN
Our suggestion for management of high grade MiNEN is shown in Fig. 1. We recommend adjuvant chemotherapy as proposed for patients with NEC [4]. Currently, no guidelines support the use of neoadjuvant therapy.
Conclusion
This review is updated after the 2019 WHO guidelines and includes the new studies which suggest that not only patients with localized and regional disease, but also selected patients with metastatic disease may profit from surgery. The studies evaluating the effect of surgery in general suggested a beneficial effect of surgery in both NEN G3 and MiNEN. In NEN G3, median PFS was up to 42 months, while median OS was up to 153 months. In MiNEN, median PFS was up to 32 months, and median OS was up to 92 months. It is a limitation that the literature before 2019 did not separate NET G3 and NEC [12, 16,17,18, 24, 29, 32], and studies pooled NEN and MiNEN [13, 15]. We did not perform a meta-analysis of data with a weighted estimate of the effect of surgery on PFS and OS in NEN G3 and MiNEN. All studies, however, seem to suggest a beneficial effect of surgery. Finally, all studies reporting the effect of surgery in patients with NENs and MiNENs are observational [9, 11,12,13,14,15,16,17,18,19,20,21,22,23,24,25, 29, 41, 44, 50, 53] and thus may have selection bias, as primarily patients with good PS undergo surgery, which may lead to overestimation of the potential beneficial effect of surgery. In conclusion, we suggest, depending on age and comorbidity, surgery in all patients with localized and regional GEP NEN G3, as well as in patients with metastatic disease, if radical resection can be obtained. Radical surgery is currently the only hope for cure and prolonged survival.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Klimstra DS, Kloppel G, La Rosa S RG. Digestive system tumours. In: WHO classification of tumours. In: IARC, Lyon. 5th edition. 2019.
Walter T, Tougeron D, Baudin E, Le Malicot K, Lecomte T, Malka D, et al. Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: are they really heterogeneous? Insights from the FFCD-GTE national cohort. Eur J Cancer. England. 2017;79:158–65.
Lloyd RV, Osamura RY, Klöppel G RJ. Pathology and genetics of tumours of endocrine organs. Fourth edition—WHO—2017. WHO classification of tumours. 2017;10:320 p.
de Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, et al. Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms. Neuroendocrinology. Switzerland. 2017;105(4):412–25.
Sorbye H, Baudin E, Borbath I, Caplin M, Chen J, Cwikla JB, et al. Unmet needs in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Neuroendocrinology. Switzerland. 2019;108(1):54–62.
Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol Off J Eur Soc Med Oncol. England. 2013;24(1):152–60.
Carlsen EA, Fazio N, Granberg D, Grozinsky-Glasberg S, Ahmadzadehfar H, Grana CM, et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: a multicenter cohort study. Endocr Relat Cancer. BioScientifica Ltd. 2019;26(2):227–39.
Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39(6):799–800.
Galleberg RB, Knigge U, Tiensuu Janson E, Vestermark LW, Haugvik S-P, Ladekarl M, et al. Results after surgical treatment of liver metastases in patients with high-grade gastroenteropancreatic neuroendocrine carcinomas. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. England. 2017;43(9):1682–9.
Merola E, Rinke A, Partelli S, Gress TM, Andreasi V, Kollár A, et al. Surgery with radical intent: is there an indication for G3 neuroendocrine neoplasms? Ann Surg Oncol. United States. 2020;27(5):1348–55.
Merola E, Falconi M, Rinke A, Staettner S, Krendl F, Partelli S, et al. Radical intended surgery for highly selected stage IV neuroendocrine neoplasms G3. Am J Surg. United States. 2020;220(2):284–9.
Mosquera C, Koutlas NJ, Fitzgerald TL. Localized high-grade gastroenteropancreatic neuroendocrine tumors: defining prognostic and therapeutic factors for a disease of increasing clinical significance. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. England. 2016;42(10):1471–7.
Pommergaard H-C, Nielsen K, Sorbye H, Federspiel B, Tabaksblat EM, Vestermark LW, et al. Surgery of the primary tumour in 201 patients with high-grade gastroenteropancreatic neuroendocrine and mixed neuroendocrine-non-neuroendocrine neoplasms. J Neuroendocrinol. United States. 2021;33(5):e12967.
Shi H, Qi C, Meng L, Yao H, Jiang C, Fan M, et al. Do neuroendocrine carcinomas and mixed neuroendocrine-non-neuroendocrine neoplasm of the gastrointestinal tract have the same prognosis? A SEER database analysis of 12,878 cases. Ther Adv Endocrinol Metab. 2020;11:2042018820938304.
van der Veen A, Seesing MFJ, Wijnhoven BPL, de Steur WO, van Berge Henegouwen MI, Rosman C, et al. Management of resectable esophageal and gastric (mixed adeno)neuroendocrine carcinoma: a nationwide cohort study. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. England. 2018;44(12):1955–62.
