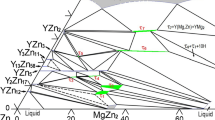
Abstract
Phase equilibria of the Mg-Gd-Zn system at 320°C are important because it has been reported that the aging treatment is carried out at that temperature. The phase equilibria at 320°C for the Mg-Zn side (x(Gd) < 50 at.%) were investigated with equilibrated alloys using electron probe microanalysis and X-ray diffraction. The partial isothermal section (x(Gd) < 50 at.%) was constructed. Eight ternary phases, τ1 to τ8, were observed at 320°C. The W phase (Gd2Mg3Zn3) which was found at high temperatures in the literature was not observed at 320°C. The analysis of the present data also indicates that the icosahedral quasicrystalline (I phase) in the Mg-Gd-Zn system is not stable. One important solid transition was found in the present work: the three-phase equilibria (Mg) + GdMg3 + GdMg5 and (Mg) + GdMg3 + τ1 at 500°C transform to (Mg) + GdMg5 + τ1 and GdMg5 + τ1 + GdMg3 at 320°C, due to the invariant reaction of (Mg) + GdMg3 → GdMg5 + τ1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mg-Gd-Zn magnesium alloys have received much attention due to their high strength at high temperatures and good corrosion resistance.1,2 Many studies have been carried out on the long period stacking ordered (LPSO) phase,3,4,5 icosahedral quasicrystalline phase (I-phase),6,7,8,9 and aging precipitation10,11 in Mg-Gd-Zn magnesium alloys. It is generally recognized that the applications of phase diagrams can significantly accelerate the development of high-performance magnesium alloys, through the predictions of the phase transitions, microstructure evolutions, and heat treatment processes (e.g., homogenization and aging, etc.).
The phase equilibria of the Mg-Gd-Zn system above 400°C have been studied by several groups of researchers.12,13,14,15,16,17 For example, the phase equilibria at 500°C in the region of x(Gd) < 50 at.% were investigated in our previous work.17 However, the solid phase equilibria at lower temperatures (below 400°C) have not been studied. It has been reported that the aging treatment for the Mg-Zn-RE(rare earth) alloys in the temperature range of 200-350°C can significantly improve strength. Thus, understanding the phase equilibria of the Mg-Gd-Zn system in that temperature range is important. Thus, this study aims to determine the solid-state phase equilibria at 320°C in the region of x(Gd) < 50 at.%.
Experimental Details
Thirty-eight Mg-Gd-Zn alloys, listed in Table I, were synthesized. High-purity magnesium (99.99 wt.%), zinc (99.999 wt.%), and gadolinium (99.9 wt.%) were used as the raw materials. Before weighing, the oxide layers on the surfaces of the magnesium, zinc, and gadolinium blocks were mechanically removed. The accurately weighed and cleaned materials of the Zn, Mg, and Gd blocks were sealed into Ta crucibles with argon gas (99.999 wt.%) which were then separately sealed into quartz tubes with argon gas (99.999 wt.%). The preparation of the alloys was as described in our previous study.18 Alloys that are marked with superscript “c” in Table I were taken from the previous work.17 These alloys were cooled from 500°C to 320°C in 20 h, and then were re-sealed individually in the Ta crucibles, which were then put into in the quartz tubes. All the alloys in Table I were annealed at 320°C for 100 ~ 300 days, and then quenched in cold water. To increase the cooling rate of the samples, the quartz tubes were quickly broken after being dropped into the cold water.
The metallographic samples of the alloys were prepared under ethanol, and then characterized by an electron probe microanalyzer (JXA-8530F; JEOL, Japan) at 15 kV and 2 × 10-8 A, with pure Mg (99.99 wt.%), Zn (99.999 wt.%), and GdF3 as standard materials for calibration. The equilibrium compositions of the phases in Table I were obtained by the method described in Ref. 19. X-ray powder diffraction (XRD) was performed for the alloys.
Experimental Results and Discussion
Table I presents the nominal alloy compositions, the identified phases, and the determined phase compositions.
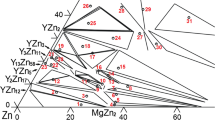
Figure 1 shows the phase compositions as listed in Table I. The XRD patterns and backscattered electron (BSE) images of some representative alloys are presented in Figs. 2 and 3, respectively. The partial isothermal section (x(Gd) < 50 at.%) at 320°C is constructed in Fig. 4. The GdMg and GdZn phases with the same crystal structure form a continuous solid solution, denoted as Gd(Mg, Zn). The solid and dashed lines in Fig. 4 represent the measured tie-triangles in the present work and the extrapolated ones, respectively.
Ternary Compounds
Eight ternary compounds, labeled as τ1 to τ8, were observed. Table II summarizes the compositions and crystallographic data of the ternary compounds. The W and I phases, which were found in the literature, were not found in the present work.
