Abstract
Hydrometallurgy is a flexible and efficient process for recovering heavy and valuable metals from industrial residues. In this study, various parameters of cobalt reductive leaching were investigated using the Taguchi experiment design (DOE) method to determine the best conditions for dissolving cobalt from zinc plant residue. The experiment parameters included: initial sulfuric acid concentration (0.1 M, 0.316 M and 1 M), reaction temperature (70, 80 and 90°C), reaction time (15, 30 and 60 min), and solid/liquid ratio (1:5, 1:6 and 1:7 g/L). The iron sulfate was used as a reducing agent, and the sulfuric acid was used as the solvent. The experiment results showed that the optimal conditions for dissolving cobalt were initial sulfuric acid concentration 1 M, temperature 90°C, time 60 min, and solid/liquid ratio 1:7. Under these conditions, there was good agreement between predicted (97.5%) and experimental results (97.2%) in terms of cobalt dissolution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cobalt is an important industrial metal which is used in various devices such as gas turbine blades, aircraft engines, hard alloys, magnetic alloys, nuclear power plants, and medical equipment because of its unique properties including being ferromagnetic and cut and wear resistant. In addition, cobalt metal is used in rechargeable batteries such as lithium-ion batteries and other electrical devices. In modern industry, it is a component of green energy, and the demand for cobalt metal is increasing. Cobalt is a strategic metal and is one of the rarest industrial metals. It comprises about 0.001% of the earth's crust, and > 60% of cobalt is mined in Congo.1,2,3,4,5,6,7 Cobalt does not exist freely in nature and is mostly extracted from primary minerals containing cobalt and secondary sources. Primary minerals include Ni (Cu, Co), Cu (Co), and Co (As) and lateritic ores Ni (Co). Depending on cobalt grade, it is mined in three open-pit, underground, and combined open-pit and underground methods.8,9,10,11,12 In recent years, researchers and producers have reported the recovery of cobalt from secondary sources. The secondary sources include sludge, catalyst residues, zinc smelting slag, spent batteries, cobalt alloy scrap, sea nodules, sulfides deposited in nickel metal production process, and residue from zinc processing plants which contain about 0.5 to 1% cobalt, depending on the plants' raw materials input.3,4,13,14,15,16,17 This metal is mostly extracted from both primary and secondary sources by three methods: pyrometallurgy, hydrometallurgy, and vapor metallurgy. The pyrometallurgical process is costly and requires more energy. Therefore, researchers and producers have employed hydrometallurgical methods to recover cobalt from secondary sources.5,18,19,20,21 Globally, > 75% of cobalt is recovered from secondary source using hydrometallurgical processes. Hydrometallurgical flowsheets have been used for recovering cobalt from secondary sources such as zinc plant residues.2,15,22,23,24,25 Most research projects for recovering cobalt from primary and secondary sources have used solvent extraction (SX) using D2EHPA, Cyanex302 and Cyphos IL101, citric acid, sodium salt of di-decylphosphinic acid (DDPA), borax, phenol dosage, ammonium bifluoride, and bis (2,4,4- trimethylpentyl) dithiophosphinic acid.9,12,15,26 In Zanjan Zinc KhalesSazan Company (ZZKICo), Iran, the cobalt filter cake is obtained at hot purification stage of hydrometallurgical production process of zinc (Fig. 1). In the first stage of zinc production process, the zinc oxide mineral is dissolved using sulfuric acid. Most of the zinc enters the solution with impurity elements such as cobalt, nickel, cadmium, and iron. These impurities are removed from solution or reduced to an acceptable level. The first stage of purification is called hot purification. At this stage, the potassium permanganate is added to the leach solution at 80–85°C to convert Co2+ to Co3+. Adjustment of pH to 5.0-5.2 using calcium carbonate precipitates cobalt as cobalt hydroxide. The entire pulp is filtered, and the solid phase is called cobalt cake. The following reactions are responsible for the formation of cobalt hydroxide (Co(OH)3) in hot purification stage.26,27,28
After oxidation and adding Ca(OH)2, cobalt precipitates as cobalt hydroxide.
