Abstract
Introduction
Butyrylcholinesterase (BChE), an important biomarker of exposure to anticholinesterases, varies its activity according to the intensity and duration of exposure to these agents. Their normal values may vary in different populations. It is important to determine the reference values for the local population, mostly black/brown.
Objective
The objective was to investigate the baseline values of BChE activity in a sample of the Salvador city population (Bahia, Brazil), evaluating the sociodemographic characteristics.
Method
A descriptive, quantitative study with a cross-sectional approach was carried out in 304 voluntary and healthy blood donors. BChE activity was determined using the integrated chemical system Dimension RxLMax and analyses of sociodemographic characteristics were performed.
Results
For the 304 participants (18 to 67 years old), BChE activity values range were 7.4 to 19.8 U/mL (male) and 6.0 to 19.6 U/mL (female), without significant inter-racial differences (p = 0.986; Mann–Whitney). The participates were predominantly black (44.7%) and brown (40.5%), with higher levels of BchE activity in males (64.8%) (p-value = 0.01) than females (35.2%). There was no relationship between alcohol use and lower BChE activity (p = 0.725, Mann–Whitney). Women using hormonal contraceptives had a median activity 9.2% lower than the non-users.
Conclusion
Despite the high miscegenation and predominance of the black race in Salvador, contrary to what was expected, the sample did not show statistically significant intra-racial differences in BChE activity, being able to use the same reference values currently used, observing factors such as sex, use of contraceptives, and drinking alcohol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholinesterases are enzymes in the group of hydrolases that catalyze the hydrolysis of choline esters, playing a fundamental role in the transmission of nerve impulses. There are three types: neurotoxic esterase, acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) [1]. BChE, also called acylcholine acylhydrolase (EC 3.1.1.8), plasma cholinesterase, or pseudocholinesterase, predominates in the plasma, liver, neuroglia, pancreas, and digestive tract walls. BChE is a tetrameric glycoprotein, with a molecular mass of 342 kDa, synthesized in the liver and renewed for 7 to 60 days. In the central nervous system (CNS), it is present in glial cells, but not in neurons [2,3,4,5,6].
Several studies claimed that the physiological functions of BChE were not yet well-known, unlike acetylcholinesterase, which has a clearly defined role in neurotransmission. Numerous mutations observed in the human BChE gene are mentioned, many of which are inactive, which may suggest that the enzyme be redundant. However, its toxicological and pharmacological importance in the detoxification or metabolization of drugs containing ester such as succinylcholine, suxamethonium, mevacurium, procaine, tetracaine, heroin, and cocaine is recognized [4, 6, 7].
Studies show the relationship of BChE with lipid metabolism and association with body mass index (BMI), cholesterol, and triglyceride levels [5]. More recently, BChE has been found to hydrolyze the gut neuropeptide hormone, ghrelin, the only orexigenic hormone known to date to stimulate food intake and promote obesity and insulin resistance. Thus, Chen et al. [8] and Pope and Brimijoin [9] believe that ghrelin modulation plays an important physiological role for this enzyme.
Therefore, BChE controls plasma ghrelin by regulating eating behavior. When the human organism has low levels of the enzyme, the desire to eat can be increased, having a strong impact on weight gain [8, 9].
BChE activity varies widely in people not exposed to anticholinesterase agents according to some characteristics such as age, sex, reproductive status, health status, and ethnic and genetic characteristics. In the specific case of females, the variations are related to the hormonal status (puberty, pregnancy, menstruation, menopause, abortion, and consumption of hormonal contraceptives) [10, 11].
The demographic variable race or skin color may be related to discrete tissue adaptations of the internal environment (genotype) at the level of membranes, ion channels, enzymes, and receptors that can lead to biochemical, physiological, biophysical, and kinetic and dynamic modifiers of a drug, with potential change in the final effect observed in the patient (phenotype) that can be expressed through biochemical measurements. The genotype, in turn, determines the individual’s specific genetic code that leads to different responses in the organism [12,13,14,15].
According to some studies, compared to other races, black Americans show, for example, a lower response to some antihypertensives (such as angiotensin conversion enzyme inhibitors – ACE inhibitors) and better response to others (loop diuretics and drug antagonists calcium channels, for example): lesser fibrinolytic action of tissue plasminogen activator (t-PA), slower recovery from intravenous anesthesia in the combined use of remifentanil and propofol, lower glucuronidation of paracetamol, and higher risk of hemolysis to oxidative drugs due to a deficit of G-6-PD in red blood cells, which is observed in 10% of the black population [14, 15].
Studies conducted by Jones [16] and Wood [12] show that heterogeneity in the distribution of polymorphic characteristics, which occur at different frequencies in different populations, resulting from genetic differences between racial and ethnic groups, may involve, in addition to enzyme activity, the drug metabolism and its receptors, for example.