The τ1 phase, labeled as the X-phase in some references,12,13,14 has a LPSO. It has been reported that the precipitation of the LPSO τ1 phase can significantly increase the strength of Mg-Zn-RE alloys. The homogeneity range of the τ1 phase has been determined to be Gd5.5-7.8Mg86.7-90.2Zn4.3-5.5 at 320°C according to the present results.
Three hexagonal phases were found and named τ2, τ3, and τ4. The τ2 phase has been named as the Z-phase16,17 or the μ-phase20 in previous studies. Abe et al.21 named τ2, τ3 and τ4 as the S-phase, M-phase, and L-phase, respectively. The homogeneity ranges of τ2, τ3, and τ4 were determined to be Gd6.4-8.5Mg25.2-31.4Zn60.8-67.4, Gd10.5-10.6Mg24.0-24.6Zn65.0-65.5, and Gd11.3-11.5Mg23.5-24.2Zn64.4-65.1, respectively.
The τ5 phase, labeled the F-phase,16,17 has a composition range of Gd13.6-15.1Mg18.4-24.6 Zn61.1-66.7. The composition range of τ7 was determined to be Gd16.4-16.7Mg10.4-17.2Zn66.3-73.3. This phase is identical to the Gd16.6Mg17.8 Zn65.6 phase reported by Li et al.22
The phases τ6 and τ8 were measured to have the composition ranges of Gd13.3-14.4Mg11.5-15.8 Zn70.4-74.2 and Gd15.9-16.1Mg5.3-6.5 Zn77.4-78.6, respectively. Their crystal structures have yet to be determined.
The icosahedral quasicrystalline phase (I-phase) was observed in the as-cast alloys,6,8,9,15 especially under rapid solidification conditions such as melt-quenching.9 Fisher et al.8 and Gröbner et al.15 considered the I-phase as a metastable phase, whereas Liu et al.7 concluded that it is a stable phase, as they observed it in their as-cast and annealed alloys (at 440°C for 8 h). The composition of the I-phase was measured to be Gd11Mg29Zn60 by Gröbner et al.,15 Gd8.58Mg41.81Zn49.61 by Liu et al.,7 and Gd7-10Mg30Zn60-63 by Saito et al.9 In the present work, no stable icosahedral quasicrystalline phase was found. In fact, the ternary compound τ2 (Gd6.4-8.5Mg25.2-31.4Zn60.8-67.4) partially covers the compositions (Gd7Mg30Zn63 and Gd8Mg30Zn62) of Saito et al.9 Thus, the I-phases reported by Saito et al.9 are metastable. In addition, the relatively short annealing time (8 h) by Liu et al.7 is not sufficient to confirm that the I-phase is stable. Thus, it is concluded that the I phase in the Mg-Gd-Zn system is not stable.
Phase Equilibria
Figure 3a and b show the BSE images of alloys #1 and #3, respectively. As labeled in Table I, the two alloys were taken from our previous work and were annealed at 500°C for 80 days,17 and were re-annealed at 320°C for 300 days. According to our previous work,17 alloys #1 and #3 are located in the two-phase fields of (Mg) + GdMg3 at 500°C. At 320°C, these two alloys are in the three-phase equilibrium fields of (Mg) + GdMg5 + τ1 and of GdMg5 + τ1 + GdMg3, respectively. Thus, there is an invariant reaction (Mg) + GdMg3 → τ1 + GdMg5 between 500°C and 320°C. The invariant transition (Mg) + GdMg3 → τ1 + GdMg5 in the Mg-rich corner demonstrates the reason for the age hardening of the Mg-Gd-based magnesium alloys. In addition, the homogeneity range of the ternary phase τ1 (14H) at 320°C (Gd5.5~7.8Mg86.7~90.2Zn4.3~5.5) becomes significantly larger than at 500°C (Gd5.5-6.2Mg89.2-90.0Zn4.3-4.7),17 as shown in Fig. 4 and listed in Table II. It should be noted that (Mg), GdMg3, τ1, and GdMg5 were found in alloys #1 and #3. One possibility is that the invariant reaction is at this temperature. However, it is more likely that these two alloys did not reach the equilibrium state. Increasing the annealing time of these alloys can certainly bring them to the full equilibrium state. However, in the present work, when the annealing time has reached 300 days, significant amounts of the metastable GdMg3 phase (in alloy #1) and (Mg) phase (in alloy #3) were found in the present work. Therefore, no attempt was made to further increase the annealing time.
