Abstract
Objective
To observe the preventive and therapeutic effects of diosgenin on retinoic acid-induced osteoporosis in rats.
Methods
A total 50 Sprague-Dawley rats were randomly divided into 5 groups: control group, model group (osteoporosis rats), low (10 mg kg−1), middle (30 mg kg−1), and high-dose diosgenin (90 mg kg−1)-treated groups. The osteoporosis rats model was induced by retinoic acid. The BMD and physical parameters of femoral including length, wet weight, and dry weight in each group were measured. The hematoxylin–eosin staining was used for bone histomorphology analysis. Besides, the bone calcium (Ca) and phosphorus (P) contents were measured. In order to detect the biochemical index in different treatment groups, the serum tartrate-resistant acid phosphatase (TRAP), alkaline phosphatase (ALP), estradiol, and osteocalcin were compared among different groups.
Results
The osteoporosis rat model was successfully induced by retinoic acid. Compared with the model group, the lessening of femoral length and weight and the loss of BMD were significantly improved in diosgenin groups. Both contents of Ca and P were much more increased when induced by retinoic acid (p < 0.05). The estradiol and osteocalcin levels in the middle and high-dose treatment groups were significantly higher than that of the model group, while the ALP and TRAP levels were much lower than the model group (p < 0.05).
Conclusion
Diosgenin can prevent the loss of bone in experimental rats. The mechanism may be that it improves the level of estrogenic hormone of estradiol and inhibits the high bone turnover.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a bone disease that leads to an increased risk of fracture with reduced bone mineral density (BMD), deteriorated bone microarchitecture, and alters amounts and types of proteins in bone [1, 2]. The purpose of osteoporosis treatment is to prevent the bone fractures by decreasing bone loss or, preferably, by enhancing bone density and strength [3, 4]. Early detection and treatment of osteoporosis can sufficiently decrease the risk of future bone diseases, while it is difficult to cure the osteoporosis by rebuilding the bone. In addition, there is no available treatment to cure osteoporosis completely. Therefore, early prevention of osteoporosis is as important as treatment [5].
Natural compounds in plants, such as polyphenols and flavonoids, have proved to be preventive efficacy to bone loss in osteoporosis rats [6–9]. Diosgenin, a steroid sapogenin (Fig. 1), extracting from Dioscorea wild yam tubers, such as the Kokoro [10], has been shown to inhibit proliferation, suppress inflammation, and induce apoptosis in tumor cells [11–13]. The aglycone (sugar-free), diosgenin is used for the commercial synthesis of steroid products, such as pregnenolone, cortisone, progesterone, etc. Previous studies have shown that diosgenin could be used to prevent and treat osteoporosis [14, 15]. All these studies indicate the safety and efficacy of diosgenin using as a certain alternative treatment modality for osteoporosis, and it is available for diosgenin being used therapeutically for postmenopausal women who attempt to reduce osteoporotic progression. However, the molecular mechanism of diosgenin activity in bone-derived cells remains largely unknown.
The model of retinoic acid-induced osteoporosis in rats is used in several studies to evaluate the influence of substances on bone loss in human, for its easy operation, high successful rate, short time consumption, and type symptoms of osteoporosis [16–19]. Early studies observed that large dose of vitamin A was toxic to the skeletal system of rats [20, 21]. Further studies also showed that retinoic acid causes constant decrease of BMD in a short period of 1–3 weeks [20]. All these findings demonstrated that short-term effects of retinoic acid could act as an appropriate revulsive of osteoporosis [22].
In the present study, we investigated the influence and mechanisms of diosgenin activity in preventing and treating osteoporosis rat model induced by retinoic acid. Our study also provides further information to the possible therapeutic use of diosgenin on the treatment of bone-related diseases.
Materials and methods
Animal grouping
A total of 50 female Sprague-Dawley rats (National Grade A experimental animal) aged 90 days old weighing between 190 and 260 g were offered by the Center of Experimental Animals, China Pharmaceutical University (Nanjing, China). The animal care and animal experimentations were processed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research and Council, 1996).