The variation in butyrylcholinesterase activity related to interracial differences and lower levels of BChE in black people compared to levels in white people of the same sex has been evidenced by some researchers, explained by the frequency of enzyme variants [17]. The occurrence of atypical mutations in human BChE has been extensively studied, resulting in the identification of more than seventy variants. The possibility of multiple mutations within a single BChE gene or combination of mutations is reported [6, 13, 18,19,20,21,22,23].
These genetic alterations result, in general, in a deficiency in the enzymatic activity; however, it is important to emphasize that a low level of BChE, or even its complete absence, is perfectly compatible with the normal development of the human being. Thus, individuals with a deficiency of this enzyme are asymptomatic; however, they have a greater sensitivity to muscle relaxants suxamethonium, mivacurium, and other related drugs, including cocaine [6, 13, 18, 24, 25].
Patients with BChE variants may exhibit markedly prolonged paralysis after administration of succinylcholine, suxamethonium, or mivacurium. These muscle relaxants used in the preoperative period lead to paralysis of the striated muscles for a few minutes, and then they are metabolized by normal cholinesterase. When the patient presents this enzyme with its activity diminished or absent, the paralysis may have its effect prolonged, leading to an apnea for half an hour or more, with risk of death. However, these variants are less frequent in the black race [13, 15, 18, 19, 24].
The best known alleles are as follows: the usual, wild, or “normal” BChE (BChEU), the atypical or “dibucaine-resistant” (BChEA), the “silent” (BChES), and the “fluoride-resistant” (BChEF). The genes that control their synthesis are alleles in relation to each other. The usual form, homozygous (BChEU/BChEU) or heterozygous with the BChES allele (BChEU / BChES), determines the normal BChE, which is the most frequent type in all populations. “Atypical” homozygotes have much lower levels of BChE [24, 26, 27].
It is hypothesized that the atypical allele (BCHEA) has a selective advantage due to its considerable frequency: Eastern Jews, 10%; North American and Brazilian Caucasoids, 2 to 3%; mixed Brazilians, 1.5%; and among Negroids and Mongoloids from other populations, less than 1%. In this case, since the mutant enzyme is less sensitive to anticholinesterases, its carriers would be more resistant to the poisoning of solanine (a naturally toxic glycoalkaloid present in plants of the Solanaceas genus, such as potatoes and green tomatoes) [24, 26,27,28].
Cocaine hydrolysis (COC) by BChE occurs slowly. Its variants prolong the half-life of this drug in the body, with rare clinical problems. However, with the continued use of COC, especially in forms of rapid absorption, such as “crack,” toxic levels of the drug accumulate, which can lead to death, which is more frequent among homozygotes and compound heterozygotes for variants of the BChE [24, 26, 27, 29]. Negrão et al. (2013) found evidence of an association between cocaine dependence and the presence of polymorphism in drug-dependent patients treated at specialized clinics in São Paulo (Brazil).
Given this variability, it is difficult to establish a normal mean value of enzyme activity that can be applied to the general population, disregarding the factors that influence cholinesterase activity [26, 30, 31].
Brazil suffered a very diversified colonization and, as a consequence, there is a predominance of different ethnic groups in different regions of the country [32]. In this country, public statistics use a color classification system composed of five categories: white, black, brown, indigenous, and yellow [33]. The Racial Equality Statute (instituted by Law No. 12,288/2010), in turn, defines the black population as “the group of people who call themselves black and brown, according to the color or race used by the Brazilian Institute of Geography and Statistics Foundation (IBGE), or that adopt similar self-definition.”
According to the IBGE, based on the last Census (2010), the largest proportions of browns are found in the North and Northeast Regions, while blacks are proportionally more present in the Northeast Region, notably in Bahia, with 2.4 million people (17.1%) who declared themselves to be that color (BRASIL, 2010). IBGE National Household Sample Survey (PNAD Continuous), in turn, shows that 48.3% and 33.4% of Salvador’s residents, in 2018, declared themselves to be brown and black, respectively [34].
Thus, this study sought to investigate the baseline values of butyrylcholinesterase activity in a sample of the population of Salvador made up of healthy people not exposed to anticholinesterase pesticides, considering their sociodemographic characteristics.
Materials and methods
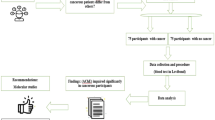
This descriptive, quantitative, cross-sectional study was approved by the Research Ethics Committee of the State of Bahia Health Department (Trial-Code No. 2,133,618/2017), with a sample of 304 adult volunteers blood donors at the Hematology and Hemotherapy Foundation of Bahia (Hemoba), with no history of exposure to cholinesterase-inhibiting pesticides and without physiological conditions that could affect BChE activity levels.
