Abstract
Background
Posterior spinal instrumentation and fusion for correction of adolescent idiopathic scoliosis (AIS) typically requires lengthy operating time and may be associated with significant blood loss and subsequent transfusion. This study aimed to identify factors predictive of duration of surgery, intraoperative blood loss and transfusion requirements in an Irish AIS cohort.
Methods
A retrospective review of 77 consecutive patients with AIS who underwent single-stage posterior spinal instrumentation and fusion over a two-year period at two Dublin tertiary hospitals was performed. Data were collected prospectively and parameters under analysis included pre- and postoperative radiographic measurements, intraoperative blood loss, surgical duration, blood products required, laboratory blood values and perioperative complications.
Results
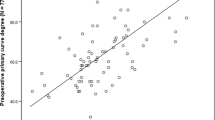
Mean preoperative primary curve Cobb angle was 62.3°; mean surgical duration was 5.6 h. The perioperative allogeneic red blood cell transfusion rate was 42.8 % with a median requirement of 1 unit. Larger curve magnitudes were positively correlated with longer fusion segments, increased operative time and greater estimated intraoperative blood loss. Preoperative Cobb angles greater than 70° [Relative Risk (RR) 4.42, p = 0.003] and estimated intraoperative blood loss greater than 1400 ml (RR 3.01, p = 0.037) were independent predictors of red blood cell transfusion risk.
Conclusion
Larger preoperative curve magnitudes in AIS increase operative time and intraoperative blood loss; preoperative Cobb angles greater than 70o and intraoperative blood loss greater than 1400 ml are predictive of red blood cell transfusion requirement in this patient group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical intervention in adolescent idiopathic scoliosis (AIS) aims to limit curve progression and achieve maximum permanent correction of the deformity [1]. Multilevel posterior spinal instrumentation and fusion (PSF) has been well established as a means to attain these goals [2–7]. Spinal fusion is a major procedure, often associated with prolonged operating times and significant morbidity. Operative time has been correlated with increasing patient age and number of vertebral levels fused [8]. Although perioperative blood loss has been demonstrated to be significantly greater in correction of scoliosis where there is an underlying neuromuscular disorder or associated poor bone mineral density, particularly during insertion of pedicle screws, patients with AIS may still lose large blood volumes intraoperatively and require transfusion to maintain adequate end organ function [8–12].
Despite modern screening procedures and transfusion practices, blood transfusion still presents some risks to the patient including exposure to blood-borne pathogens, altered levels of circulating clotting factors, haemodynamic instability, immunological reactions and decreased cardiac, pulmonary or renal function secondary to fluid shifts [12–15]. Transfusion requirements are directly related to blood loss in correction of paediatric spinal deformities which may be determined by the severity of the preoperative Cobb angle and the number of fused vertebral segments relative to the patient’s weight; however, not all studies have shown an increased risk of transfusion with greater numbers of fused vertebrae [16, 17].
Many factors have been shown to influence blood loss in spinal surgery and several techniques are employed to minimise this. Methods of reducing intraoperative blood loss with substantial supporting evidence include controlled intraoperative hypotension, acute normovolaemic haemodilution, intraoperative autologous blood salvage and use of antifibrinolytic agents such as tranexamic acid [18–23].
The ability to identify those patients at greatest risk of blood loss sufficient to require transfusion may allow better targeting of these techniques and also facilitate better preoperative counselling of patients and their families. To our knowledge, this is the first study of its kind in an Irish context. The aim of this study was to identify factors predictive of surgical duration, blood loss and transfusion requirements in a relatively homogenous cohort of patients with AIS undergoing PSF as a single-stage procedure.
Patients and methods
Study design
A retrospective review of data pertaining to a cohort of patients with AIS who underwent PSF at two Dublin tertiary hospitals [Our Lady’s Children’s Hospital Crumlin (OLCHC) and the Blackrock Clinic (BRC)] from January 2010 to April 2012 was performed. Study eligibility criteria included no prior spine surgery and the absence of complex morbidities including diagnosed endocrine, cardiac, neurological and psychiatric disorders. The ethics committees of the respective hospitals approved this study prior to commencement.
