Abstract
A pyrometallurgical process for the direct reduction of molten high-lead smelting slag obtained by the Shuikoushan (SKS) method was reported in this article using solid anthracite as the fuel and reductant. The chemical composition, the lead phase composition, and the physical properties of the molten high-lead slag were examined. The effects of the process parameters on the recovery rate of valued metals were investigated in the laboratory. According to the experimental results, a new efficient bottom blow reduction furnace was employed in the pilot-scale test for high-lead slag reduction. The results showed the average recovery rate of lead was more than 96.0% with lower Pb and high Zn content of the reducing slag under the condition of reduction temperature 1100–1200°C, coal ratio 5.5–7.5%, reduction time 90–150 min, CaO/SiO2 ratio 0.35–0.45, and FeO/SiO2 ratio 1.4–1.55. Moreover, nearly 250 kg of standard coal per ton of crude Pb output was reduced compared with the blast furnace reduction process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead is considered to be an important metal, and it is widely used in chemical power sources, metallurgy, and radiation protection.1 China is the largest producer of lead in the world. More than 4647,000 t of lead metal was produced in China in 2012, which ranked first in the world. Most lead is recovered by pyrometallurgical methods, which traditionally involve sintering roasting, followed by blast furnace smelting. Nevertheless, this process has exposed many problems, such as long process time, high sinter return rate (up to 70%), low heat utilization, poor operating environment, and serious Pb and SO2 pollution problems.2 Because of a lead resource shortage and the stricter policies on energy saving and environmental protection, there is an urgent demand for energy-efficient and environmentally friendly lead extraction technologies.
Some advanced direct smelting technologies have been explored to improve the operating environment and to enhance heat utilization. These methods include the Kivcet method,3 Kaldo method,4 Queneau-Schuhmann-Lurgi (QSL) method,5,6 Shuikoushan (SKS) method,7 Isasmelt,8,9 Ausmelt, and Vanyukov. Most studies have focused on the lead concentrate smelting process and on the increase of the concentration of SO2 in the fume for H2SO4 production based on the QSL method by domestic enterprises, i.e., oxygen-rich bottom blow furnace smelting process (OBBF) and oxygen-rich side blow furnace smelting process (OSBF).10 Therefore, the SKS technology has been rapidly expanded based on the QSL method in last 20 years, and its production capacity has accounted for 60% of the total production capacity of lead in China. During the SKS process, OBBF was employed for lead concentrate smelting to replace the sintering roasting process and to obtain the high-lead slag, and the blast furnace (BF) was still used for high-lead slag reduction. Nevertheless, the molten high-lead slag from OBBF requires cooling and casting before it can be processed in the BF. Obviously, this two-step operation is overall thermally inefficient and causes serious environmental pollution. Moreover, the BF slag should be further cleaned with an electric heating well to reduce its heavy metal content, resulting in high capital investment and energy consumption for Pb extraction in SKS technology. To ensure full use of the latent heat of the molten high-lead slag and to strengthen smelting slag reduction, a direct reduction process of molten high-lead smelting slag has become the subject of concern.
Inspired by this approach, a novel process of direct reduction smelting for molten high-lead smelting slag using solid anthracite as reductant and fuel is proposed and the effects of the operating parameters on the recovery rate of valued metal are investigated in the lab. On this basis, an efficient reduction reactor of the variable diameter bottom blow furnace is used in a pilot-scale test. This process would be more energy efficient and improve the valued metal recovery.
Experimental
The high-lead smelting slag was obtained from Henan Yuguang Gold and Lead Co., Ltd., Henan, China. The mixture consisting of solid anthracite, CaO, ferrite, quartz, and 400-g smelting slag was loaded into a corundum crucible (Φ50 mm × 110 mm) and then put in the high-temperature furnace. After a set reaction schedule, the slag cooled to room temperature and then was crushed, weighted, and analyzed. The concentration of metals mentioned in this article was analyzed by dissolving the solid sample. The volume of filtrate was recorded and sampled for chemical analysis using inductively coupled plasma emission spectrometry (ICP). The melting point of the high-lead smelting slag was measured by an intelligent melting point apparatus (2DHR-200). The viscosity of high-lead smelting slag was measured by the rotating cylinder method.