The participants were selected for convenience, considering as inclusion criteria: being over 17 years old, accept to participate in the research, with the signature of the Free and Informed Consent Form (ICF), being able to donate blood, established by clinical screening performed by doctors from Hemoba, and the absence of a history of exposure to anticholinesterase substances. The non-inclusion criteria were not to live in Salvador, under the age of 18, inability to donate blood and a history of exposure to cholinesterase inhibitors.
Participants, after clinical screening conducted by doctors from the Hemoba Foundation and approval for the donation, were invited to participate in the research. Having accepted the invitation, after signing the Free and Informed Consent Term (ICF), they were interviewed just before the blood collection by a previously trained team, through the use of a structured script, elaborated with questions that address socio-demographic characteristics, health and lifestyle, considering the following variables: age, sex, color/race (self-declared, based on the classification used by the Brazilian Institute of Geography and Statistics – IBGE [34]); alcohol consumption habits and using hormonal contraceptives (CH).
Blood samples were collected through the device used in venipuncture for blood bags, immediately before collection for donation, consisting of a volume of 5 mL, in a tube with heparin, for the determination of butyrylcholinesterase. Volunteers whose samples were hemolyzed or lipemic were excluded. These samples were processed at the Emergency Toxicological Analysis Laboratory (Labtox) of the Bahia Toxicological Information and Assistance Center (CIATox-BA). After centrifugation, an aliquot of 200 µL of plasma was separated to determine the activity of BChE in triplicate and the rest was stored in a freezer at − 20 °C.
The determination of BChE activity was performed using the Siemens Healthcare Diagnostics Dimension® RxL Max® clinical chemistry system, with reagent cartridge ready for analysis, whose method is based on the coupled oxidation–reduction reaction described by Gal and Roth [35]. The procedure is based on the hydrolysis of butyrylthiocoline (BTC) by BChE, resulting in the release of thiocoline, which directly reduces 2,6-dichlorophenol-indophenol (DIP), blue dye, to its colorless form. The resulting change in absorbance at 600 nm is directly proportional to BChE activity, being measured using a bichromatic rate technique (600 and 700 nm) [36]. The manufacturer recommended reference value is 7 to 19 U/mL.
In order to validate the BCHE enzyme activity kit, measurements of selectivity, linearity, detection limits, quantification limit, trend/recovery, precision, and robustness were determined following the American Agency Food and Drug Administration (FDA) criteria [37].
The results obtained were plotted in an Excel® spreadsheet and later analyzed using the SPSS V.9.0 and Minitab V.17 software. Values were considered statistically significant when p < 0.05. The normality analysis of the distribution of quantitative variables was performed by the Kolmogorov–Smirnov test. From this information, parametric association tests were used, by comparing means, such as Student’s t (2 variables) or ANOVA (3 or more variables) tests or non-parametric, by comparing medians, such as Mann–Whitney U (2 groups) or Kruskal–Wallis (for evaluations of averages in groups of three or more categories) tests. The analysis of qualitative or categorical variables from three or more groups was performed using the non-parametric chi-square test, duly corrected by the Mantel–Haenszel and Yates tests. Fisher exact test was used for the analysis of values below 5.
Results
The validation of the kit used to determine the activity of butyrylcholinesterase showed excellent linearity (y = 1.0507x − 0.4227; R2: 0.9997), showing the strong relationship between the enzyme activity and the readings obtained. Furthermore, its limits have been guaranteed, which demonstrates that the kit is effective for this study.
In the period from January to June 2019, 329 samples were obtained, however, 25 were excluded due to presenting hemolysis or lipemia or, still, with insufficient data or with incongruities for analysis. Therefore, 304 samples were evaluated.
When assessing the basic demographic characteristics and BChE activity levels of the survey participants, it was observed that 64.8% (197) were male. Men had a median age greater than women (p = 0.001; Mann–Whitney) (Table 1).
More than 50% of the participants were between 18 and 35 years old. The age group from 26 to 35 years old predominated in both sexes, being 29.0% for women and 27.4% for men.
Of the 304 participants, there was a predominance of individuals of the self-declared black color/race (44.7%), followed by brown (40.5%).
The study showed individual variations in BChE activity in the population studied (Table 2).
The percentile distribution of the levels found in this study showed that the 2.5th percentile position corresponded to the values of 8.2 U/mL and 9.3 U/mL for females and males, respectively. At the 97.5th percentile, the values found were 18.8 U/mL for females and 18.4 U/mL for males. Therefore, all values obtained were inside of the manufacturer recommended reference range (7–19 U/mL).
This study did not observe a statistically significant difference (p = 0.8878; Mann–Whitney) of BChE activity between races, with an average of 13.6 ± 2.5 U/mL and 13.6 ± 2.5 U/mL for both black and brown, when compared to white color (13.6 ± 2.7 U/mL). The three groups (white, black, and brown) were compared separately in relation to sex to verify the influence of this variable on BChE activity in different colors/races (Table 3), with no significant difference (p = 0.633; Kruskal–Wallis test, females, and p = 0.380; Kruskal–Wallis test, males).