Outcome measures
Factors assessed included surgical duration, number of fused vertebrae, estimated intraoperative blood loss (EBL) and blood products required including the allogeneic red blood cells (RBC), platelet and fresh-frozen plasma (FFP) transfusions. These data were collated from the surgical and anaesthetic records. Consultant surgeon and anaesthetist details were also noted. Socio-demographic (age at surgery, gender, race) and anthropometric factors, patients’ preoperative co-morbidities and length of hospital stay were collected from the hospital admission assessment and medical charts and recorded.
The following immediate preoperative laboratory blood values, all of which were complete for each case, were ascertained: prothrombin time (PT), activated partial thromboplastin time (APTT), platelet count, white cell count (WCC) and haemoglobin.
Immediate pre- and postoperative anterior–posterior radiographs were obtained and curve magnitude assessed using the Cobb method. Data relating to curve type, primary and secondary curve magnitude, and number of fused vertebrae were documented.
Independent variables
The definition of a perioperative (i.e. intra and post) complication in the present study was broad and included adverse events occurring within the initial 30 days after surgery. Major perioperative complications included adverse events necessitating longer hospital/intensive care stay, or requiring further interventions. Minor perioperative complications referred to any event producing only transient adverse effects without requiring further significant intervention.
Statistical analysis
Analyses were performed using the Statistical Package for the Social Sciences (version 19; SPSS Inc., Chicago, IL, USA). Data are summarised using numerical descriptive statistics including means with standard deviations (SD), medians with interquartile range (IQR), or number of observations (%). Comparison of mean values between dichotomous variables was performed using Student’s t test or Mann–Whitney U test. The Chi-square test was used to compare categorical variables. Relationships between continuous variables were examined using the Spearman’s rank (r s) correlation coefficient. Only the significant variables identified in the correlation analysis were retained for multivariate modelling; multiple linear regression models were developed for each of the three principal dependent variables.
To assess the factors predictive of ≥1 RBC transfusion (vs. the non-RBC transfusion group), only the factors that were significant in the univariate analysis were entered singly into a stepwise binary logistic regression analysis. The relative risk (RR) and 95 % confidence intervals (CI) were calculated for each predictor factor in the final model. Statistical significance was defined as p < 0.05.
Results
Patient characteristics are presented in Table 1. In total, 78 consecutive patients with AIS underwent PSF during the study period; one case was excluded due to a diagnosis of hypothyroidism—the remaining 77 cases successfully completed follow-up. All 77 patients underwent a single-stage PSF with multiple osteotomies and instrumentation with pedicle screw-rod constructs to achieve curve correction. Three surgeons were involved in these cases; however, over 80 % of patients were operated on by a single surgeon. All received a bolus of tranexamic acid at anaesthetic induction.
The mean preoperative primary Cobb angle was 62.3o (SD 13.2). Severe curves (>70°) were noted in 21 patients (27.3 %) (3 of these patients had curves >90°). Preoperative traction X-rays were used to assess curve flexibility in a majority of patients; however, these data were only complete for 50 individuals within the cohort. Within these 50 patients, mean major curve corrections of 35° and lumbar curve corrections of 22° were attained on traction films. No significant relationship between curve flexibility and blood loss was noted.
Median duration of surgery and EBL were 5.6 h and 1012 ml, respectively. Thirty-six patients (46.8 %) received a perioperative blood product transfusion; of these, 33 patients (42.8 %) received an allogeneic RBC transfusion. The median RBC transfusion requirement for the sample was 1 unit (IQR 1–2); 15 patients (19.5 %) in the cohort required ≥2 RBC units.
The median preoperative PT and APTT values for the sample were 13.9 and 31.5 s, respectively. A bleeding disorder (Factor VII/Factor VIII deficiency) was identified in 2 cases (2.6 %). Preoperative platelet counts were within the reference range (150–400 × 109/L) in all with the exception of one subject in whom it was 131 × 109/L. Prolonged preoperative PT and APTT values were observed in 48.1 % (n = 37) and 37.7 % (n = 29) of cases, respectively.
As shown in the correlation analysis in Table 2, larger preoperative major curve Cobb angles were significantly correlated with longer fusions (r s = 0.524, p < 0.0001), increased surgical duration (r s = 0.474, p < 0.0001) and greater EBL (r s = 0.371, p < 0.01). No significant correlation between any of the dependent factors under analysis, minor or major perioperative complications, or length of hospitalisation, was observed.