It has been reported that a “ternary compound” W phase with the same crystal structure as GdMg3 forms at 500°C.12,13,14 Thus, a two-phase equilibrium of the W and GdMg3 phases were observed, which was termed “phase separation”. In the present work, alloys #4-7 and #34-37 were designed to investigate the phase relationships between the W and GdMg3 phases at 320°C. However, the so-called phase separation was not found at 320°C. The maximum solubility of Zn in GdMg3 was measured to be 51.7%. Thus, whether the W phase is stable is questionable.
Figure 3c and d exhibit the BSE images of alloys #14 and #15, respectively. A typical peritectic microstructure formed during solidification was observed, as shown in Fig. 3c and d. Although the samples have been annealed at 500°C for 120 days and then at 320°C for up to 300 days, the peritectic microstructure remained clear due to the slow kinetics in alloy #14. Nevertheless, the three-phase conjunctions of MgZn2 + τ2 (Z-phase) + Gd2Zn17 were detected in alloy #14. Four phases (MgZn2, GdZn12, Mg2Zn11, and Gd2Zn17) were observed in alloy #15. According to the microstructure, the Gd2Zn17 phase was the primary phase of a peritectic reaction and almost disappeared. Therefore, it is considered that Gd2Zn17 is not a stable phase. A three-phase equilibrium of MgZn2 + GdZn12 + Mg2Zn11 was determined in alloy #15, as shown in Fig. 3d.
Alloy #18 was taken from the work at 500°C17 and re-annealed at 320°C. This alloy was still composed of the four phases τ2 to τ5, as shown in Fig. 3e. However, the equilibrium compositions of the specific phases changed slightly when the temperature was lowered from 500 to 320°C, indicating sluggish reaction kinetics. As shown in Fig. 4, it is hard to see the tie-triangles corresponding to the two three-phase equilibria of τ2 + τ3 + τ4 and of τ3 + τ4 + τ5, as the two three-phase fields are very narrow. Since the alloy is far from the full equilibrium state, the present results for alloy #18 are tentative.
Figure 3f and g presents the BSE images of alloys #29 and #30, respectively. Alloy #29 was composed of four phases (τ5, τ7, GdMg3, and Gd3Zn11). From the microstructure, the Gd3Zn11 is the primary precipitated phase and is transforming to other phases. In addition, the volume fraction of the Gd3Zn11 is unusually small. Thus, it is concluded that the Gd3Zn11 is not a stable phase. The three-phase equilibrium of τ5 + τ7 + GdMg3 was measured in alloy #29. The former was also detected in the above alloy #24 and is accepted in Fig. 4. Similarly, the three-phase equilibrium of GdMg3 + Gd13Zn58 + Gd3Zn11 determined in alloy #30 is accepted in Fig. 4.
Compared with the isothermal section at 500°C of the Mg-Gd-Zn by Xu et al., the major difference is that the W phase was not stable at 320°C.
Discussions on the rest alloys can be found in the Supplementary Material (see supplementary Figure S1).
Based on the experimental data obtained in the present work, the partial isothermal section (x(Gd) < 50 at.%) at 320°C is constructed in Fig. 4.
Conclusion
The solid-state phase equilibria below 400°C of the Mg-Gd-Zn system is essential information for the understanding of the aging treatment of Mg-RE alloys. The phase equilibria at 320°C of the Mg-Gd-Zn system were studied, and the isothermal section at 320°C was constructed. Eight stable ternary phases (τ1 to τ8) formed at 320°C. The stabilities of the ternary compounds W and I were studied. The W phase (Gd2Mg3Zn3) phase was not found at 320°C according to the present results. The I phase was not found in the present work which indicates that the I phase is not stable in the Mg-Gd-Zn system.
Although the samples were annealed at 320°C for 310 days, some alloys (1, 3, 15, 18) have still not reached equilibrium, since more than three phases have been observed. Further studies are necessary.
The homogeneity ranges of the eight ternary compounds (τ1 to τ8) were determined: Gd5.5-7.8Mg86.7-90.2Zn4.3-5.5 (τ1), Gd6.4-8.5Mg25.2-31.4Zn60.8-67.4 (τ2), Gd10.5-10.6Mg24.0-24.6Zn65.0-65.5 (τ3), Gd11.3-11.5Mg23.5-24.2Zn64.4-65.1 (τ4), Gd13.6-15.1Mg18.4-24.6Zn61.1-66.7 (τ5), Gd13.3-14.4Mg11.5-15.8 Zn70.4-74.2 (τ6), Gd16.4-16.7Mg10.4-17.2Zn66.3-73.3 (τ7), and Gd15.9-16.1Mg5.3-6.5 Zn77.4-78.6 (τ8), respectively.