The relationship between BChE activity and the age group (p = 0.002; test = Kruskal–Wallis) showed an increase in the median of activity as the age group increased, what happened until the group of 46 to 55 years old, decreasing in the age 56 to 65 years old. The age group was then divided into two groups by sex, to assess the effect of this factor on enzyme activity, which was evidenced only in males (p = 0.006; Kruskal–Wallis test), where there was an increase in activity from BChE to the group of 46 to 55 years, decreasing in the range of 56 to 65 years (Table 4). In females (p = 0.293; Kruskal–Wallis test), the mean of enzymatic activity does not differ between age groups (Table 4).
Of the total number of female participants (n = 107), thirty-one (29.2%) used HC. The median levels of BChE activity in women using HC (11.8 U/mL; 10.5–13.0) were lower when compared to those not using the hormone (13.0 U/mL; 11.8–14.8; p = 0.0083; Mann–Whitney test); however, no sample showed activity below 7.2 U/mL (Table 2). This finding corroborates the results found by Carmona-Fonseca [10], Caro-Gamboa et al. [11], Sidell and Kaminskis [38], Vásquez and Osorio [39], and Whittaker et al. [40].
The influence of menopause (n = 18) on enzyme activity was also evaluated, with no significant difference (p = 0.0897; Mann–Whitney test).
Regarding the alcohol consumption habits, 171 (56.3%) participants reported using it with some frequency, and the levels of enzyme activity were higher in those who did not report using these drinks. However, there was no significant difference in BChE activity between the two groups (13.8 U/mL (12.0–15.4); 13.5 U/mL (11.8–14.8); p = 0.725, Mann–Whitney), as seen in Table 2, differently from what was found in the literature [22, 41, 42].
Discussion
The present study reports the activity levels of butyrylcholinesterase in a sample of the population of Salvador city (capital of the state of Bahia) and its realization is justified by the need to know the baseline levels and establish reference values in relation to this laboratory parameter for that population, considering their ethnic characteristics and genetics resulting from high miscegenation, with a predominance of individuals of black and brown ethnicity [43,44,45]. The scientific literature shows evidence that the black population, whether from Africa or the Americas, may show differences in the activity of enzymes, in the metabolism of drugs and their receptors.
There are few studies carried out in Brazil in recent years with populations not occupationally exposed to cholinesterase inhibitor pesticides and without association of specific pathology that assess the activity of this enzyme among different racial groups. Twenty-three articles have been identified between 1965 and 2013 and all of them studied the frequency of genetic variants of BChE (which lead to changes in enzyme activity), but few related the frequency of these variants to different racial groups. The majority of these surveys (61%) took place before the year 2000, with nine of these specifically involving samples of indigenous populations. Among the others, only two used samples from the population of the Northeast Region of Brazil, one of which was carried out by Chautard-Freire-Maia et al. [17] and consisted of evaluating the frequency of BChEA in samples of 856 women classified by racial group in two hospitals in the Salvador city [17, 45].
Therefore, it is clear that there is a scarcity of studies involving the analysis of cholinesterases in a population not exposed to anticholinesterase drugs in the state of Bahia (Brazil).
Although Salvador is one of the capitals with the highest frequency of black population in Brazil, contrary to what was expected, this study did not observe lower levels of BChE activity in people with this characteristic, compared to whites of the same sex, although it was found by Chautard-Freire-Maia et al. [17] a frequency of 0.394 ± 0.227% of the atypical BChE gene in black people in their study.
The activity of the BChE enzyme, as well as acetylcholinesterase, is a relevant parameter for assessing exposure to anticholinesterase agents, both in chronic and acute use; however, for a good interpretation of its results, it is important that the professional is aware of the inter factors and intraindividuals that can interfere with the activity of these enzymes, such as age, sex, body mass, lipid levels, pregnancy, and use of contraceptives [11, 26, 31, 46, 47].
The results obtained in the present study, when comparing ethnic groups, did not find evidence, therefore, to reject the null hypothesis (equal activity between racial groups) as a single group. On the other hand, median BChE values for men were significantly higher than those found for women, similar to the results obtained by Câmara et al. [30], Jiménez-Diaz and Schosinsky-Nevermann [47], and Carmona-Fonseca [48], but diverging from the results obtained by other authors where there was no significant difference between genders [31, 49].
The scientific literature has reported lower levels of BChE activity in women [30, 44, 47, 50,51,52,53], which was found in the present study. According to Sidell and Kaminskis [38] and Lepage et al. [54], as mentioned by Lockridge and Masson [55], these levels are around 36 to 50% lower than in males.