Multiple linear regression analysis models are summarised in Table 3. Increasing preoperative Cobb angle (p = 0.008) and surgical duration (p < 0.0001) were both significantly associated with fused vertebrae frequency (Model 1; R 2 = 0.479). The only independent predictor of surgical duration was increased number of fused vertebrae—a factor that explained 47.4 % of the variance in surgical duration (Model 2; R 2 = 0.474). After adjustment, increased RBC transfusion frequency was the only factor significantly associated with increased blood loss (Model 3; R 2 = 0.426).
In the univariate analysis, longer surgical duration, greater estimated blood loss and larger preoperative major Cobb curve angles were identified as the strongest and most significant factors influencing RBC transfusion risk. No significant difference in preoperative PT, APTT or platelet count levels between the RBC and non-RBC transfusion groups was found.
A significantly higher RBC transfusion rate in subjects who attended OLCHC compared with BRC (69.7 vs. 30.3 %, p = 0.008) was demonstrated. In a separate analysis, OLCHC cases were identified as a more complex patient group, having larger preoperative Cobb curve angles [mean 67.3° (SD 13.2) vs. 57.2° (SD 11.2), p = 0.001] and higher number of fused vertebrae (median 13 [IQR 11–14] vs. 10 [IQR 8.75–12], p < 0.0001) with subsequent longer surgical duration (median 7 h [IQR 6–7.1] vs. 4.3 h [IQR 3.8–4.9], p < 0.0001) and increased intraoperative blood loss (median 1215 ml [IQR 950–1550] vs. 945 ml [682–1254], p = 0.012) (Table 4).
In view of the greater surgical complexities of patients who attended OLCHC, and to limit confounding, ‘hospital site’ as a dichotomous variable (i.e. OLCHC vs. BRC) was excluded from the regression analysis. There was no difference in the same intraoperative protocol between the two sites. After adjustment in the final binary logistic regression model, preoperative Cobb curve angles greater than 70° (RR 4.42, 95 % CI 1.95–5.82, p = 0.003) and total EBL greater than 1,400 ml (RR 3.01, 95 % CI 1.07–5.79, p = 0.037) independently predicted risk of perioperative RBC transfusion (Table 5).
Discussion
This is the first Irish study to evaluate the impact of available preoperative predictive factors on the duration of surgery, extent of blood loss and transfusion requirements in a cohort of patients with AIS. The current cohort was relatively homogenous as all included patients with AIS were otherwise healthy. Those with other forms of scoliosis or significant medical co-morbidities were excluded, and all patients underwent single-stage PSF procedures. There are no previously published studies evaluating these factors in an Irish cohort; however, our findings are largely consistent with reported international findings in other populations. Mean EBL was 1012 ml (interquartile range 791–1400 ml) in our study, which is comparable to mean EBLs of 750 ml to 1500 ml reported in other studies of paediatric spinal fusions [12]. This mean EBL of 1012 mL represents a substantial blood loss in an adolescent population and, unsurprisingly, just over 40 % of patients (n = 33 patients, 42.8 %) received an allogeneic RBC transfusion. Within this group, 15 patients required 2 or more units, which conferred added costs and associated risks. It is also notable that significantly increased intraoperative blood loss was observed in those who received a RBC transfusion vs. the non-RBC group (1305 vs. 947 ml, p < 0.0001).
In the current study, longer duration of surgery, increased estimated blood loss and larger preoperative Cobb angles (>70°) were identified as the most important determinants of RBC transfusion risk among the factors analysed here. Larger preoperative curve magnitudes were also associated with longer fusion segments and increased operative time. These findings are consistent with other studies showing greater blood loss with higher numbers of fused vertebrae and longer duration of surgery, particularly when more osteotomies are performed [8, 16, 24–27]. Overall, the current study’s findings strongly indicate that the severity of the deformity (measured by the preoperative Cobb angle) is predictive of the need for perioperative blood transfusion.
Other factors may also influence the duration of surgery and overall blood loss such as curve flexibility, vertebral rotation and preoperative kyphosis and surgeon experience; however, this study did not address these factors [25, 28]. Longer surgical duration is of course multifactorial and not always predictable; however, it is important where possible to recognise in advance those cases which are likely to be of greater duration.