Shen C, Chen H, Chen H, Yin Y, Han L, Chen J, et al. Surgical treatment and prognosis of gastric neuroendocrine neoplasms: a single-center experience. BMC Gastroenterol. 2016;16(1):111.
Xie J-W, Sun Y-Q, Feng C-Y, Zheng C-H, Li P, Wang J-B, et al. Evaluation of clinicopathological factors related to the prognosis of gastric neuroendocrine carcinoma. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. England. 2016;42(10):1464–70.
Crippa S, Partelli S, Bassi C, Berardi R, Capelli P, Scarpa A, et al. Long-term outcomes and prognostic factors in neuroendocrine carcinomas of the pancreas: morphology matters. Surgery. United States. 2016;159(3):862–71.
Feng T, Lv W, Yuan M, Shi Z, Zhong H, Ling S. Surgical resection of the primary tumor leads to prolonged survival in metastatic pancreatic neuroendocrine carcinoma. World J Surg Oncol. 2019;17(1):54.
Partelli S, Inama M, Rinke A, Begum N, Valente R, Fendrich V, et al. Long-term outcomes of surgical management of pancreatic neuroendocrine tumors with synchronous liver metastases. Neuroendocrinology. Switzerland. 2015;102(1–2):68–76.
Yoshida T, Hijioka S, Hosoda W, Ueno M, Furukawa M, Kobayashi N, et al. Surgery for pancreatic neuroendocrine tumor G3 and carcinoma G3 should be considered separately. Ann Surg Oncol. United States. 2019;26(5):1385–93.
Conte B, George B, Overman M, Estrella J, Jiang Z-Q, Mehrvarz Sarshekeh A, et al. High-grade neuroendocrine colorectal carcinomas: a retrospective study of 100 patients. Clin Colorectal Cancer. 2016;15(2):e1–7.
Fields AC, Lu P, Vierra BM, Hu F, Irani J, Bleday R, et al. Survival in patients with high-grade colorectal neuroendocrine carcinomas: the role of surgery and chemotherapy. Ann Surg Oncol. 2019;26(4):1127–33.
Shafqat H, Ali S, Salhab M, Olszewski AJ. Survival of patients with neuroendocrine carcinoma of the colon and rectum: a population-based analysis. Dis Colon Rectum. United States. 2015;58(3):294–303.
Smith JD, Reidy DL, Goodman KA, Shia J, Nash GM. A retrospective review of 126 high-grade neuroendocrine carcinomas of the colon and rectum. Ann Surg Oncol. 2014;21(9):2956–62.
Casas F, Ferrer F, Farrús B, Casals J, Biete A. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer. United States. 1997;80(8):1366–72.
Brenner B, Shah MA, Gonen M, Klimstra DS, Shia J, Kelsen DP. Small-cell carcinoma of the gastrointestinal tract: a retrospective study of 64 cases. Br J Cancer. 2004;90(9):1720–6.
Halfdanarson TR, Strosberg JR, Tang L, Bellizzi AM, Bergsland EK, O’Dorisio TM, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas. United States. 2020;49(7):863–81.
Haugvik S-P, Janson ET, Österlund P, Langer SW, Falk RS, Labori KJ, et al. Surgical treatment as a principle for patients with high-grade pancreatic neuroendocrine carcinoma: a Nordic multicenter comparative study. Ann Surg Oncol. United States. 2016;23(5):1721–8.
Howe JR, Merchant NB, Conrad C, Keutgen XM, Hallet J, Drebin JA, et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49(1):1–33.
Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang Y-Z, et al. The surgical management of small bowel neuroendocrine tumors: consensus guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46(6):715–31.
Ishida M, Sekine S, Fukagawa T, Ohashi M, Morita S, Taniguchi H, et al. Neuroendocrine carcinoma of the stomach: morphologic and immunohistochemical characteristics and prognosis. Am J Surg Pathol. United States. 2013;37(7):949–59.
Panzuto F, Merola E, Pavel ME, Rinke A, Kump P, Partelli S, et al. Stage IV gastro-entero-pancreatic neuroendocrine neoplasms: a risk score to predict clinical outcome. Oncologist. 2017;22(4):409–15.
Assi HA, Mukherjee S, Kunz PL, Machiorlatti M, Vesely S, Pareek V, et al. Surgery versus surveillance for well-differentiated, nonfunctional pancreatic neuroendocrine tumors: an 11-year analysis of the National Cancer Database. Oncologist. 2020;25(2):e276–83.
Pape U-F, Berndt U, Muller-Nordhorn J, Bohmig M, Roll S, Koch M, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. England. 2008;15(4):1083–97.