Based on the recommendations of the International Federation of Clinical Chemistry and Laboratory Medicine [44, 56], the authors propose as reference values for the population of the city of Salvador the ranges from 8.2 to 18.8 and 9.3 to 18.4 U/mL for females and males, respectively. Unlike these findings, some studies have found no statistically significant difference in BChE activity between genders [31, 45, 48, 57]. Study carried out by Zlatković et al. [49], who developed a similar study using Dimension RxL© in a sample of the population of the Republic of Serbia, in the 2.5th percentile found the values of 9.0537 U/mL (9,053.7 U/L) for both sexes, close to those found by these authors. On the other hand, in the 97.5 percentile the values obtained by them were higher, that is, 23.6718 U/mL (23,671.8 U/L) for both sexes.
In a study developed in Colombia by Sanchez et al. [45], using a modification of the Ellman method, there was also no difference in enzymatic activity between the sexes, with values between 9,945.8 and 10,462.2 U/L. Siqueira et al. [31], in turn, developed a study in academics from the University of São Paulo using the method of Michel and Caraway, obtaining results slightly higher than those found at the time in the literature, from 77 U for BChE. These differences, possibly, are related to demographic, geographic and genetic factors, as well as the use of different laboratory methods and techniques, which makes it difficult to compare these results [45, 56].
This study showed that women who used hormonal contraceptives had lower BChE activity, thus corroborating the results found by Caro-Gamboa et al. [11], Macqueen and Plaut [26], Câmara et al. [30], Sidell and Kaminskis [38], Vásquez and Osorio [39], Whittaker et al. [40], and Jiménez-Diaz and Schosinsky-Nevermann [47].
Of the total number of female participants (n = 107), thirty-one (29.2%) used HC. The median levels of BChE activity in women using HC were lower when compared to those not using the hormone; however, no sample showed activity below 7.2 U/mL. This finding corroborates the results found by Caro-Gamboa et al. [11], Sidell and Kaminskis [38], Vásquez and Osorio [39], and Whittaker et al. [40] and Jiménez-Diaz and Schosinsky-Nevermann [47].
The activity levels of BChE increased as the age group increased, up to the limit of 46 to 55 years. It was evident that this influence was due to males, since the difference was not significant for females. Some studies carried out by other authors also found virtually no significant difference in both sexes, except for the youngest age group (18 to 29 years) in females [58, 59].
When analyzing the activity of BChE in relation to alcohol consumption habits, there was not statistical significance between the means of the two groups (“alcohol intake” and “no alcohol intake”), diverging from the results reported in the literature [42, 58]. We assume that this divergence may be related to a possible moderate consumption of alcoholic beverages.
In the monitoring of occupationally exposed individuals, it is important to have baseline or pre-exposure values, so that an assessment of the laboratory result can be made with greater safety, reducing the influence of inter-individual variables.
Local reference values can be an important tool to support a safer interpretation of the results obtained from cholinesterase activity, based on the characteristics of the regional population.
Conclusion
The results presented by this study can be useful in the clinical routine when the determination of BChE is necessary for the diagnosis and prognosis of intoxications by pesticides cholinesterase inhibitors in occupational or accidental exposures, either in individual or mass events, considering the sociodemographic characteristics population of Salvador, in particular, ethnic characteristics.
For the studied sample constituted of individuals considered healthy, not occupationally exposed to anticholinesterase substances, the baseline values of plasma cholinesterase (BChE) were determined as ranges from 8.2 to 18.8 U/mL for females and 9.3 to 18.4 U/mL for males, for both black and non-black individuals, since no differences were observed between these two groups.
Women using hormonal contraceptives had median BChE levels (11.8 U/mL; 10.5–13.0) lower than those who did not use them (13.0 U/mL; 11.8–14.8) (p = 0.008; Mann–Whitney test), however, with levels of enzyme activity not less than 7.2 U/mL.