The solubilities of Zn in GdMg2, GdMg3, and GdMg5 were measured to be 0.8, 51.7, and 1.6 at.% Zn, respectively. The solubilities of Mg in Gd3Zn11, Gd13Zn58, Gd3Zn22, Gd2Zn17, and GdZn12 were determined to be up to 3.3, 6.9, 0.7, 3.1, and 0.1 at.% Mg, respectively. The solubilities of Gd in MgZn, Mg2Zn3, MgZn2, and Mg2Zn11 are about 0.1, 0.5, 0.7, and 0.1 at.% Gd, respectively. The solubilities of Mg in the GdZn2 and GdZn3 phases are negligible.
The two previously reported equilibria (Mg) + GdMg3 + GdMg5 and (Mg) + GdMg3 + τ1 in the Mg-rich corner at 500°C transform to (Mg) + GdMg5 + τ1 and GdMg5 + τ1 + GdMg3 at 320°C, due to the invariant reaction of (Mg) + GdMg3 → GdMg5 + τ1. This phase transition is expected to favor the age hardening of the Mg-Gd-based magnesium alloys, as more volume fraction of the strengthening phase τ1 (14H) emerges during the aging treatment.
References
W. Rong, Y. Zhang, Y. Wu, Y. Chen, T. Tang, L. Peng, and D. Li, Mater. Charact. 131, 380 (2017).
D. Wu, R. Chen, and E. Han, J. Alloy. Compd. 509, 2856 (2011).
J. Liu, L. Yang, C. Zhang, B. Zhang, T. Zhang, Y. Li, K. Wu, and F. Wang, J. Alloy. Compd. 782, 648 (2019).
J. Wang, W. Jiang, Y. Ma, Y. Li, and S. Huang, Mater. Chem. Phys. 203, 352 (2018).
Y. Wu, D. Lin, X. Zeng, L. Peng, and W. Ding, J. Mater. Sci. 44, 1607 (2009).
Y. Tian, H. Huang, G. Yuan, and W. Ding, J. Alloy. Compd. 626, 42 (2015).
Y. Liu, G. Yuan, C. Lu, and W. Ding, Scripta Mater. 55, 919 (2006).
I. Fisher, Z. Islam, A. Panchula, K. Cheon, M. Kramer, P. Canfield, and A. Goldman, Philos. Magaz. B 77, 1601 (1998).
H. Saito, K. Fukamichi, T. Goto, A. Tsai, A. Inoue, and T. Masumoto, J. Alloy. Compd. 252, 6 (1997).
D. Wang, P. Fu, L. Peng, Y. Wang, and W. Ding, Mater. Charact. 153, 157 (2019).
J.F. Nie, K. Oh-Ishi, X. Gao, and K. Hono, Acta Mater. 56, 6061 (2008).
H. Qi, G. Huang, H. Bo, G. Xu, L. Liu, and Z. Jin, J. Mater. Sci. 47, 1319 (2012).
Z. Zhu, and A.D. Pelton, J. Alloy. Compd. 652, 426 (2015).
J. Gröbner, A. Kozlov, X.-Y. Fang, S. Zhu, J.-F. Nie, M.A. Gibson, and R. Schmid-Fetzer, Acta Mater. 90, 400 (2015).
J. Gröbner, S. Zhu, J.-F. Nie, M.A. Gibson, and R. Schmid-Fetzer, J. Alloy. Compd. 675, 149 (2016).
B. Liu, H. Li, Y. Ren, H. Xie, H. Pan, M. Jiang, and G. Qin, J. Alloy. Compd. 870, 159502 (2021).
H. Xu, H.-L. Chen, P. Wang, and T. Zhou, J. Alloy. Compd. 884, 161048 (2021).
H. Xu, J. Fan, H.-L. Chen, R. Schmid-Fetzer, F. Zhang, Y. Wang, Q. Gao, and T. Zhou, J. Alloy. Compd. 603, 100 (2014).
H. Xu, Y. Du, Y. Tan, Y. He, S. Li, and Z. Xiang, J. Alloy. Compd. 425, 153 (2006).
K. Sugiyama, K. Yasuda, T. Ohsuna, and K. Hiraga, Zeit. für Kristallogr. Cryst. Mater. 213, 537 (1998).
E. Abe, H. Takakura, A. Singh, and A. Tsai, J. Alloy. Compd. 283, 169 (1999).
M. Li, D. Deng, and K. Kuo, J. Alloy. Compd. 414, 66 (2006).
Acknowledgements
The financial supports from the China Hunan Provincial Science& Technology Department (Grant No 2020GK2051), Major Special Projects in Changsha Science and Technology Bureau (Grant No kh2103011), National key research and development program of China (2021YFB3502600) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, H., Zhou, T., Wang, P. et al. Solid-State Phase Equilibria at 320°C of the Mg-Gd-Zn System in the Region of Less Than 50 at.% Gd. JOM 75, 3025–3032 (2023). https://doi.org/10.1007/s11837-023-05737-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-023-05737-2