Several investigators have identified abnormal coagulation profiles at higher than expected frequencies in both adolescent idiopathic and neuromuscular scoliosis [29, 30]. Although prolonged preoperative PT and APTT values were present in 48.1 and 37.7 % of patients, respectively, these abnormalities did not significantly influence RBC transfusion risk in this study. High preoperative values may be characteristic in this population (as shown previously [29]); however, clinically, these data suggest that such high values do not appear to be clinically relevant in relation to blood loss or transfusion requirement.
Routine use of blood conservation measures can decrease the need for allogeneic blood transfusion [18]. These techniques are varied and include intraoperative controlled hypotension (directly reducing the volume of blood lost) and acute normovolaemic haemodilution which reduces the proportion of red cells lost intraoperatively [18, 31, 32]. Infusion of antifibrinolytic drugs such as tranexamic acid can also decrease the need for allogeneic blood transfusion by competitive inhibition of the activation of plasminogen to plasmin [22, 33]. The evidence for the clinical benefit and cost-effectiveness of intraoperative blood salvage is more variable, with many studies showing a decreased need for transfusion with its use [18]. However, Weiss et al. [34] demonstrated no benefit in use of a cell saver when comparing 95 consecutive patients undergoing PSF for scoliosis correction. It is notable, however, that the aforementioned study included patients with all types of scoliosis, and not AIS exclusively. In the present cohort, intraoperative blood salvage and regular on-table haemoglobin analysis were used to allow a real-time assessment of intraoperative blood loss. Intraoperative blood loss approaching 20 % of estimated blood volume (weight in kg × 70 mls/kg) coupled with intraoperative haemoglobin of 8 g/dL or less, prompted the administration of a second bolus of tranexamic acid (all patients having received a first bolus at induction), and transfusion of one RBC unit. Additional newer techniques such as use of ultrasonic bone scalpels for facetectomies and Ponte osteotomies also show promise in reducing blood loss, although there are limited clinical data to date [35–37]. Ultrasonic bone cutters were not in routine use in our institutions during the period of this study.
The current study’s findings are valuable for preoperative planning, and in particular, when counselling patients and their families regarding the risks associated with surgical intervention for AIS. The increased RBC transfusion risk associated with a preoperative Cobb angle greater than 70° is noteworthy, given the progressive nature of scoliosis and the prolonged waiting times many of our patients endure between referral, assessment and surgery. It may be the case that these patients would benefit from intervention at an earlier stage while their deformities are still of a lower magnitude. This also has economic implications as correction of more severe curves may result in greater utilisation of healthcare resources through these and other factors [38]. In addition, the identification of ‘greater than 1400 ml EBL’ as an independent predictive factor for transfusion risk is of practical clinical importance in the management of these patients in the perioperative period, highlighting the need for careful postoperative monitoring and intervention when necessary. While these findings have not changed surgical technique, they have affected the type of information conveyed to patients and families during preoperative counselling regarding risks of intervention, particularly for those with very large curves.
This study has some limitations including the moderate sample size and predominantly female cohort. Male gender has been shown to be predictive of higher intraoperative blood loss in PSF for AIS; however, our cohort did not contain a sufficient number of males to perform a meaningful gender comparison [25]. In addition, assessment of intraoperative blood loss is based on estimation of losses incurred rather than direct measurement. Another limiting factor is the lack of detailed data regarding the number of vertebral osteotomies performed in each case as this has previously been shown to significantly affect the total EBL [27].
In conclusion, this is the first Irish study to examine potential predictors of operative time, blood loss and transfusion requirements in a cohort of patients with AIS. Intraoperative EBL >1400 ml and preoperative Cobb angles greater than 70° were independently predictive of perioperative transfusion requirements. These findings have practical implications when planning for surgery and counselling patients with AIS and their families, particularly for those patients with more severe deformities.