References
Makhaeva GF, Rudakova EV, Richardson RJ (2018) Investigation of the esterase status as a complex biomarker of exposure to organophosphorus compounds. biomedical chemistry: research and methods. Institute of Biochemistry 1(3):e00028. https://doi.org/10.18097/bmcrm00028
Alonzo HGA, Corrêa CL (2003) Praguicidas. In: OGA, Seizi. Fundamentos de toxicologia. São Paulo: Atheneu (2):437–458
Goodal R (2004) Cholinesterase: phenotyping and genotyping. Ann Clin Biochem 41(Pt 2):98–110. https://doi.org/10.1258/000456304322879971 (PMID: 15025799)
Johnson G, Moore SW (2012) Why has butyrylcholinesterase been retained? Structural and functional diversification in a duplicated gene. Neurochem Int 61(5):783–797. https://doi.org/10.1016/j.neuint.2012.06.016 (Epub 2012 Jun 28 PMID: 22750491)
Lockridge O (2015) Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther 148:34–46. https://doi.org/10.1016/j.pharmthera.2014.11.011 (Epub 2014 Nov 20 PMID: 25448037)
Delacour H, Dedome E, Courcelle S et al(2016) Butyrylcholinesterase deficiency. Ann Biol Clin (Paris) 74(3):279–85. English. https://doi.org/10.1684/abc.2016.1141 (PMID: 27237801)
Lockridge O (1990) Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. Pharmacol Ther 47(1):35–60. https://doi.org/10.1016/0163-7258(90)90044-3 (PMID: 2195556)
Chen VP, Gao Y, Geng L, Brimijoin S (2017) Butyrylcholinesterase regulates central ghrelin signaling and has an impact on food intake and glucose homeostasis. Int J Obes (Lond) 41(9):1413–1419. https://doi.org/10.1038/ijo.2017.123 (Epub 2017 May 22. PMID: 28529331; PMCID: PMC5585042)
Pope CN, Brimijoin S (2018) Cholinesterases and the fine line between poison and remedy. Biochem Pharmacol 153:205–216. https://doi.org/10.1016/j.bcp.2018.01.044 (Epub 2018 Jan 31. PMID: 29409903; PMCID: PMC5959757)
Carmona-Fonseca J (2006) Relación entre los niveles de colinesterasa y los grupos sanguíneos ABO y Rh. Acta Med Colomb 31(3):104–112
Caro-Gamboa LJ, Forero-Castro M, Dallos-Báez AE (2020) Cholinesterase inhibition as a biomarker for the surveillance of the occupational population exposed to organophosphorus pesticides. Ciencia y Tecnología Agropecuaria 21(3). https://doi.org/10.21930/rcta.vol21_num3_art:1562
Wood AJ (2001) Racial differences in the response to drugs–pointers to genetic differences. N Engl J Med 344(18):1394–1396. https://doi.org/10.1056/NEJM200105033441811 (PMID: 11336055)
Lockridge O, Norgren RB Jr, Johnson RC, Blake TA (2016) Naturally occurring genetic variants of human acetylcholinesterase and butyrylcholinesterase and their potential impact on the risk of toxicity from cholinesterase inhibitors. Chem Res Toxicol 29(9):1381–92. https://doi.org/10.1021/acs.chemrestox.6b00228 (Epub 2016 Aug 31. PMID: 27551784; PMCID: PMC5030680)
Vale NB, Delfino J (2003) Anestesia na população negra [Anesthesia in the afro-american population]. Rev Bras Anestesiol 53(3):401–18. Portuguese. https://doi.org/10.1590/s0034-70942003000300013 (PMID: 19475293)
Vale NB, Delfino J, Vale LFB (2003) O Conhecimento de Diferenças Raciais pode Evitar Reações Idiossincrásicas na Anestesia? Rev Bras Anestesiol 53(2):258–277
Jones DS (2013) How personalized medicine became genetic, and racial: Werner Kalow and the formations of pharmacogenetics. J Hist Med Allied Sci 68(1):1–48. https://doi.org/10.1093/jhmas/jrr046 (Epub 2011 Sep 10 PMID: 21908852)
Chautard-Freire-Maia EA, Carvalho RD, da Silva MC et al (1984) Frequencies of atypical serum cholinesterase in a mixed population of northeastern Brazil. Hum Hered 34(6):364–370. https://doi.org/10.1159/000153497 (PMID: 6510933)
Ceppa F, Gidenne S, Benois A et al (2002) Rapid identification of atypical variant of plasma butyrylcholinesterase by PCR. Clin Chem Lab Med 40(8):799–801. https://doi.org/10.1515/CCLM.2002.138 (PMID: 12392308)
Delacour H, Lushchekina S, Mabboux I et al (2014) Characterization of a novel BCHE “silent” allele: point mutation (p.Val204Asp) causes loss of activity and prolonged apnea with suxamethonium. PLoS One 9(7):e101552. https://doi.org/10.1371/journal.pone.0101552 (PMID: 25054547; PMCID: PMC4108472)
Goodall R, Association of Clinical Biochemists Analytical Investigations Standing Committee (2004) Cholinesterase: phenotyping and genotyping. Ann Clin Biochem 41(Pt 2):98–110. https://doi.org/10.1258/000456304322879971 (PMID: 15025799)
