Abstract
Osteoarthritis is a significant cause of chronic pain in the elderly population with hip osteoarthritis as one of the main causes of functional disability and joint pain in adults older than 55 years. Recently, platelet rich plasma (PRP) injections have been introduced for treatment of osteoarthritis. The aim of this systematic review is to assess its effectiveness in the management of hip osteoarthritis. We performed a search of the literature for published prospective studies that assessed the effectiveness of PRP injections in the treatment of hip osteoarthritis, with a minimum follow-up of 3 months. Primary outcome measures were WOMAC and VAS scores. Five trials were identified with 185 patients undergoing treatment with ultrasound-guided intra-articular injections of PRP, compared with patients treated with hyaluronic acid alone (n = 148) or hyaluronic acid combined with PRP (n = 31) in one study. PRP was shown to improve patient outcome scores at follow-up at 6 and 12 months; however, there was no significant difference seen between patients treated with PRP or hyaluronic acid alone. Following this systematic review, we cannot currently recommend the use of intra-articular injections of PRP for the treatment of hip OA. Given that intra-articular steroid injections are the only such injection recommended by international guidelines for the treatment of hip OA, further studies comparing PRP to steroid would be of benefit to determine the value of PRP injections in hip OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is one of the most common joint diseases, and it is one of the major causes of pain and disability in older adults [1]. This disability threatens the independence of older adults and has significant social and economic costs. It typically affects weight-bearing joints, with the hip the second most frequently involved large joint after the knee. The incidence of hip osteoarthritis continues to increase secondary to an ageing and overweight population [2].
Treatments for osteoarthritis include conservative measures (weight loss, physiotherapy, activity modification, supports) and pharmacological treatments (analgesics, steroids, NSAIDs) administered topically, orally, or intra-articularly [3]. Oral medications have limitations with regard to the degree of efficacy and associated side effect profiles. Nonsteroidal anti-inflammatory drugs (NSAIDs) can be associated with significant exacerbation of co-morbidities commonly seen in the OA population and opioid-based medication can cause nausea, constipation, and drowsiness, which are not well tolerated by patients [4] as well as an increasingly recognised risk of long-term dependency.
Intra-articular injections offer a potential useful therapy as it directly targets the affected joint with low risk of systemic effects. Steroid injections for hip OA are supported by treatment guidelines from the National Institute for Clinical Excellence (NICE), American College of Rheumatology (ACR), and Osteoarthritis Research Society International (OARSI). A recent systematic review shows that steroid injections may be useful for short-term pain reduction although the study does note that the quality of the evidence was poor [5]. Hyaluronic acid has been introduced as an intra-articular injection for hip OA with some positive results in the literature, but it is not currently recommended by the NICE for the treatment of either knee or hip OA [6,7,8].
The use of platelet-rich plasma (PRP) has expanded significantly in recent years, with positive results seen in the treatment of tendon and ligament injuries [9, 10] and in the treatment of knee arthritis. In rabbit studies, it has been shown to improve osteochondral healing, on the macro- and microscopic scale [11, 12]. This study reviews the recent published evidence of PRP injections for hip OA and discusses the findings.
Methods
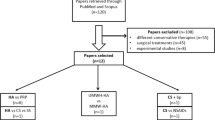
A search was performed of PubMed (up to 16 April 2019), Embase (up to 17 April 2019), and the Cochrane Central Registrar of Controlled Trials (CENTRAL) (latest) for studies which assessed the effectiveness of intra-articular hip injections using PRP in the treatment of hip osteoarthritis with a minimum of 3 months follow-up. Keywords and MeSH terms used were “PRP”, “platelet-rich plasma”, “hip osteoarthritis”, and “hip arthritis”. Inclusion criteria included all English language human clinical trials published in the last 10 years. All study design types, apart from case reports, were included. Five studies met our inclusion criteria, including four RCTs (Fig. 1).
Search strategy
We searched the following databases in order to identify eligible studies:
-
1.
The Cochrane Central Register of Controlled Trials (CENTRAL) (latest issue)
-
2.