Forty of the rats were treated with 70 mg/kg retinoic acid suspension (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) once daily for 14 days. The rat osteoporosis model induced by retinoic acid was examined by the cortex, size, and beam of bone. Thirty of the these rats were randomly allocated to low (10 mg/kg), middle (30 mg/kg), and high (90 mg/kg) dose-treated groups with administration of three doses of diosgenin (National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China), respectively, by oral gavage for another 14 days. The doses of diosgenin are similar to 1 time, 2 times, and 4 times dose of steroidal saponins in clinical prescription for treatment of patients with myocardial ischemia or coronary heart disease (1.44 g/60 kg weight/day) [23]. Others feed with 0.5 % 70 mg/kg sodium carboxymethylcellulose (CMC-Na, Longhe food ingredient co., LTD, Nanjing, China) were defined as model group.
Additionally, another 10 healthy rats were supplemented as the healthy control group and treated with 0.5 % 70 mg/kg CMC-Na once daily for 28 days. The dosing was adjusted according to the daily weight conditions. All rats were raised under consistent conditions during the study.
Bone physical parameters
Bilateral femur bones were got from the killed rats for histomorphology analysis. The right one was used to analyze the length and wet weight. For dry weight determination, the femur bone was first dehydrated and then carbonized by burning into ashes at 800 °C for 8 h. The left femur bone was cut at the mid-diaphysis to test BMD using dual energy X-ray bone densitometer (Hologic, USA).
Bone histomorphometry
The femoral bones of the selected rats from each group were used to analyze the bone histomorphometry by hematoxylin–eosin (HE) staining. Briefly, the bone samples were fixed in 4 % formalin for 24 h followed by 2-week decalcification at 4 °C by 10 % ethylene diamine tetraacetic acid (EDTA) solution. After that, bone samples were trimmed and embedded in paraffin. They were cut into 5-µm-thick sections and stained with HE for light microscope examination.
Bone mineral detection
The levels of Ca and P (mmol/g) were determined by inorganic calcium and phosphorus assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) when some ashes were dissolved in 6 mol/L HCl (Norman Biotechnology Co. Ltd., Nanjing, China).
Biochemical indexes of serum ALP, TRAP, estradiol, and osteocalcin
The serum samples were obtained from the rats 24 h after the last dose of the study drugs given. The levels of ALP and TRAP were measured with reagent kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively. The colorless para-nitrophenyl phosphate (pNPP) is converted to yellow encounter with ALP and TRAP at the base condition and acid condition, respectively. Thus, the enzymatic activities of ALP and TRAP could be evaluated by colorimetric analysis of pNPP. The absorbance of serum samples was measured at 400–415 nm under the base and acid conditions, respectively, to detect their enzymatic activity. The levels of estradiol (ng/mL) and osteocalcin (pg/mL) were detected by radioimmunoassay [24] with 125I-labeled tracer (Aladdin reagent Co., Shanghai, China).
Statistical analysis
Statistical comparison analysis was performed by ANOVA. Results were expressed as mean ± SE with significance defined as p < 0.05.
Results
Effect of diosgenin on bone physical parameters
As shown in Table 1, the BMD, length, wet weight, and dry weight of femur bones in model group were much less than that in the control group (p < 0.05), indicating a successful osteoporosis model was constructed. Compared with the model group, different dose of diosgenin-treated groups showed significant anti-osteoporosis effect (p < 0.05), especially the rats treated with high dose of diosgenin.
Effect of diosgenin on bone histomorphology
In order to detect the effect of diosgenin on bone histomorphology, the histomorphometry was performed by HE staining (Fig. 2). Compared with the control group, rats in model group showed sparse and thin trabeculae, as well as loss of connectivity. However, the trabeculae were relatively wider and reticulate structure was more obvious after treated with different concentrations of diosgenin.