Howard TD, Hsu FC, Grzywacz JG et al (2010) Evaluation of candidate genes for cholinesterase activity in farmworkers exposed to organophosphorus pesticides: association of single nucleotide polymorphisms in BCHE. Environ Health Perspect 118(10):1395–9. https://doi.org/10.1289/ehp.0901764 (Epub 2010 Jun 8. PMID: 20529763; PMCID: PMC2957918)
McQueen MJ (1995) Clinical and analytical considerations in the utilization of cholinesterase measurements. Clin Chim Acta 237(1–2):91–105. https://doi.org/10.1016/0009-8981(95)06067-n (PMID: 7664482)
Lockridge O, Bartels CF, Vaughan TA et al (1987) Complete amino acid sequence of human serum cholinesterase. J Biol Chem 262(2):549–557 (PMID: 3542989)
Borges-Osório MR, Robinson WM (2001) Genética humana. Porto Alegre: Artmed (3):243–244
Ramaiah M, Prudhivi RK (2020) Pseudocholinesterase deficiency in an Indian community. Journal of Pharmacy Practice and Community Medicine 3(1):27–30. https://doi.org/10.5530/jppcm.2017.1.6
Macqueen J, Plaut D (1973) A review of clinical applications and methods for cholinesterase. Am J Med Technol 39(7):279–287 (PMID: 4354600)
Henry JB (1989) Diagnósticos clínicos e conduta terapêutica por exames laboratoriais. Ed Manole 1(16):414–416
Janeway CA (1980) Review: biological function of cholinesterase. Clin Biochem 13(6):239–243
Negrão AB, Pereira AC, Guindalini C et al (2013) Butyrylcholinesterase genetic variants: association with cocaine dependence and related phenotypes. PLoS ONE 8(11):e80505. https://doi.org/10.1371/journal.pone.0080505.PMID:24312228;PMCID:PMC3842332
Câmara SA, Silva I, Pontes ER, Barbosa AM (2012) Exposição a agrotóxicos: determinação dos valores de referência para colinesterase plasmática e eritrocitária. Brasília Med 49(3):163–169
Siqueira MEPB, Fernícola NAGG, Borges EL (1978) Determinação de níveis normais de colinesterase plasmática e eritrocitária. Rev Saúde Pública São Paulo 12(3). https://doi.org/10.1590/S0034-89101978000300008.
Salzano FM, Sans M (2014) Interethnic admixture and the evolution of Latin American populations. Genet Mol Biol 37(1 Suppl):151–170. https://doi.org/10.1590/s1415-47572014000200003.PMID:24764751;PMCID:PMC3983580
Petrucelli JL, Saboia AL (2013) Características Étnico-raciais da População: classificações e identidades. IBGE Rio de Janeiro 2:208
Brasilde Geografia e Estatística - IBGE. Censo Demográfico, IB (2010) Características gerais da população, religião e pessoas com deficiência. Rio de Janeiro 2010:66
Gal EM, Roth E (1957) Spectrophotometric methods for determination of cholinesterase activity. Clin Chim Acta 2(4):316–326. https://doi.org/10.1016/0009-8981(57)90009-8 (PMID: 13473097)
Siemens Healthcare Diagnostics Ltd (2018) Dimension clinical chemistry system. Flex reagent cartridge. Instruction manual. Camberley
US Department of Health and Human Services Food and Drug Administration (2019) Draft guidance document: M10 bioanalytical method validation. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/m10-bioanalytical-method-validation. Accessed 9 Jul 2021
Sidell FR, Kaminskis A (1975) Influence of age, sex, and oral contraceptives on human blood cholinesterase activity. Clin Chem 21(10):1393–1395 (PMID: 1157304)
Vásquez L, Osorio J (2000) Variación de la Actividad de la Enzima Butirilcolinesterasa en Usuarias de Anticonceptivos Hormonales. An Fac Med (Perú) 61(4):271–277
Whittaker M, Charlier AR, Ramaswamy S (1971) Changes in plasma cholinesterase isoenzymes due to oral contraceptives. J Reprod Fertil 26(3):373–375. https://doi.org/10.1530/jrf.0.0260373 (PMID: 5569652)
Huang A, Chang B, Sun Y et al (2017) Disease spectrum of alcoholic liver disease in Beijing 302 hospital from 2002 to 2013: a large tertiary referral hospital experience from 7422 patients. Medicine (Baltimore) 96(7) (PMID: 28207552; PMCID: PMC5319541)
Hemantha Kumara DS, Muralidhara Krishna CS, Vishwanath H (2018) A comparative study in assessing the usefulness of serum cholinesterase, high sensitivity C-reactive protein with Liver Function Tests in Alcoholic Liver disease. Indian J Med Biochem 22(2):147–153. https://doi.org/10.5005/jp-journals-10054-0073
Giolo SR, Soler JM, Greenway SC et al (2012) Brazilian urban population genetic structure reveals a high degree of admixture. Eur J Hum Genet 20(1):111–6. https://doi.org/10.1038/ejhg.2011.144 (Epub 2011 Aug 24. PMID: 21863058; PMCID: PMC3234512)
Queiroz EM, Santos AM, Castro IM et al (2013) Genetic composition of a Brazilian population: the footprint of the Gold Cycle. Genet Mol Res 12(4):5124–5133. https://doi.org/10.4238/2013.October.29.6 (PMID: 24301772)