Results and Discussion
Mineralogical Analysis
The main chemical composition of high-lead smelting slag is as follows: Pb 51.7%, Fe 12.4%, Cu 0.37%, Zn 7.93%, SiO2 9.6%, CaO 1.6%, As 0.9%, and S 0.29%. The mineralogical composition of high-lead smelting slag is as follows: PbO 49%, PbSO4 0.42%, Pb 2.4%, PbS 0.32%, Pb3(AsO4)2 0.6%, and Pb3(FeO3)2 0.13%. The chemical composition of solid anthracite has calorific value of 29.5 MJ/kg and 75.4% fixed carbon.
Melting Point of High-Lead Smelting Slag
The melting point of high-lead smelting slag is important for choosing the temperature of the operation. The melting point of high-lead smelting slag was 886°C, and the flowing temperature was 898°C. The flowing temperature is the minimum temperature to enable the metallurgical melt to flow. Thus, the operation temperature should be above 898°C.
Viscosity of High-Lead Smelting Slag
Figure 1 presents the effect of temperature on the viscosity of high-lead smelting slag. The viscosity of high-lead smelting slag decreased with increasing temperature. To ensure good fluidity of high-lead smelting slag, the viscosity should be lower than 1.0 Pa s. Thus, the operating temperature should be greater than 1130°C.
Reduction Smelting
The reduction smelting system is a complicated process with many physical and chemical changes. The probable reactions representing reduction smelting systems are listed as follows:
PbO was mainly reduced by C in the reduction smelting process, whereas 2PbO × SiO2 and PbO × SiO2 were reduced in the presence of CaO and FeO. Solid lead oxide and molten lead oxide were both easily reduced. In the presence of CaO and FeO, the more stable phase of CaO × FeO × SiO2 forms and the molten lead silicate contacts more fully with the reducing agent, C or CO, making PbO much easier to reduce. This result is mainly attributed to the stronger affinity among CaO, FeO, and SiO2. The reduction processes of molten smelting slag include liquid (molten lead oxide)–solid (hot coke) and liquid (molten lead oxide)–gas (CO) reactions. The liquid–solid reaction is dominant at the high smelting temperature because it is an endothermic reaction. The ΔH andΔG of reaction (1) are 35.7 kJ/mol and −404.2 kJ/mol at 1250 K, respectively.
Effect of Coal Ratio
It is known that most Pb and Cu will be reduced and transferred into the metal phase, whereas most Zn and Fe will remain in the slag phase during the reducing process. Therefore, the concepts of recovery of Pb, Cu and enrichment of Zn, Fe are used. In Fig. 2, the recovery rate of related metals from the molten slag differed with different coal ratios. The coal ratio was calculated as the percentage of total input. In this study, the recovery rate of lead and copper increased when the coal ratio was increased from 2.5% to 3.5%. This result corresponds to the fact that the affinity of O for heavy metals is in the order of Cu < Pb < Fe < Zn. The oxides of Pb and Cu were reduced by the C and CO, and the corresponding products formed the molten metal phase. Meanwhile, most Fe and Zn was maintained as the corresponding metal oxides and entered into the slag. Thus, Zn would be mainly recovered from the reduced slag. Nevertheless, ZnO could also be partly reduced by C and CO. The resulting Zn has a low boiling point of 908°C, which is much lower than the operating temperature. Thus, the higher coal ratio increases the possibility of reduction of ZnO and further increases the volatilization loss of Zn. The recoveries of Pb were shown to be 98.1% and 98.9% when the coal ratio was 3.2% and 3.5%, respectively. The optimum ratio of coal was thus chosen to be between 3.2% and 3.5%.
Effect of Reduction Temperature
Figure 3 presents the change of the recovery of metals with different temperatures from the molten high-lead slag in 60 min under the condition that solid anthracite was added to the slag sample at coal ratios of 3.2% and 3.5%. Figure 3 shows that the recovery rate of Pb increased slightly with increasing reduction temperature. The recovery rate of Pb was slightly higher when the coal ratio was 3.5% compared with that when the coal ratio was 3.2%.
The content of Zn in slag phase decreased with increasing temperature. This observation may be attributed to the accelerated zinc volatilization with increased temperature. Higher temperatures improved the rate of Zn volatilization and the transport process. Thus, temperature played a key role in the zinc recovery process. Moreover, the content of Zn in slag phase at a coal ratio of 3.5% was slightly lower than that at a coal ratio of 3.2%. When the temperature ranged from 1175 K to 1225 K, the effect of temperature on the recovery of copper was slight. When the temperature was between 1250 K and 1275 K, the recovery of copper had an obvious decrease. The reason remains to be further studied. The proposed reduction temperature is thus 1200–1225°C, which is also high enough to maintain the slag in the molten state throughout the reduction process.