References
Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA (2008) Adolescent idiopathic scoliosis. Lancet 371(9623):1527–1537
Bjerkreim I, Steen H, Brox JI (2007) Idiopathic scoliosis treated with Cotrel-Dubousset instrumentation: evaluation 10 years after surgery. Spine (Phila Pa 1976) 32(19):2103–2110
Helenius I, Remes V, Yrjonen T, Ylikoski M, Schlenzka D, Helenius M et al (2003) Harrington and Cotrel-Dubousset instrumentation in adolescent idiopathic scoliosis. Long-term functional and radiographic outcomes. J Bone Joint Surg Am 85-A(12):2303–2309
Lenke LG, Bridwell KH, Blanke K, Baldus C, Weston J (1998) Radiographic results of arthrodesis with Cotrel-Dubousset instrumentation for the treatment of adolescent idiopathic scoliosis. A five to ten-year follow-up study. J Bone Joint Surg Am 80(6):807–814
Luque ER (1982) Segmental spinal instrumentation for correction of scoliosis. Clin Orthop Relat Res 163:192–198
Storer SK, Vitale MG, Hyman JE, Lee FY, Choe JC, Roye DP Jr (2005) Correction of adolescent idiopathic scoliosis using thoracic pedicle screw fixation versus hook constructs. J Pediatr Orthop 25(4):415–419
Wang Y, Fei Q, Qiu G, Lee CI, Shen J, Zhang J et al (2008) Anterior spinal fusion versus posterior spinal fusion for moderate lumbar/thoracolumbar adolescent idiopathic scoliosis: a prospective study. Spine (Phila Pa 1976) 33(20):2166–2172
Zheng F, Cammisa FP Jr, Sandhu HS, Girardi FP, Khan SN (2002) Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine (Phila Pa 1976) 27(8):818–824
Edler A, Murray DJ, Forbes RB (2003) Blood loss during posterior spinal fusion surgery in patients with neuromuscular disease: is there an increased risk? Paediatr Anaesth 13(9):818–822
Nuttall GA, Horlocker TT, Santrach PJ, Oliver WC Jr, Dekutoski MB, Bryant S (2000) Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine (Phila Pa 1976) 25(5):596–601
Modi HN, Suh SW, Hong JY, Song SH, Yang JH (2010) Intraoperative blood loss during different stages of scoliosis surgery: a prospective study. Scoliosis 5:16
Shapiro F, Sethna N (2004) Blood loss in pediatric spine surgery. Eur Spine J 13(Suppl 1):S6–S17
Triulzi DJ, Vanek K, Ryan DH, Blumberg N (1992) A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion 32(6):517–524
Triulzi DJ (2009) Transfusion-related acute lung injury: current concepts for the clinician. Anesth Analg 108(3):770–776
Guay J, de Moerloose P, Lasne D (2006) Minimizing perioperative blood loss and transfusions in children. Can J Anaesth 53(6 Suppl):S59–S67
Hassan N, Halanski M, Wincek J, Reischman D, Sanfilippo D, Rajasekaran S et al (2011) Blood management in pediatric spinal deformity surgery: review of a 2-year experience. Transfusion 51(10):2133–2141
van Popta D, Stephenson J, Patel D, Verma R (2014) The pattern of blood loss in adolescent idiopathic scoliosis. Spine J 14(12):2938–2945
Verma RR, Williamson JB, Dashti H, Patel D, Oxborrow NJ (2006) Homologous blood transfusion is not required in surgery for adolescent idiopathic scoliosis. J Bone Joint Surg Br 88(9):1187–1191
Bowen RE, Gardner S, Scaduto AA, Eagan M, Beckstead J (2010) Efficacy of intraoperative cell salvage systems in pediatric idiopathic scoliosis patients undergoing posterior spinal fusion with segmental spinal instrumentation. Spine (Phila Pa 1976) 35(2):246–251
Bess RS, Lenke LG (2006) Blood loss minimization and blood salvage techniques for complex spinal surgery. Neurosurg Clin N Am 17(3):227–234
Xu C, Wu A, Yue Y (2012) Which is more effective in adolescent idiopathic scoliosis surgery: batroxobin, tranexamic acid or a combination? Arch Orthop Trauma Surg 132(1):25–31
Shapiro F, Zurakowski D, Sethna NF (2007) Tranexamic acid diminishes intraoperative blood loss and transfusion in spinal fusions for duchenne muscular dystrophy scoliosis. Spine (Phila Pa 1976) 32(20):2278–2283
