Abstract
Introduction
Although the role of vitamin D in the prevention of rickets has long been well established, controversies still exist on the ideal dose of vitamin D supplementation in infants.
Objective
We assessed serum 25-hydroxyvitamin D (25OHD) status simultaneously in maternal and cord samples and the response to vitamin D3 supplementation in neonates.
Methods
Serum 25OHD levels were evaluated from maternal, and umbilical cord samples from term normal pregnancies. Repeat 25OHD levels were assessed in neonates with 25OHD below 30 nmol/L following vitamin D3 200 IU daily after 6 weeks.
Results
Blood samples were taken including 57 cord samples and 16 follow-up neonatal samples. Maternal and cord serum 25OHD were 43 ± 21 and 29 ± 15 nmol/L, respectively. Infants with 25OHD < 30 nmol/L (19.8 ± 4.7 nmol/L) had a significant increase in serum 25OHD (63.3 ± 14.5 nmol/L) following vitamin D3 200 IU daily after 6 weeks.
Conclusion
Healthy Irish infants born at term are at high risk of vitamin D deficiency, but vitamin D3 200 IU daily, rapidly corrects poor vitamin D status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitamin D is essential for maintenance of bone health and deficiency is linked causally to rickets in children [1]. A resurgence of vitamin D deficiency rickets has been reported across most countries [2]. Vitamin D deficiency is linked to extraskeletal disorders in infants and children such as type 1 diabetes mellitus and inflammatory diseases [3]. Infants at birth are entirely dependent on maternal supply of vitamin D. The natural dietary sources of vitamin D are quite limited, and in high latitude countries like Ireland, synthesis of vitamin D is absent for nearly 6 months from late autumn to early spring [4, 5]. Once born, an infant is dependent on oral intake of vitamin D; while formula feeds are fortified to varying degrees with vitamin D (approximately 50 IU per 100 ml). Normally, breast milk is the best and natural source of nutrition for newborn infants, but vitamin D content of breast milk is very low. Breast milk of mothers who are even replete in vitamin D contains only 15–50 IU/L of vitamin D, which is not sufficient enough to meet recommended daily vitamin D intake [2]. In 2010 in Ireland, a national policy was implemented mandating that all infants, both breast fed and formula fed, be administered vitamin D3 200 IU daily for 12 months using a list of approved medications only [6].
The Institute of Medicine (IOM), following a systematic review of all available scientific evidence, revised the dietary reference intakes for calcium and vitamin D in the North American population, both USA and Canada [7]. They specified intakes for all age groups, and they advised about corresponding 25OHD levels that matched these intakes. For those over 1 year, the estimated average vitamin D requirement is 400 IU daily, corresponding to a median serum 25OHD level of 40 nmol/L. The recommended daily allowance (RDA) for vitamin D that meets the requirement of 97.5 % of the population was specified to be 600 IU daily, corresponding to a 25OHD level of 50 nmol/L. For infants, since there was insufficient evidence to specify an RDA, the IOM specified that an adequate intake (AI) of vitamin D 400 IU daily is likely to meet the needs of the majority of infants. The IOM went on to state that a 25OHD level per se was not diagnostic of vitamin D deficiency but they deemed that a 25OHD level below 30 nmol/L indicated an increased risk of deficiency. The IOM also highlighted the tendency towards inflation of the prevalence of vitamin D deficiency, and that levels in excess of 125 nmol/L could be associated with harm [7]. Some reports have shown that people living in sun-enriched environment can attain higher levels without toxicity [8]. Although the levels of vitamin D associated with toxicity were much higher than 125 nmol/L in these reports, it is sensible to have a cut-off level as a margin of safety for the public. The IOM view is prudent as there are no reports of added benefit for 25OHD levels higher than 125 nmol/L, rather there may be harm from chronically higher concentrations [7]. In a survey of 148 preterm infants <32 weeks gestation in whom we assessed the response to intake from all sources of vitamin D (D2 and D3) 400 IU daily over a median of 15 weeks, we demonstrated that such an intake achieved 25OHD levels above 50 nmol/L in 86 % and that nearly 10 % had levels above 125 nmol/L [9].
In this study, we sought to evaluate vitamin D status in healthy mothers, who delivered at term, and their infants. We also sought to evaluate the early response to supplementation in those infants with low 25OHD levels (below 30 nmol/L) using the dose recommended by Food Safety Authority of Ireland (FSAI), namely vitamin D3 200 IU daily [6].
Methods
This was a prospective study carried out in a cohort of full-term normal infants and their mothers in Ireland. This study was conducted at the National Maternity Hospital, Dublin in 2010. Approval to conduct the study was obtained from the Ethics Committee of National Maternity Hospital. All parents were informed of the nature of the study and informed written consent was obtained prior to recruitment.