Adding potassium permanganate to leach solution for cobalt oxidation increases the amount of manganese in the cobalt cake. As a result, it complicates the separation of manganese and cobalt.24,25 In this research, the different parameters of cobalt reductive leaching are investigated using Taguchi experiment design method with 95% confidence level to determine the optimal conditions for cobalt dissolution from hot filter cake (HFC). The Taguchi method has advantages such as fewer tests, less time, and low cost of raw materials compared to other methods. In the reductive leaching experiment, the effects of initial sulfuric acid concentration, reaction temperature, reaction time, and solid/liquid ratio are investigated.
Experimental Methods
Materials and Characterization of the Hot Filter Cake (HFC)
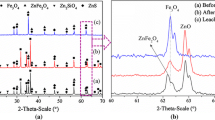
The cobalt filter cake (hot filter cake) was obtained from Zanjan, Zinc KhalesSazan Company (ZZKICo), Iran. First, the cobalt cake was dried in an oven at 105 °C for 24 h and then mixed completely. The mixed sample was then used for chemical and mineralogical analysis using digestion/AAS and XRD. The chemical analysis of the dried sample was conducted to determine the composition of the cobalt filter cake using an atomic absorption spectroscopy (Varian, AA240, Australia) device; the chemical composition of cobalt filter cake is shown in Table I. The mineralogy analysis was conducted by x-ray diffractometry (XRD) (inel, 2000X, France, voltage, 40 KV; current, 30 mA) device. The results showed gypsum (CaSO4.0.5H2O) as the major phase of mineralogy in cobalt filter cake. The mirroring of the phases indicated that that the remaining phases in cobalt cake include manganese oxide (MnO2, Mn3O4), cobalt oxide (CoO, Co3O4), iron oxide (Fe3O4), and zinc oxide (ZnO) (Fig. 2). The sulfuric acid from Merck Company (purity = 95-98%, density = 1.84 g/mL) and iron (II) sulfate from Rad Chemistry Alborz Company (purity = 97-98%, density = 1.85 g/cm3) were used in reductive leaching of cobalt.
Taguchi Design of Experiment
For the first time, an English researcher named Fisher developed a method for designing experiments with different parameters and levels, known as the factorial method which involved full factorial and partial factorial. The full factorial design method is complicated as the number of parameters rises by enhancement of the number of parameters. To reduce the number of experiments, partial factorial experiments were developed to obtain the most information from minimum experiments. However, this method decreased the accuracy of the results. In 1940, Taguchi, a Japanese researcher, developed a method based on orthogonal arrays, known as the Taguchi method. The use of this method not only resolved the defects of the factorial method, but also reduced the number of experiments and thus reduced the costs while offering more accurate results by conducting fewer experiments.22,23,24,25,26 In this study, cobalt reductive leaching was explored by the Taguchi experiment design method with L9(34) orthogonal array (OA) with four factors, each in three levels.25,26,27,28,29 The results were analyzed using Minitab18 software. The positions of parameters and levels at Taguchi experiment design method in orthogonal L9array are shown in Table II. Table III also lists the design of Taguchi L9, (OA)34 adopted in this study.27,28,29,30,31,32,33,34
Experimental Procedure
A magnetic stirrer (MSH600 – SHAFQ) with the ability to digitally control the temperature, time, and stirring speed, a 1000 mL beaker, a pH meter (Mettler Toledo, S220 Seven Compact pH/ion meter), an oven (Model 50L, Behdad Equipment Company, Iran) with the ability to adjust temperature and time, and a mechanical stirrer (TAT-2500) with the ability to digitally control the time and speed of stirring with three blades and stainless coating were used to conduct the reductive leaching experiments.