PubMed (up to 16 April 2019)
The search terms used were as follows:
(“platelet-rich plasma” [MeSH Terms] or (“platelet-rich” [All Fields] and “plasma” [All Fields]) or “platelet-rich plasma” [All Fields] or (“platelet” [All Fields] and “rich” [All Fields] and “plasma” [All Fields]) or “platelet- rich plasma” [All Fields]) and (“osteoarthritis, hip” [MeSH Terms] or (“osteoarthritis” [All Fields] and “hip” [All Fields]) or “hip osteoarthritis” [All Fields] or (“hip” [All Fields] and “osteoarthritis” [All Fields])) [ptyp]
-
1-
Embase (up to 17 April 2019)
The search strategy involved the following:
#9 #7 and #8 (1)
#8 randomised and control and trial (35,350)
#7 #5 and #6 (74)
#6 #1 or #2 (12,187)
#5 #3 or #4 (15,345)
#4 “hip arthritis”/exp or “hip arthritis” (3728)
#3 “hip osteoarthritis”/exp or “hip osteoarthritis” (12,127)
#2 “platelet-rich plasma cell”/exp or “platelet-rich plasma cell” (266)
#1 “thrombocyte rich plasma”/exp (12,011)
We reviewed all titles and abstracts; duplicates were removed, and a full-text review was subsequently performed. Any ambiguity in relation to the inclusion/exclusion of a specific study was discussed with the second investigator, and any disagreements were resolved by reaching a consensus.
Outcome measures
Primary outcome measures were pain, assessed using the visual analog scale (VAS) and the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and functional capacity and stiffness, assessed by WOMAC. The secondary outcomes were any local or systemic adverse events recorded which were related to the interventions.
Results
A total of 4 RCTs were identified comparing PRP with HA, one of which also compared it against an injection of both HA and PRP (Table 1). A prospective study of PRP treatment without control or comparison was also identified.
The earliest study found was a non-randomized, prospective case series study by Sanchez et al. in 2012 of 40 patients treated with PRP [13]. All patients suffered with unilateral hip OA of at least mild severity (20 mm on VAS) with average pain score of 52 mm at baseline (on a 100 mm scale). The average age was 56 years (range 33–84). Three injections were performed under US guidance, with the interval between injections ranging between 1 to 2 weeks. WOMAC and VAS scores were evaluated at baseline, 6–7 weeks and 6 months post-treatment. A clinically significant reduction in pain was defined in this study as an improvement of 30% in WOMAC or VAS. Twenty-three (58%) patients had a clinically significant improvement in their pain which was sustained for 6 months. The remaining 17 patients either had a small change in pain which was not deemed clinically relevant (n = 6) or no change (n = 11).
Three studies performed comparative RCTs of PRP and HA. In 2013, Battaglia et al. carried out a prospective, randomized, single-centre trial with strict inclusion and exclusion criteria, with all patients having a history of unilateral hip pain between 6 and 24 months duration with associated radiographic findings of hip OA [14]. One hundred four patients were recruited with 4 lost to follow-up (12 months). The degree of OA severity was based on Kellgren-Lawrence score, with 39 patients with early OA, 44 with moderate OA, and 17 with severe OA [15]. Venous samples underwent centrifuge twice for preparation of PRP with 3 samples of 5 ml stored frozen before thawing for injection. All patients underwent 3 injections (once every 2 weeks) of 5ml of PRP or HA (30 mg/2 ml of high-molecular-weight HA—1500kD). Fifty underwent treatment with PRP while 50 underwent injections with HA. Follow-up was at 1, 3, 6, and 12 months post-treatment with assessment of VAS and Harris Hip Score (HHS) as primary outcome measures. Both groups experienced significant improvements in VAS and HHS early on (1 and 3 months follow-up) with PRP producing a 32% decrease in VAS score at 1 month, compared with 40% in the HA group. Both groups then had progressive worsening of pain between 6 and 12 months; however, final clinical scores remained above baseline. No statistically significant difference was seen between HA and PRP for the treatment of hip OA.