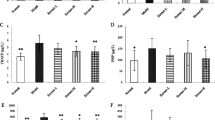
Effect of diosgenin on bone calcium (Ca) and phosphorus (P)
Both bone Ca and P levels in model group and diosgenin-treated groups were significantly increased compared with that in control group (p < 0.05). As shown in Fig. 3, there was a significant difference in bone Ca content between high-dose-treated group and model-treated group (p < 0.05).
Effect of diosgenin on ALP and TRAP
As shown in Fig. 4, enzymatic activities of ALP and TRAP in serum of model group were much more than those of control group (p < 0.05). However, ALP and TRAP activities in serum of middle and high-dose-treated groups were evidently less than those of model group (p < 0.05).
Effect of diosgenin on estradiol and osteocalcin
Figure 5 shows estradiol and osteocalcin contents in model group were evidently lower than those of control group (p < 0.05). Both estradiol and osteocalcin contents increased in a dose-dependent manner. In addition, estradiol content in high-dose-treated group and osteocalcin content in middle and high groups were much more than model group (p < 0.05), indicating diosgenin could reduce estradiol and osteocalcin loss induced by retinoic acid.
Discussion
In the present research, we investigated whether diosgenin intake inhibits osteoporosis in retinoic acid-induced rats by regulating bone metabolism toward negative balance. The retinoic acid-induced rats showed a decreased femur length and estradiol and osteocalcin level, and increased level of ALP and TRAP, indicating a successful model. Indeed, significant promotion of bone formation and inhibition of bone absorption were observed in diosgenin-treated groups, indicating the improvement of bone metabolism and bone mineralization. All these changes closely imitate the human bone metabolism.
The histopathological studies have already revealed the sparse and disrupted bone in osteoporosis rats, and this disruption may lead to the decline of bone strength [25]. The experimental results showed the reduced weight of femoral shaft, shortened width of bone, and decreased bone mineral substances, and collagen in rats model group administered retinoic acid for 14 days, implying successfully induced osteoporosis-like changes. All the indexes have obvious improvement in middle, high dose of diosgenin-treated groups compared with model group. These changes support with our hypothesis that there is a protective effect of diosgenin against osteoporosis induced by retinoic acid in rats.
Nutritional factors also contribute to the development of osteoporosis. Contents of Ca and P in model group were higher than in control, and the reason could be explained by the slow lost process of these mineral substances. Previous study has also proved that the phases of bone resorption were much longer than bone formation when estrogen level declined caused by ovariectomization [26]. Contents of Ca and P were much higher in diosgenin-treated group than in model group, especially the bone Ca content between high-dose group (90 mg/kg) and model group. These changes may be due to the botanical composition (e.g., flavonoids, phenolics) in diosgenin, which could exhibit strong bone protective properties in rat models of osteoporosis [27].
ALP, a biomarker of bone information, releasing from human osteoblast cells, can reflect the activity of osteoblast [28]. TRAP mainly expressed by osteoclast, having a pivotal role in many biological processes including bone mineralization, skeletal development, etc., can indicate the amount and activity of osteoclast [29]. As bone metabolic markers, the serum ALP and TRAP level associated with bone formation was increased in osteoporosis and other bone metabolic disorders [30]. Similar changes happened in our study, the increased activity levels of ALP and TRAP under osteoporosis conditions were significantly decreased by diosgenin-mediated suppression, indicating an antagonistic effect of diosgenin on bone absorption and bone loss, and an enhancement of bone absorption. Our study also showed a compensated bone formation in diosgenin-treated group.