According to Reaction 7 and Eq. 2, 254.7 g of iron sulfate II (2FeSO4.7H2O) was first weighed and poured into a 1000-mL beaker. The volume of the solution was then increased to 350 mL using distilled water. The mixture was magnetically stirred at 600 rpm and the iron sulfate was completely dissolved in distilled water. Afterward, 100 g dried cobalt cake (with a grain size < 100 microns) was added to the iron sulfate solution. Using distilled water and sulfuric acid, the desired volumes were obtained, and the experiment was conducted. It should be noted that in all experiments, the temperature and volume of the solution were constant, and the initial pH was controlled by a pH meter. At the end of the experiment, the leaching solution was filtered and cobalt was analyzed using AAS. The cobalt dissolution efficiency was calculated according to Eq. 1.
where R is the dissolution percentage of cobalt; V1 denotes the concentration of cobalt ion in the leaching solution; X represents the volume of leaching solution; V2 stands for the cobalt concentration in cobalt filter cake, and M shows the mass of cobalt filter cake.

Results and Discussion
To determine the optimal conditions of cobalt dissolution, the Taguchi design method was used. Based on the principles of quality control and the array of primary theories, orthogonal arrays, and factor design, Taguchi established this method, leading to standard designs for examining different factors and levels. Taguchi lists 18 basic orthogonal arrays that are known as standard orthogonal arrays. In this research, Taguchi L9 test design method has been used.
Reductive leaching has been extensively used for the recovery of valuable metals from industrial wastes such as ocean nodules, manganese nodules, and residue from zinc production plants. Some methods for extracting valuable metals from industrial wastes are based on hydrometallurgical processes, because the hydrometallurgical processes are relatively simple and the energy consumption for extraction is low. According to the previous research and the analysis performed on the cobalt cake, the initial structure of the cobalt filter cake is spinel. To release cobalt from the compound and enter it into the solution, a strong reducing agent along with a solvent is required. In the present study, ferrous sulfate was used as the reducing agent and sulfuric acid was used to dissolve cobalt. According to the Reaction 7 and Eq. 2, ferrous sulfate II converts Mn4+ to Mn2+ and the cobalt is released from the compound. Existing sulfuric acid also dissolves cobalt and other impurities in the compound. The impurities can be removed from the leaching solution via various methods such as SX using the ammonium persulfate to remove the iron and zinc from the solution and using the NN reagent to separate manganese from Refs. 24, 25, and 35,36,37,38,39,40. According to the experimental data presented in Table III, the lowest efficiency of cobalt dissolution is related to experiments 7 and 8. This low dissolution efficiency can be attributed to the experimental conditions, especially the low pH and temperature required for the reaction, which reduces the penetration of ferrous sulfate in the composition and reduction of manganese. The highest efficiency of cobalt dissolution is related to experiment 3. This is due to the high concentration of sulfuric acid and high temperature simultaneously. According to the observations, it can be concluded that with increasing initial concentration acid and temperature, the dissolution rate of cobalt increases linearly and reaches its maximum at 90°C. It is clear that in addition to the type and concentration of solvent, the temperature and concentration of sulfuric acid have a direct effect on the dissolution process according to the Arrhenius equation.40,41 Therefore, with increasing temperature and concentration of sulfuric acid, the dissolution rate also increases (according to Table III and Fig. 3). As shown in Figs. 3 and 4, the pH curve affects the dissolution of cobalt more than other factors. ANOVA analysis (Table IV) calculated the effect of this factor as p-value = 0.025. In contrast, the ratio of solid to liquid had the least effect on dissolution, so two factors of sulfuric acid concentration and temperature were selected as the two factors affecting the dissolution of cobalt from zinc plant waste.23,27,29 In the Taguchi experimental design, the S/N ratio is used to determine the degree of deviation from the desired conditions to find the best experiment conditions. Numerous equations have been developed to determine the S/N ratio; in reductive leaching, "the larger the S/N ratio, the better" was used (Eq. 3, Table III). In this equation, the S/N is the experiment specification. yi is the desired response of process in the i-the experiment and n denotes the number of repetitions of the experiments (n = 1, 2, 3, ..., n).26,27,28
The effects of each factor at each level can be calculated by averaging. For example, the effect of pH on first level was calculated by Eq. 4 (Fig. 3a and Table II).24,27
Based on signal-to-noise ratio analysis, the S/L ratio was more effective than other parameters. Primary pH was found to be much more effective. Therefore, as seen in Fig. 3 A and B, sulfuric acid primary concentration was the most effective factor chosen in this study. The efficiency of reductive leaching increased by raising the temperature.