Janson ET, Knigge U, Dam G, Federspiel B, Grønbaek H, Stålberg P, et al. Nordic guidelines 2021 for diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. Acta Oncol. England. 2021;60(7):931–41.
Park JY, Ryu M-H, Park YS, Park HJ, Ryoo B-Y, Kim MG, et al. Prognostic significance of neuroendocrine components in gastric carcinomas. Eur J Cancer. England. 2014;50(16):2802–9.
La Rosa S, Marando A, Sessa F, Capella C. Mixed adenoneuroendocrine carcinomas (MANECs) of the gastrointestinal tract: an update. Cancers (Basel). 2012;4(1):11–30.
Volante M, Righi L, Asioli S, Bussolati G, Papotti M. Goblet cell carcinoids and other mixed neuroendocrine/nonneuroendocrine neoplasms. Virchows Arch. Germany. 2007;451(Suppl):S61–9.
Apostolidis L, Bergmann F, Jäger D, Winkler EC. Efficacy of topotecan in pretreated metastatic poorly differentiated extrapulmonary neuroendocrine carcinoma. Cancer Med. 2016;5(9):2261–7.
Frizziero M, Wang X, Chakrabarty B, Childs A, Luong TV, Walter T, et al. Retrospective study on mixed neuroendocrine non-neuroendocrine neoplasms from five European centres. World J Gastroenterol. 2019;25(39):5991–6005.
La Rosa S, Marando A, Furlan D, Sahnane N, Capella C. Colorectal poorly differentiated neuroendocrine carcinomas and mixed adenoneuroendocrine carcinomas: insights into the diagnostic immunophenotype, assessment of methylation profile, and search for prognostic markers. Am J Surg Pathol. United States. 2012;36(4):601–11.
McCusker ME, Coté TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. United States. 2002;94(12):3307–12.
Zheng M, Li T, Li Y, Zhang T, Zhang L, Ma W, et al. Survival profile and prognostic factors for appendiceal mixed neuroendocrine non-neuroendocrine neoplasms: a SEER population-based study. Front Oncol. 2020;10:1660.
Maru DM, Khurana H, Rashid A, Correa AM, Anandasabapathy S, Krishnan S, et al. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am J Surg Pathol. United States. 2008;32(9):1404–11.
Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38(4):437–47.
La Rosa S, Adsay V, Albarello L, Asioli S, Casnedi S, Franzi F, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol. United States. 2012;36(12):1782–95.
Kim J, Lee WJ, Lee SH, Lee KB, Ryu JK, Kim Y-T, et al. Clinical features of 20 patients with curatively resected biliary neuroendocrine tumours. Dig liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. Netherlands. 2011;43(12):965–70.
Harada K, Sato Y, Ikeda H, Maylee H, Igarashi S, Okamura A, et al. Clinicopathologic study of mixed adenoneuroendocrine carcinomas of hepatobiliary organs. Virchows Arch. Germany. 2012;460(3):281–9.
Laenkholm IT, Langer SW, Andreassen M, Holmager P, Kjaer A, Klose M, et al. A short report of 50 patients with gastroenteropancreatic mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN). Acta Oncol (Stockholm, Sweden). England. 2021;60:808–12.
La Rosa S, Sessa F, Uccella S. Mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr Pathol. United States. 2016;27(4):284–311.
Brathwaite S, Yearsley MM, Bekaii-Saab T, Wei L, Schmidt CR, Dillhoff ME, et al. Appendiceal mixed adeno-neuroendocrine carcinoma: a population-based study of the surveillance, epidemiology, and end results registry. Front Oncol. 2016;6:148.
Nießen A, Schimmack S, Weber TF, Mayer P, Bergmann F, Hinz U, et al. Presentation and outcome of mixed neuroendocrine non-neuroendocrine neoplasms of the pancreas. Pancreatol Off J Int Assoc Pancreatol . [et al]. Switzerland; 2021;21(1):224–235.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Pernille Holmager declares that she has no conflict of interest. Seppo Langer declares that he has no conflict of interest. Andreas Kjaer declares that he has no conflict of interest. Lene Ringholm declares that she has no conflict of interest. Rajendra Singh Garbyal declares that he has no conflict of interest. Hans-Christian Pommergaard declares that he has no conflict of interest. Carsten Palnaes Hansen declares that he has no conflict of interest. Birgitte Federspiel declares that she has no conflict of interest. Mikkel Andreassen declares that he has no conflict of interest. Ulrich Knigge declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuroendocrine Cancers
Rights and permissions
About this article
Cite this article
Holmager, P., Langer, S.W., Kjaer, A. et al. Surgery in Patients with Gastro-Entero-Pancreatic Neuroendocrine Carcinomas, Neuroendocrine Tumors G3 and High Grade Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Curr. Treat. Options in Oncol. 23, 806–817 (2022). https://doi.org/10.1007/s11864-022-00969-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-022-00969-x