Sánchez LH, Medina OM, Gómez G et al (2015) Laboratory genetic-based reference values for cholinesterase activity in a Colombian population: a step forward in personalized diagnostics. Biomedica 35 Spec:20–9. https://doi.org/10.1590/S0120-41572015000500003 (PMID: 26535739)
Simpson NE (1996) Factors influencing cholinesterase activity in a Brazilian population. Am J Hum Genet 18(3):243–52 (PMID: 5944418; PMCID: PMC1706078)
Jiménez-Díaz M, Schosinsky-Nevermann K (2000) Valores de referencia de colinesterasa plasmática y eritrocítica en población costarricense: comparación del desempeño clínico de ambas enzimas. Rev costarric cienc méd 21(3–4):117–126
Carmona-Fonseca J (2006) Correlación y conversión entre valores de colinesterasa eritrocitaria medida con las técnicas de Michel y EQM. Biomedica 26(4):546–555. https://doi.org/10.7705/biomedica.v26i4.324
Zlatković M, Krstić N, Subota V et al (2017) Determination of reference values of acetyl and butyryl cholinesterase activities in Serbian healthy population. Vojnosanit Pregl 74(8):736–741. https://doi.org/10.2298/VSP160303101Z
Karasova JZ, Maderycova Z, Tumova M et al (2017) Activity of cholinesterases in a young and healthy middle-European population: relevance for toxicology, pharmacology and clinical praxis. Toxicol Lett 5(277):24–31. https://doi.org/10.1016/j.toxlet.2017.04.017 (Epub 2017 Apr 30 PMID: 28465191)
Worek F, Schilha M, Neumaier K et al (2016) On-site analysis of acetylcholinesterase and butyrylcholinesterase activity with the ChE check mobile test kit-determination of reference values and their relevance for diagnosis of exposure to organophosphorus compounds. Toxicol Lett 13(249):22–28. https://doi.org/10.1016/j.toxlet.2016.03.007 (Epub 2016 Mar 29 PMID: 27033775)
Carmona-Fonseca J, Henao S, Garcés R (2000) Valores de referencia de actividad colinesterásica sanguínea en población laboral activa no expuesta a plaguicidas inhibidores de colinesterasa. Rev Fac Nal Sal Públ (Medellín) 18:55–72
Jensen FS, Skovgaard LT, Viby-Mogensen J (1995) Identification of human plasma cholinesterase variants in 6,688 individuals using biochemical analysis. Acta Anaesthesiol Scand 39(2):157–162. https://doi.org/10.1111/j.1399-6576.1995.tb04035.x
Lepage L, Schiele F, Gueguen R, Siest G (1985) Total cholinesterase in plasma: biological variations and reference limits. Clin Chem 31(4):546–550 (PMID: 3978785)
Lockridge O, Masson P (2000) Pesticides and susceptible populations: people with butyrylcholinesterase genetic variants may be at risk. Neurotoxicology 21(1–2):113–26 (PMID: 10794391)
Ichihara K, Ozarda Y, Barth JH et al (2017) A global multicenter study on reference values: 1. Assessment of methods for derivation and comparison of reference intervals. Clin Chim Acta 467:70–82. https://doi.org/10.1016/j.cca.2016.09.016 (Epub 2016 Sep 22. PMID: 27666761)
Küçükosman G, Pişkin Ö, Hancı V et al (2018) Pseudocholinesterase levels in patients under electroconvulsive therapy. Saudi Med J 39(1):103–106. https://doi.org/10.15537/smj.2018.1.21307 (PMID: 29332117; PMCID: PMC5885109)
Abou-Hatab K, O’Mahony MS, Patel S, Woodhouse K (2001) Relationship between age and plasma esterases. Age Ageing 30(1):41–45. https://doi.org/10.1093/ageing/30.1.41 (PMID: 11322671)
Hosseini J, Firuzian F, Feely J (1997) Ethnic differences in the frequency distribution of serum cholinesterase activity. Ir J Med Sci 166(1):10–2. https://doi.org/10.1007/BF02939767 (PMID: 9057423)
Acknowledgements
The authors thank the institutions Toxicological Information and Assistance Center of Bahia (CIATox-BA), the Secretariat of Health of the State of Bahia (SESAB), the Federal University of Bahia (UFBA), and the Hematology and Hemotherapy Foundation of Bahia (HEMOBA) who kindly authorized the access and use of their structures, necessary to carry out this work. This research had the support of Anicele de Jesus and Leonardo Buffone in collecting material, Agnaldo de Souza Orrico in the statistical analysis, and Siemens Helthcare in providing kits for determination of dosages of BChE activity. The authors would also like to thank each of the collaborators and donors participating in the research, without whom it would not materialize.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Conceição Filho, J.N., dos Santos, I.C., Gonçalves, D.P.d. et al. Black and non-black population: investigation of the difference in butyrylcholinesterase activity in a healthy population in Salvador, Bahia. Ir J Med Sci 192, 1311–1319 (2023). https://doi.org/10.1007/s11845-022-03087-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-03087-7