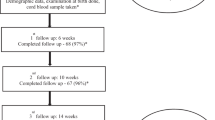
Serum 25OHD levels were evaluated from maternal and umbilical cord samples from term normal pregnancies within the first one hour of delivery. Newborns with a congenital anomaly, maternal chorioamnionitis, intra-uterine growth restriction, maternal substance abuse and/or maternal intrapartum fever were excluded from the study. The FSAI recommend supplements that contain only vitamin D3; there are three vitamin D3-only liquid formulations that are available in Ireland, namely Baby D vitamin D3 supplement, BabyvitD3 vitamin D and Abidec vitamin D3 drops. Baby D vitamin D3 supplement contains vitamin D3 formulated in miglyol oil (extract of palm oil) and also contains trace amount of vitamin E, which acts as an anti-oxidant to vitamin D3. Baby D comes with a single oral dosing syringe of 0.2 ml/200 IU vitamin D3 and is given once a day. BabyvitD3 contains vitamin D3 also formulated in miglyol oil and each drop has 100 IU vitamin D3, it is given as two drops (i.e. 200 IU) once daily. Abidec vitamin D3 drops contain vitamin D3 with potassium sorbate and anise oil as a preservative and one drop contains 40 IU of vitamin D3, it is given as 5 drops (200 IU) once per day. Pregnant mothers were not routinely supplemented with vitamin D during antenatal care in the hospital. Parents of infants with serum 25OHD levels <30 nmol/L were invited to attend for repeat sampling at 6 weeks postnatally.
Serum 25OHD levels were measured by competitive radioimmunoassay (Immunodiagnostic Systems Limited, Boldon, Tyne and Wear, UK) as previously described [10]. In order to ensure a high standard of analysis, we participate in the Vitamin D External Quality Assessment Scheme [11]. Descriptive statistics are presented as mean ± standard deviation, unless stated otherwise. Difference between mean values is tested by independent or paired t test, whichever is appropriate. A value of p < 0.05 was considered statistically significant. Statistics were performed using PASW Statistics for Windows version 18.0 (SPSS Inc, Chicago).
Results
Maternal and cord (n = 57) samples were taken and 47 were matched samples. There were 32 male and 25 female infants of whom 50 were Caucasian and 7 non-Caucasian (Table 1). The characteristics of the infants were as follows: gestational age 40.0 ± 1.8 weeks; birth weight 3.6 ± 0.5 kg; body length 52.3 ± 1.7 cm; head circumference 35.2 ± 1.1 cm; and Apgar scores 9. The mean maternal 25OHD status was 42.8 ± 21.0 nmol/L and cord 25OHD was 29.3 ± 15.1 nmol/L. The prevalence of 25OHD levels below 30 nmol/L was 24.5 % in maternal samples and 63.2 % in cord samples. In matched maternal-cord samples, there was a positive correlation between maternal and cord serum 25OHD levels (r = 0.6, p < 0.001) (Fig. 1). There was no significant difference in 25OHD level when gender, birth weight and season of birth were considered; however, there was a difference in 25OHD level in view of ethnicity with Caucasians having higher serum 25OHD levels than non-Caucasians (p = 0.04) (Table 1). The general practitioners of all the infants with cord 25OHD level <30 nmol/L were contacted, and all families were invited to bring the infants for a follow-up assessment. Only 16 of 36 neonates with 25OHD <30 nmol/L returned for repeat samples, but levels increased significantly from 19.9 ± 4.7 to 63.2 ± 14.5 nmol/L (p < 0.001). All the 16 but 3 infants had 25OHD < 50 nmol/L of which 2 were formula fed and 1 was breast fed. Post supplementation, formula-fed infants had significantly higher serum 25OHD levels compared to their breast-fed counterparts (67.1 ± 14.7 versus 51.8 ± 5.2, p = 0.008). The individual increments in 25OHD level at 6 weeks repeat samples for the 16 infants are shown in Fig. 2.
Discussion
We demonstrated low 25OHD levels among a cohort of healthy Irish mothers with nearly 25 % having levels at risk for vitamin D deficiency, similar to our earlier report [12]. Cord 25OHD levels were about two-thirds of maternal levels leaving 63 % of the newborn infants at risk of deficiency unless remedial action was taken. Low dose supplementation with vitamin D3 200 IU daily readily corrected low 25OHD levels. No infant achieved a 25OHD level above the safe threshold.
There has been considerable controversy about the ideal dose and duration of neonatal vitamin D supplementation. The IOM, the American Academy of Pediatrics, the Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society, the Canadian Paediatric Society, and the European Society for Paediatric Endocrinology (ESPE) recommend total 400 IU daily of vitamin D intake for infants [1, 7, 13]. The Endocrine Society advocates vitamin D 1500–2000 IU daily to correct low 25OHD levels [14]. The UK Department of Health recommend a daily vitamin D supplements of 280–340 IU daily in the form of drops for all children aged 6 months to 5 years; however it is advised that babies fed infant formula will not need vitamin D supplements unless receiving <500 ml of formula per day. The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) suggested 800–1000 IU daily for infants [15].