Di Sante et al. (2016) then compared PRP with HA in 43 patients [16]. This was a well-powered, prospective, randomized, single-centre study involving patients diagnosed with hip OA, according to the American College of Rheumatology criteria [17]. Patients with mild and severe OA on radiographs were excluded (Kellgren and Lawrence score of I and IV). Primary outcome was pain reduction, as assessed by WOMAC and VAS scores at baseline and follow-up (4 and 16 weeks post-treatment). PRP was produced by twice centrifuging a peripheral venous blood sample (as per instructions of Regen Kit used in this study). The patient received either PRP (3 ml) or HA (30 mg/2 ml with molecular weight 1000–2900 kD) injections, with one injection each week for 3 weeks. Twenty-two patients underwent treatment with HA and 21 with PRP, both groups well matched for clinical and demographic characteristics with no patient lost to follow-up. PRP produced a clinically and statistically significant improvement in VAS score between baseline and 4-week follow-up (7.08 vs 4.73, p < 0.01), but this effect was lost at the 16-week review (6. 36). No significant change was seen in WOMAC score in the PRP group, either early (4 weeks) or late (16 weeks). HA did not significantly improve VAS or WOMAC scores at 4 weeks but produced a significant change from baseline in both at 16 weeks (VAS—6. 32 vs 3.63, p < 0.01). Overall, PRP provided very limited clinical relief for patients with effects lost at 16 weeks post-treatment.
Doria et al. published their study in 2017, a prospective double-blinded RCT carried out in a single centre. Eighty patients were included in the study with 40 receiving PRP and 40 receiving HA. PRP was produced by twice centrifuging venous blood with 5 ml samples given at each injection (1/week). The HA group each received three injections over the same period (Hyalubrix—30 mg/2 ml with molecular weight 1000–2900 kD). Follow-up was at 6 and 12 months post-treatment with WOMAC and VAS scores for pain as primary outcome. Inclusion criteria were symptomatic early OA (Kellgren-Lawrence grade 0–2) and age range 40–72 years. Exclusion criteria included previous intra-articular injections within 3 months, recent or chronic NSAID use, and patients with BMI > 30. Age, BMI, and OA severity were not significantly different between the groups. This study found statistically significant improvement in WOMAC and VAS scores at 6 and 12 months for both PRP and HA without any significant difference between the two treatments in pain relief. VAS scores for PRP improved from 7.5 (+ / − 2.1) to 6.3 (+ / − 3.3) at 6 months and 6.4 (+ / − 2.9) at a year while HA was 7.8 (+ / − 1.9), 6.3 (+ / − 2.9) at 6 months and 6.1 (+ / − 2.3) at a year. The authors did not recommend PRP for treatment of hip OA based on their findings.
Dallari et al. in 2016 performed an adequately powered, prospective, randomized, single-centre study of PRP, HA, and PRP with HA in the treatment of hip OA [18]. One hundred eleven patients were included, 44 received PRP alone, 36 received HA alone, with 31 receiving an injection of PRP with HA. The patients included met the American College of Rheumatology criteria for OA (uni-/bilateral joint disease chronic pain, functional impairment > 4 months). They also needed to score > 2 on VAS scale, age 18–65 years, and Kellgren-Lawrence grade 1–4. Patients were not blinded to treatment, while data collectors and outcome assessors were blinded throughout. PRP again produced with twice centrifuged venous blood. All patients received three injections 1 week apart (PRP, 5 ml; HA, 2 ml; PRP + HA, 7 ml). Patients were then followed up at 2, 6, and 12 months with primary outcome being pain reduction assessed by VAS score. Secondary outcomes were HHS, WOMAC scores, and proportion of responders (reduction in clinical scores of > 30% from baseline at 12 months). Groups were well matched for age and OA grade. At 2 and 6 months, patients treated with PRP alone had a significant improvement compared with HA and PRP + HA. At 12 months, it showed loss of significance in terms of WOMAC score but still significant difference in VAS score. There were few positive responders, those who had significant pain relief up to 12 months post-treatment when assessed with WOMAC: PRP alone (10 of 47, 21.2%), HA alone (6 of 37, 16.2%), and PRP + HA (13 of 33, 39.4%). No significant difference was noted between groups for positive responders when assessed by VAS or HHS. The authors conclude that PRP can offer significant improvements over HA and HA + PRP in the treatment of hip OA.
Discussion
Reviewing the literature presented, it is difficult to recommend PRP for the management of hip OA. While it is a safe procedure for patients, this review would not support its introduction for the treatment of hip OA currently. PRP was not found to be superior to HA, which is a significant finding given that HA itself is not currently recommended by guidelines for hip OA management.
Another issue noted is the level of heterogeneity across the 5 studies with significant variability in patient cohort demographics, the extent of their disease, preparations used, timing of treatments, and duration of follow-up. Certainly, the concern regarding the variability of PRP preparations and the role that has on its effect has been highlighted in reviews of its use in other conditions [19].