Yang B, Li H, Wang D, He X, Zhang C, Yang P (2013) Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spinal surgery. PLoS One 8(2):e55436
Guay J, Haig M, Lortie L, Guertin MC, Poitras B (1994) Predicting blood loss in surgery for idiopathic scoliosis. Can J Anaesth 41(9):775–781
Ialenti MN, Lonner BS, Verma K, Dean L, Valdevit A, Errico T (2013) Predicting operative blood loss during spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop 33(4):372–376
Yu X, Xiao H, Wang R, Huang Y (2013) Prediction of massive blood loss in scoliosis surgery from preoperative variables. Spine (Phila Pa 1976) 38(4):350–355
Koerner JD, Patel A, Zhao C, Schoenberg C, Mishra A, Vives MJ et al (2014) Blood loss during posterior spinal fusion for adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 39(18):1479–1487
Cahill PJ, Pahys JM, Asghar J, Yaszay B, Marks MC, Bastrom TP et al (2014) The effect of surgeon experience on outcomes of surgery for adolescent idiopathic scoliosis. J Bone Joint Surg Am 96(16):1333–1339
Ryan KM, O’Brien K, Regan I, O’Byrne JM, Moore D, Kelly PM et al (2013) The prevalence of abnormal preoperative coagulation tests in pediatric patients undergoing spinal surgery for scoliosis. Spine J. doi:10.1016/j.spinee.2013.07.460
Stanitski CL, Whittlesey G, Thompson I, Stanitski DF, Mohan A (1998) Clotting parameters in patients with adolescent idiopathic scoliosis undergoing posterior spinal fusion and instrumentation. J Pediatr Orthop B 7(2):132–134
Fox HJ, Thomas CH, Thompson AG (1997) Spinal instrumentation for Duchenne’s muscular dystrophy: experience of hypotensive anaesthesia to minimise blood loss. J Pediatr Orthop 17(6):750–753
Copley LA, Richards BS, Safavi FZ, Newton PO (1999) Hemodilution as a method to reduce transfusion requirements in adolescent spine fusion surgery. Spine (Phila Pa 1976) 24(3):219–222 Discussion 23–4
Neilipovitz DT (2004) Tranexamic acid for major spinal surgery. Eur Spine J 13(Suppl 1):S62–S65
Weiss JM, Skaggs D, Tanner J, Tolo V (2007) Cell Saver: is it beneficial in scoliosis surgery? J Child Orthop 1(4):221–227
Al-Mahfoudh R, Qattan E, Ellenbogen JR, Wilby M, Barrett C, Pigott T (2014) Applications of the ultrasonic bone cutter in spinal surgery—our preliminary experience. Br J Neurosurg 28(1):56–60
Bartley CE, Bastrom TP, Newton PO (2014) Blood loss reduction during surgical correction of adolescent idiopathic scoliosis utilizing an ultrasonic bone scalpel. Spine Deform 2:285–290
Hu X, Ohnmeiss DD, Lieberman IH (2013) Use of an ultrasonic osteotome device in spine surgery: experience from the first 128 patients. Eur Spine J 22(12):2845–2849
Miyanji F, Slobogean GP, Samdani AF, Betz RR, Reilly CW, Slobogean BL et al (2012) Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization?: a multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am 94(9):809–813
Acknowledgments
The authors are very grateful to the National Children’s Research Centre, Dublin 12, Ireland, for funding this study. They thank Ms. Gloria Crispino-O’Connell for providing statistical support, and acknowledge the assistance of the staff at Our Lady’s Children’s Hospital Crumlin and the Blackrock Clinic throughout the study. This study was exclusively funded by The National Children’s Research Centre, Crumlin, Dublin 12, Ireland.
Conflict of interest
None.
Ethical standard
This study was approved by the Ethics Committees of Our Lady’s Children’s Hospital Crumlin, Dublin 12, Ireland and the Blackrock Clinic, Co. Dublin, Ireland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nugent, M., Tarrant, R.C., Queally, J.M. et al. Influence of curve magnitude and other variables on operative time, blood loss and transfusion requirements in adolescent idiopathic scoliosis. Ir J Med Sci 185, 513–520 (2016). https://doi.org/10.1007/s11845-015-1306-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-015-1306-5