Given that most infants may be fed either formula or breast milk, we think that the Irish policy of the FSAI is the safest and most effective approach for this population. An oral supplement of vitamin D3 200 IU daily should suffice for all, as shown in this study. Vitamin D3 rather than vitamin D2 is preferred because the former is more potent, as we have previously shown in infants [10]. None of the guidelines other than FSAI specify the form of vitamin D. With the exception of IOM, the North American guidelines do not distinguish between total intake and supplemental intake. The UK guidelines need to cover supplementation from birth, rather than waiting until 6 months; this is harder to implement than starting after birth, and likely to be less successful. The ESPGHAN guideline may be excessive and is likely to result in infants having 25OHD levels in excess of 125 nmol/L, which is the safe upper limit. Our opinion is also supported by study showing that dose of 200–250 IU daily of vitamin D3 given to breast-fed infants has been shown to prevent privational rickets, and a dose of 400 IU daily has not been shown to be superior [16, 17]. Limitation of our study is the small numbers, especially for the follow-up response to supplementation despite several invitations to parents although in all cases, parents were advised about vitamin D supplementation. However, the cord 25OHD levels are similar to 25OHD levels that we found in 274 preterm infants; and the response to low dose supplementation is also similar in 148 with 25OHD < 50 nmol/L taken at a median of 15 weeks intervention and at a median gestational age of 7 weeks [10].
In summary, hypovitaminosis D is common in healthy neonates at birth in Ireland, but this is readily corrected by supplementation with vitamin D3 200 IU daily, regardless of whether formula fed or breast fed. We recommend that guidelines of low dose vitamin supplementation be advised and implemented for all infants from birth. Surveillance by measurement of 25OHD is not warranted, but centres should audit their compliance with this public health policy.
References
Wagner CL, Greer FR (2008) Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122:1142–1152. doi:10.1542/peds.2008-1862
Misra M, Pacaud D, Petryk A et al (2008) Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 122:398–417. doi:10.1542/peds.2007-1894
Walker V, Modlin R (2009) The vitamin D connection to pediatric infections and immune function. Pediatr Res 65:106R–113R. doi:10.1203/PDR.0b013e31819dba91
McKenna MJ (1992) Differences in vitamin D status between countries in young adults and the elderly. Am J Med 93:69–77
Holick MF, Chen TC (2008) Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87:1080S–1086S
Ireland H (2010) Vitamin D Supplementation for Infants. http://www.hse.ie/eng/health/child/vitaminD/supplementation.html
Ross AC, Manson JE, Abrams SA et al (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58. doi:10.1210/jc.2010-2704
Luxwolda MF, Kuipers RS, Kema IP et al (2012) Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr 108:1557–1561. doi:10.1017/S0007114511007161
McCarthy RA, McKenna MJ, Oyefeso O et al (2012) Vitamin D nutritional status in preterm infants and response to supplementation. Br J Nutr. doi:10.1017/S0007114512004722
McCarthy RA, McKenna MJ, Oyefeso O et al (2012) Vitamin D nutritional status in preterm infants and response to supplementation. Br J Nutr. doi:10.1017/S0007114512004722
Carter GD (2009) 25-Hydroxyvitamin D assays: the quest for accuracy. Clin Chem 55:1300–1302. doi:10.1373/clinchem.2009.125906
Walsh JM, Kilbane M, McGowan CA et al (2013) Pregnancy in dark winters: implications for fetal bone growth? Fertil Steril 99:206–211. doi:10.1016/j.fertnstert.2012.09.010
Canadian Paediatric Society (2007) Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health 12:583–598
Holick M, Binkley N (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Clin Endocrinol 96:1911–1930. doi:10.1210/jc.2011-0385
Agostoni C, Buonocore G, Carnielli VP et al (2010) Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 50:85–91. doi:10.1097/MPG.0b013e3181adaee0
Shakiba M, Sadr S, Nefei Z et al (2010) Combination of bolus dose vitamin D with routine vaccination in infants: a randomised trial. Singapore Med J 51:440–445
Siafarikas A, Piazena H, Feister U et al (2011) Randomised controlled trial analysing supplementation with 250 versus 500 units of vitamin D3, sun exposure and surrounding factors in breastfed infants. Arch Dis Child 96:91–95. doi:10.1136/adc.2009.178301
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no conflict of interest.
Funding
National Maternity Hospital Fund.
Rights and permissions
About this article
Cite this article
Onwuneme, C., Diya, B., Uduma, O. et al. Correction of vitamin D deficiency in a cohort of newborn infants using daily 200 IU vitamin D supplementation. Ir J Med Sci 185, 683–687 (2016). https://doi.org/10.1007/s11845-015-1341-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-015-1341-2