According to the experimental data, the optimal conditions for reductive leaching of cobalt from cobalt filter cake are shown in Table V. The optimal conditions for dissolving cobalt from cobalt cake were calculated using Eqs. 5 and 6 as follows.
YOpt represents optimal value, YT (746.35) denotes the response totals in experiments, and N is the number of the experiments (9) in L9(34). Primary pH (1 M), temperature (90°C), time (60 min), and S/L ratio (1:7) were selected to maximize the reductive leaching efficiency. At the end of the test, confirmation was performed under optimal conditions to ensure that the response variable is within the range obtained. If the results of this experiment are not within the confidence range, they may not be able to count several interactions and should be considered in further studies. However, a confirmatory experiment was conducted based on the optimal condition as determined by reductive leaching experiment. There was a good agreement between predicted (97.5%) and experimental (97.2%) cobalt dissolution efficiency (Table VI).
In the field of extraction and recycling of valuable metals from secondary waste sources of zinc plants, hydrometallurgical methods are one of the most efficient methods. The first step in this method is the dissolution of the desired metal with the highest efficiency. Since cobalt and manganese metals are present in the cake filter as 3+ and 4+, respectively, a reducing agent is necessary to increase the leaching rate and efficiency and dissolve them at normal temperatures and pressures. Leaching can be performed in water, dilute sulfuric acid, dilute hydrochloric acid, or ammonia solution using a suitable reducing agent. Other important reductants include hydrogen peroxide and citric acid. It has also been shown that the use of a suitable reducing agent converts quaternary manganese to divalent manganese, thus speeding up the leaching process. Furthermore, some metals such as zinc and copper are easily soluble in weak sulfuric acid. Cobalt and manganese form; however, strong oxides require strong acids and a reducer agent for dissolution. Therefore, these two metals should be leached and dissolved under reductive conditions. The amount of consumed sulfuric acid is also one of the important factors in cobalt dissolution from the cobalt filter cake. The sulfuric acid has shown higher reactivity for cobalt. Higher sulfuric acid consumption accelerates the dissolution of cobalt from the cobalt filter cake. Overuse of sulfuric acid, however, is not economically justified and may be environmentally problematic.4,5,6,7,20,21,32,38 Figure 3a and b present the S/N ratio and the mean effects of each factor calculated for each parameter at three levels in the dissolution of cobalt from the cobalt filter cake. According to the previous reports on cobalt reductive leaching,20 the initial concentration of sulfuric acid has the highest effect on dissolution of cobalt from cobalt filter cake. The results indicate that an increase in the initial concentration of sulfuric acid from 0.1 to 1 M further increased the dissolution of cobalt from cobalt cake. However, the time and solid/liquid ratio showed lower influences on cobalt dissolution from the cobalt cake.5,16,20 According to results, the solid/liquid ratio and the initial concentration of sulfuric acid have the least and greatest effect on reductive leaching of cobalt from cobalt filter cake, respectively (Fig. 3a and b, Table 4). In reductive leaching, the pooling action is carried out on the parameter with the least effect on cobalt dissolution. In this experiment, the solid/liquid ratio had the least effect on cobalt dissolution; thus, the pooling action was conducted on that (Table IVa and b).
The effect of initial pH, temperature, and time on cobalt dissolution is shown in Fig. 4a–d. An increase in initial sulfuric acid concentration, temperature, and time enhanced the cobalt dissolution. The dissolution also linearly rose with temperature elevation and reaches the maximum level at 90ºC. According to the Arrhenius equation, the temperature is exponentially associated with the acid dissociation constant of a reaction. Therefore, it is expected that the dissolution rate of cobalt will increase with rising temperature. The reaction time, like the temperature, increased the dissolution of cobalt, which reached its maximum at 60 min. However, the further increase of reaction time may have less effect on the dissolution of cobalt. Given that the acid is used based on the initial concentration, the hypothesis can be proposed that consumption of acid in other early stages may result in a condition in which there will be no acid to react; thus, acid content could not have a signification effect on the dissolution of cobalt in longer periods.20,21,30,36 However, in reductive cobalt leaching, the temperature of 90 ºC, time of 60 min, and sulfuric acid initial concentration of 1 M were determined as optimal conditions for cobalt dissolution. Figure 5 illustrates a conceptual flowsheet for reductive leaching of Co from hot filter cake (cobalt cake).