As we know, osteoporosis is a direct result of a disorder balance between bone formation and its resorption. The imbalance of the two processes is acted as basic regulatory mechanism contributing to bone construction and reconstruction [31]. In addition, osteocalcin, secreted by osteoblasts, plays an important role in bone regeneration progress [31]. Upon evolution of the different osteocalcin levels between the control and treatment groups, a significant decrease was observed in model group; however, osteocalcin in diosgenin-treated groups was gradually increased with dose up, indicating diosgenin could induce ossification, producing much bone matrix, and leading to the normal balance of bone metabolism. From a mechanistic point of view, the diosgenin treatment can either raise the amount of osteoblasts, or activate the activity of the existed cells [32]. In fact, recent published data have already proved that consumption diet with rich olive oil was associated with the increase in serum osteocalcin levels [33, 34]. It seems that the diosgenin-mediated increase of osteocalcin is likely to be a key factor associated with the inhibition of bone loss.
Estradiol is an estrogenic hormone with two hydroxyl groups in molecular structure. The absence of estradiol is a well-known and probably the most important cause of osteoporosis in premature and menopause female [35]. Our results indicate that the protective effect of estradiol on diosgenin-induced bone loss occurs, and estradiol content in diosgenin-treated groups was increased in different degrees. Ferretti et al. [36] suggested that the estradiol can prevent osteoblast apoptosis by suppression of estrogen receptor β (ERβ). Our results suggest the possible mechanism that diosgenin improves the level of estradiol and inhibits the high bone turnover, while the inhibition principle was still completely blunted. The role of diosgenin influencing estradiol expression needs further study.
Recently, the possibility that naturally phytochemicals from edible materials may reduce the risk of bone diseases has gained considerable interest. Zhang et al. [25] demonstrated that higher dose of ethanol extract of Lepidium meyenii was effective in preventing bone loss. Rao et al. [37] reported that lycopene in tomato inhibited osteoclastic mineral resorption by decreasing tartrate-resistant acid phosphatase formation. Citrus fruits are rich in micronutrients, limonoids, and polyphenolic compounds, which were important in improving the BMD and bone biomechanical properties and decreasing the bone resorption [38]. A recent report showed that an extract of Prunus mume affected the proliferation and differentiation of pre-osteoblastic MC3T3-E1 cells [39]. Almost all these researches based on the mechanisms that antioxidant vitamins may exert favorable effects on BMD and osteoporotic risk by scavenging free radicals and thereby reducing oxidative stress [40]. The present study also proved that diosgenin has certain prevention and cure function for osteoporosis rats, however, by promoting bone formation, inhibiting bone absorption, and regulating bone metabolism. Although Shishodia and Aggarwal [41] suggest that the diosgenin suppresses osteoclastogenesis through restrain of NF-κB-regulated gene expression and reinforce of cytokines-induced apoptosis, the detailed mechanisms of inhibiting effect of diosgenin are still unknown; therefore, further studies are needed to identify the bioactive components and the mechanisms of the action.
Conclusions
According to the experimental results mentioned above, we can make the following conclusions. First, diosgenin possesses a potential inhibitory effect against osteoporosis via promoting bone formation, inhibiting bone absorption, and regulating bone metabolism toward negative balance. Second, diosgenin has certain prevention and cure function for osteoporosis in rats induced by retinoic acid. Our study provides a theoretical base for the further development of diosgenin.