However, given that some studies have shown a positive response in patient pain levels up to 12 months post-treatment, it would be advisable to undertake further research to ascertain if PRP may have a potential as a future treatment in hip OA. We recommend large prospective RCTs with homogeneity of patient population, a standard protocol for PRP preparation, and timing of treatment, with long-term follow-up (up to 12 months). Ideally, PRP would be compared with both placebo and intra-articular steroid injection to determine its efficacy.
References
Song J, Chang RW, Dunlop DD (2006) Population impact of arthritis on disability in older adults. Arthritis Rheum 55(2):248–255
Roux CH, Saraux A, Mazieres B et al (2008) Screening for hip and knee osteoarthritis in the general population: predictive value of a questionnaire and prevalence estimates. Ann Rheum Dis 67(10):1406–1411
Hochberg MC, Altman RD, Brandt KD et al (1995) Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum 38(11):1541–1546
Richy F, Bruyere O, Ethgen O et al (2004) Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis 63(7):759–766
McCabe PS, Maricar N, Parkes MJ et al (2016) The efficacy of intra-articular steroids in hip osteoarthritis: a systematic review. Osteoarthr Cartil 24(9):1509–1517
Letizia Mauro G, Scaturro D, Sanfilippo A, Benedetti MG (2018) Intra-articular hyaluronic acid injections for hip osteoarthritis. J Biol Regul Homeost Agents 32(5):1303–1309
Migliore A, Granata M, Tormenta S et al (2011) Hip viscosupplementation under ultra-sound guidance reduces NSAID consumption in symptomatic hip osteoarthritis patients in a long follow-up. Data from Italian registry. Eur Rev Med Pharmacol Sci 15(1):25–34
Dziedzic KS, Healey EL, Porcheret M et al (2018) Implementing core NICE guidelines for osteoarthritis in primary care with a model consultation (MOSAICS): a cluster randomised controlled trial. Osteoarthr Cartil 26(1):43–53
Pauly S, Klatte-Schulz F, Stahnke K et al (2018) The effect of autologous platelet rich plasma on tenocytes of the human rotator cuff. BMC Musculoskelet Disord 19(1):422
Rabago D, Best TM, Zgierska AE et al (2009) A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br J Sports Med 43(7):471–481
Altan E, Aydin K, Erkocak O et al (2014) The effect of platelet-rich plasma on osteochondral defects treated with mosaicplasty. Int Orthop 38(6):1321–1328
Sun Y, Feng Y, Zhang CQ et al (2010) The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop 34(4):589–597
Sanchez M, Guadilla J, Fiz N, Andia I (2012) Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology (Oxford) 51(1):144–150
Battaglia M, Guaraldi F, Vannini F, Rossi G et al (2013) Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics 36(12):e1501–e1508
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16(4):494–502
Di Sante L et al (2016) Intra-articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrason 18(4):463–468
Altman R, Alarcón G, Appelrouth D et al (1991) The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 34(5):505–514
Dallari D, Stagni C, Rani N et al (2016) Ultrasound-guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis: a randomized controlled study. Am J Sports Med 44(3):664–671
Murray DJ, Javed S, Jain N et al (2015) Platelet-rich plasma injections in treating lateral epicondylosis: a review of the recent evidence. J Hand Microsurg 7(2):320–325
Iagnocco A, Naredo E (2012) Osteoarthritis: research update and clinical applications. Rheumatology (Oxford) 51(Suppl 7):vii2–vii5
Author information
Authors and Affiliations
Contributions
BS and LG developed the idea for the study. MB and PM performed the searches and study review. All authors were involved in the analysis of the studies. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Nil.
Ethics approval and consent to participate
Not applicable (systematic review).
Consent for publication
No personal data included.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• The use of platelet-rich plasma as a treatment modality has expanded so quickly that the research into its efficacy lags far behind.
• The goal of this study is to determine if the use of PRP in hip osteoarthritis is a treatment that will benefit patients suffering from a debilitating illness.
• This study cannot support the routine use of PRP in the treatment of hip osteoarthritis based on the studies performed to date.
Rights and permissions
About this article
Cite this article
Berney, M., McCarroll, P., Glynn, L. et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci 190, 1021–1025 (2021). https://doi.org/10.1007/s11845-020-02388-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-020-02388-z