Conclusion
-
The Taguchi experimental design method with orthogonal array (OA) 34(L9) was utilized to optimize cobalt dissolution in the cobalt reductive leaching process. The following experimental results are obtained.
-
The initial concentration of sulfuric acid had the highest impact on reductive cobalt leaching. The increase of sulfuric acid enhanced cobalt dissolution. In contrast, the solid/liquid ratio had the least effect on the cobalt dissolution; thus, the pooling action was conducted on that. The optimal conditions in cobalt reductive leaching involved sulfuric acid initial concentration of 1 M, temperature of 90ºC, time of 60 min, and solid/liquid ratio of 1:7.
-
A confirmatory experiment was conducted based on optimal conditions. A proper agreement was observed between theory (97.5%) and experiment (97.2%) in terms of cobalt reductive leaching efficiency.
-
According to calculations in the confirmatory experiment, the conducted experiment was within the calculated reliability limit. Hence, it can be concluded that the experimental error had a negligible level of 0.3%.
-
The effect of initial sulfuric acid concentration (pH), temperature, and time on the reductive leaching experiment was also explored. The highest cobalt dissolution occurred when the temperature, time, and sulfuric acid initial concentration were set to 90ºC, 60 min, and 1 M, respectively.
References
K. Kongolo, M.D. Mwema, A.N. Banza, and E. Gock, Miner. Eng 16, 1371 (2003).
M. Liwen, N. Xiaoli, X. Zuoren, and H. Xin’gang, Hydrometallurgy 136, 1 (2013).
Z. Xiu-jing, L. Nai-jun, Z. Xu, F. Yan, and J. Lan, Trans. Nonferrous Met. Soc. China 21, 2117 (2011).
L. Boisvert, K. Turgeon, J.F. Boulanger, C. Bazin, and G. Houlachi, Metals 10, 1553 (2020).
M.K. Jha, V. Kumar, and R. Singh, Resources, Conserv. Recycl. 33, 1 (2001).
B. Kasongo, and H.M. Mwanat, South African J. Sci. 117(5-6), 1–8 (2021).
Y. Zhang, Q. Liu, and C. Sun, Miner. Eng. 14(5), 525–537 (2001).
M. Tanaka, K. Koyama, H. Nartia, and T. Oihi, Recycling valuable metals via hydrometallurgical routes (Design for innovative value towards a sustainable society, Springer, 2012), pp 507–512.
M. Minakshi, N. Sharma, D. Ralph, D. Appadoo, and K. Nallathamby, Solid-state lett. 14, 86 (2011).
C.C. Yong, B.R. Keith, Z. Wensheng, Z. Zhaowu, P. Yoko, and J. Chin, Eng 24, 237 (2016).
C. Fan, X. Zhai, Y. Fu, Y. Chang, B. Li, and Z. Ting-an, Hydrometallurgy 105(1-2), 191–194 (2010).
R. Ewa, B. Lidia, and G. Wanda, Miner. Eng 22, 88 (2009).
S. Shaole, S. Wei, W. Li, L. Runqing, H. Haisheng, H. Yuehua, Y. Yue, and J. Environ, Chem. Eng 7, 102777 (2019).
G. Bina, D. Akash, and S. Virendra, Hydrometallurgy 70, 121 (2003).
J.M. Kumar, K. Anjan, J.A. Kumari, K. Vinay, H. Jhumki, and D.P. Banshi, Waste Manage 33, 1890 (2013).