References
Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9(8):1137–1141
Kanis J, Black D, Cooper C, Dargent P, Dawson-Hughes B, De Laet C, Delmas P, Eisman J, Johnell O, Jonsson B (2002) A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int 13(7):527–536
Hodgson S, Watts N, Bilezikian J, Clarke B, Gray T, Harris D, Johnston C Jr, Kleerekoper M, Lindsay R, Luckey M (2003) American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract 9(6):544
Ammann P, Rizzoli R (2003) Bone strength and its determinants. Osteoporos Int 14(3):13–18
Wood AJ, Riggs BL, Melton LJ III (1992) The prevention and treatment of osteoporosis. N Engl J Med 327(9):620–627
Ueda H, Yamazaki C, Yamazaki M (2002) Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol Pharm Bull 25(9):1197–1202
Gryglewski RJ, Korbut R, Robak J, Świȩs J (1987) On the mechanism of antithrombotic action of flavonoids. Biochem Pharmacol 36(3):317–322
Chiba H, Uehara M, Wu J, Wang X, Masuyama R, Suzuki K, Kanazawa K, Ishimi Y (2003) Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J Nutr 133(6):1892–1897
Kim T-H, Jung JW, Ha BG, Hong JM, Park EK, Kim H-J, Kim S-Y (2011) The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J Nutr Biochem 22(1):8–15
Raju J, Patlolla JM, Swamy MV, Rao CV (2004) Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomark Prev 13(8):1392–1398
Gupta A, Gupta R, Lal B (2001) Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. J Assoc Phys India 49:1057–1061
Corbiere C, Liagre B, Bianchi A, Bordji K, Dauça M, Netter P, Beneytout J-L (2003) Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma cells. Int J Oncol 22(4):899–906
Liu M-J, Wang Z, Ju Y, Wong RN-S, Wu Q-Y (2005) Diosgenin induces cell cycle arrest and apoptosis in human leukemia K562 cells with the disruption of Ca2+ homeostasis. Cancer Chemother Pharmacol 55(1):79–90
Higdon K, Scott A, Tucci M, Benghuzzi H, Tsao A, Puckett A, Cason Z, Hughes J (2001) The use of estrogen, DHEA, and diosgenin in a sustained delivery setting as a novel treatment approach for osteoporosis in the ovariectomized adult rat model. Biomed Sci Instrum 37:281
Yen ML, Su JL, Chien CL, Tseng KW, Yang CY, Chen WF, Chang CC, Kuo ML (2005) Diosgenin induces hypoxia-inducible factor-1 activation and angiogenesis through estrogen receptor-related phosphatidylinositol 3-kinase/Akt and p38 mitogen-activated protein kinase pathways in osteoblasts. Mol Pharmacol 68(4):1061–1073
Wu BXB, Huang T, Wang J (1996) A model of osteoporosis induced by retinoic acid in male Wistar rats. Acta Pharm Sin 31(4):241
Wei MYZ, Li P, Zhang Y, Sse WC (2007) Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am J Chin Med 35(04):663–667
Liao EY, Luo XH, Wang WB, Wu XP, Zhou HD, Dai RC, Liao HJ, Yang C (2003) Effects of different nylestriol/levonorgestrel dosages on bone metabolism in female Sprague-Dawley rats with retinoic acid-induced osteoporosis. Endocr Res 29(1):23–42
Xu P, Guo X, Zhang YG, Li YF, Cao JL, Xiong YM (2005) The effect of retinoic acid on induction of osteoporotic model rats and the possible mechanism. Sichuan Da Xue Xue Bao Yi Xue Ban 36:229–232
Fahmy SR, Soliman AM (2009) Oxidative stress as a risk factor of osteoporotic model induced by vitamin A in rats. Aust J Basic Appl Sci 3:1559–1568
Hough SAL, Muir H, Gelderblom D, Jenkins G, Kurasi H, Slatopolsky E, Bergfeld MA, Teitelbaum SL (1988) Effects of hypervitaminosis A on the bone and mineral metabolism of the rat. Endocrinology 122:2933–2939
Oršolić N, Goluža E, Đikić D, Lisičić D, Sašilo K, Rođak E, Jeleč Ž, Lazarus M, Orct T (2013) Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur J Nutr 53:1217–1227. doi:10.1007/s00394-013-0622-7
Zhang Z, Song C, Fu X, Liu M, Li Y, Pan J, Liu H, Wang S, Xiang L, Xiao GG, Ju D (2014) High-dose diosgenin reduces bone loss in ovariectomized rats via attenuation of the RANKL/OPG ratio. Int J Mol Sci 15(9):17130–17147
Price P, Parthemore J, Deftos L (1980) New biochemical marker for bone metabolism. Measurement by radioimmunoassay of bone GLA protein in the plasma of normal subjects and patients with bone disease. J Clin Investig 66(5):878
Zhang Y, Yu L, Ao M, Jin W (2006) Effect of ethanol extract of Lepidium meyenii Walp. on osteoporosis in ovariectomized rat. J Ethnopharmacol 105(1):274–279
Zhang QYQL, Huang BK, Wang Y, Wang H, Chen L (2003) Effects and mechanisms of osthole on sciatica induced by lumber disc herniation. Chin Pharm J 5:101–104
LE Bu SY, Frankin M, Marlow D, Brachett DJ, Boldrin EA (2007) Comparison of dried plum supplementation and intermittent PTH in restoring bone in osteopenic orchidectomized rats. Osteoporos Int 18:931–942
Farley JR, Stilt-Coffing B (2001) Apoptosis may determine the release of skeletal alkaline phosphatase activity from human osteoblast-line cells. Calcif Tissue Int 68(1):43–52. doi:10.1007/s002230001181
Minkin C (1982) Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int 34(1):285–290
Deyhim F, Garica K, Lopez E, Gonzalez J, Ino S, Garcia M, Patil BS (2006) Citrus juice modulates bone strength in male senescent rat model of osteoporosis. Nutrition 22(5):559–563
Bharadwaj SNA, Betageri GV, Prasadarao NV, Naidu AS (2009) Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporos Int 20:1603–1611
Born AK, Lischer S, Maniura-Weber K (2012) Watching osteogenesis: life monitoring of osteogenic differentiation using an osteocalcin reporter. J Cell Biochem 113(1):313–321
Filip R, Possemiers S, Heyerick A, Pinheiro I, Raszewski G, Davicco MJ, Coxam V (2014) Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J Nutr Health Aging 19:77–86. doi:10.1007/s12603-014-0480-x
Fernández-Real JM, Bulló M, Moreno-Navarrete JM, Ricart W, Ros E, Estruch R, Salas-Salvado J (2012) A Mediterranean diet enriched with olive oil is associated with higher serum total osteocalcin levels in elderly men at high cardiovascular risk. J Clin Endocrinol Metab 97(10):3792–3798
Stepan JJPJ, Presl J (1987) Bone loss and biochemical indices of bone remodeling in surgically induced post-menopausal women. Bone 8:279–284
Ferretti M, Bertoni L, Cavani F, Benincasa M, Sena P, Carnevale G, Zavatti M, Di Viesti V, Zanoli P, Palumbo C (2012) Structural and histomorphometric evaluations of ferutinin effects on the uterus of ovariectomized rats during osteoporosis treatment. Life Sci 90(3):161–168
Rao LG, Krishnadev N, Banasikowska K, Rao AV (2003) Lycopene I—effect on osteoclasts: lycopene inhibits basal and parathyroid hormone-stimulated osteoclast formation and mineral resorption mediated by reactive oxygen species in rat bone marrow cultures. J Med Food 6(2):69–78
Mandadi K, Ramirez M, Jayaprakasha GK, Faraji B, Lihono M, Deyhim F, Patil BS (2009) Citrus bioactive compounds improve bone quality and plasma antioxidant activity in orchidectomized rats. Phytomedicine 16(6):513–520
Kono R, Okuno Y, K-i Inada, Tokuda A, Hashizume H, Yoshida M, Nakamura M, Utsunomiya H (2011) A Prunus mume extract stimulated the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biosci Biotechnol Biochem 75(10):1907–1911
Garrett I, Boyce B, Oreffo R, Bonewald L, Poser J, Mundy G (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Investig 85(3):632
Shishodia S, Aggarwal B (2005) Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, IκB kinase activation and NF-κB-regulated gene expression. Oncogene 25(10):1463–1473
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, S., Niu, F., Xu, CY. et al. Diosgenin prevents bone loss on retinoic acid-induced osteoporosis in rats. Ir J Med Sci 185, 581–587 (2016). https://doi.org/10.1007/s11845-015-1309-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-015-1309-2