S. Palanivel, and S. Natarajan, Met 2, 24 (2012).
Z. Yunran, T. Jia, L. Shijun, H. Fang, L. Mei, J. Wei, and H. Jiugang, Sep. Purif. Technol 101, 240 (2020).
C.C. Yong, U. Mark, D. Michael, P. Yoko, and Z. Zhaowu, Miner. Eng 77, 17 (2015).
A. ZhangL, B. NieZ, C. XiX, and D. MaL, Sep. Purif. Technol 195, 244 (2018).
R.K. Mishra, P.C. Rout, K. Sarangi, and K.C. Nathsarma, Trans. Nonferrous Met. Soc. China 26, 301 (2016).
L. Guang-hui, R. Ming-jun, L. Qian, P. Zhi-wei, and J. Tao, Trans. Nonferrous Met. Soc. China 20, 1517 (2010).
P. Rafighi, M.R. Yaftian, and N. Noshiranzadeh, Sep. Purif. Technol 75, 32 (2010).
X. Peng, W. Cheng-yan, X. Sheng-ming, and J. Zhong-jun, Trans. Nonferrous Met. Soc. China 23, 517 (2013).
F. Farahmand, D. Moradkhani, M.S. Safarzadeh, and F. Rashchi, Hydrometallurgy 95(3-4), 316–324 (2009).
A. Fattahi, F. Rashchi, and E. Abkhoshk, Hydrometallurgy 161, 185–192 (2016).
M.S. Safarzadeh, D. Nikhil, B. Mustafa, and D. Moradkhani, Hydrometallurgy 106, 51 (2011).
R. Ahmadi, and H.R. Madaah Hosseini, Mater. Sci-Poland 31, 253 (2013).
A. Babaei-Dehkordi, J. Moghaddam, and A. Mostafaei, Mater. Res. Bull 48, 4235 (2013).
G. Taguchi, E.A. Elsayed, and T.C. Hsiang, Quality engineering in production systems (McGraw-Hill, New York, 1989), pp 12–23.
B. Behnajady, and J. Moghaddam, Chem. Eng. Res. Des 117, 564 (2017).
R. Golmohammadzadeh, F. Rashchi, and E. Vahidi, Waste Manage 64, 244 (2017).
M. Kavand, P. Eslami, and L. Razeh, J. Water Process. Eng 34, 101151 (2020).
K. Ozgul, Hydrometallurgy 95, 333 (2009).
S. Karimi, F. Rashchi, and J. Moghaddam, Int. J. Miner. Process 162, 58 (2017).
M. Deniz Turan, H. Soner Altundogan, and F. Tumen, Hydrometallurgy 75, 169 (2004).
S. Basudev, J. Jinki, L. Jae-chun, L. Gae-Ho, and S. Jeong-Soo, J. Power Sources 167, 536 (2007).
D. Moradkhani, B. Sedaghat, M. Khodakarami, and I. Ataei, Miner. Process. 50(2), 735–746 (2014).
S.B. Kanungo, and R.P. Das, Hydrometallurgy. 21, 41–58 (1988).
D.W. Fuerstenau, and K.N. Han, Miner. Process. Technol. Rev. 1, 1–83 (1983).
J.C. Agarwal, N. Beecher, D.S. Davis, G.L. Hubred, V.K. Kakaria, and R.N. Kust, J. Metals. 28, 24–31 (1976).
J.M. Richardson, L.G. Stevens, and M.C. Kuhn, The recovery of metal values from nickel-bearingllaterite ores by reductive roast/ammonia leach technology (Process and fundamental considerations of selected hydrometallurgical systems, New York, 1981), pp 17–34.
Acknowledgement
The authors appreciate the support of Zanjan Zinc KhalesSazan Industries Company (ZZKICO) to provide samples and analysis used in present investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khodaei, M., Moghaddam, J. & Ahmadi, R. Optimization of Cobalt Reductive Leaching from Zinc Plant Residue (ZPR) Using Taguchi Experimental Design Method. JOM 74, 3030–3038 (2022). https://doi.org/10.1007/s11837-022-05370-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05370-5