Effect of Reduction Time
Figure 4 shows the effect of reduction time on the recovery of Pb and Cu in metal phase or content of Zn and Fe in slag phase at 1200°C under the condition that solid anthracite was added to the slag sample at coal ratios of 3.2% and 3.5%. The recovery rate of Pb increased slightly with increasing reduction time and coal ratio. The content of Zn in slag decreased when the reduction time was prolonged from 15 min to 60 min and the coal ratio was increased from 3.2% to 3.5%. The decreased Zn content was mainly attributed to the volatilization. The content of Fe increased with increased reduction time. The proposed reduction time is thus 30–60 min.
Effect of CaO/SiO2 Ratio
Figure 5 shows that higher CaO/SiO2 ratios were not beneficial for the reduction of Pb and Cu in the high-lead molten slag. This may be a result of the fact that PbO is a kind of basic oxide. More PbO remains in slag phase with higher viscosity caused by increased flowing temperature. Nonetheless, this tendency is not obvious. The initial CaO/SiO2 ratio in the high-lead smelting slag was 0.17. The recovery of Pb, Fe, Cu, and Zn decreased with increasing CaO/SiO2 ratio. Therefore, it is suggested that CaO and SiO2 is not added in the reduction process.
Effect of FeO/SiO2 Ratio
The effect of different FeO/SiO2 ratios on the reduction of high-lead smelting slag is shown in Fig. 6. The original FeO/SiO2 ratio in high-lead smelting slag was 1.66. The recoveries of Pb, Fe, and Cu changed slightly with increasing FeO/SiO2 ratio. The recovery of Zn obviously decreased with increasing FeO/SiO2 ratio because more ZnO is reduced and then volatilized. The initial FeO/SiO2 ratio in the high-lead smelting slag was 1.66. Therefore, it is not necessary that FeO and SiO2 are added during the reduction process.
Pilot-Scale Experiments
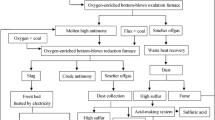
During the high-lead slag reduction process, the liquid Pb is easily reoxidized because of bath agitation and the position of the spray gun. In addition, the life span of the spray gun will be also considered during the reduction process. Therefore, a novel bottom blow reduction furnace (BBRF) is designed as the reactor. Figure 7 shows a schematic diagram of the new reduction furnace. The hearth of the furnace was built with refractories to contain the lead produced during high-lead slag reduction. The different areas of the furnace had different interactions with the process phase and, thus, needed different refractory materials. The furnace roof would be inevitably eroded by the reductant gas CO. The Fe2O3 and Cr2O3 from the refractories would be reduced and then form a substance with a low melting point. The bottom would be corroded by interaction with elements like Pb, Cu, Sb, and As. Therefore the magnesium-chromium bricks DMGe-12 (MgO% ≥ 68%; Cr2O3% ≥ 12%; Fe2O3% ≤ 6.0%) and LBMGe-20 (MgO% ≥ 60%; Cr2O3% ≥ 20%; Fe2O3% ≤ 7.0%) were applied to construct the roof and bottom of the furnace, respectively. The natural gas was injected into the molten slag phase by tuyeres from the bottom of the furnace. The flue gas then went out of the furnace through the flue gas outlet. The products, including reduced slag and crude lead, were released by a slag taphole and lead outlet, respectively. The crude lead entered the siphon room.
According to the lab experimental results, pilot-scale experiments were carried out using VDBBRF as the reactor at Henan Yuguang Gold and Lead Co., Ltd. The average recovery rate of the lead was more than 96.0% under the condition of reduction temperature, 1100–1200°C; coal ratios, 5.5–7.5%; reduction time, 90–150 min; CaO/SiO2 ratio 0.35–0.45; and FeO/SiO2 ratio 1.4–1.55. Moreover, the energy consumption was about 220 kg standard coal per ton crude Pb output. Compared with the blast furnace method (nearly 470 kg of standard coal per crude Pb output), the coal consumption was reduced by 250 kg of standard coal per ton crude. Pb output was reduced, and the flue gas volume was reduced from 39,000 m3/h to 20,000 m3/h. The average Pb content in the reduced lead slag was less than 2.0%, and the Zn content was more than 14%. At present, industrial production has operated for more than a year, and the production process has been functioning well with an annual handling capacity of 110,000 t of lead slag.
Conclusion
The method proposed herein is efficient at strengthening the direct reduction of molten high-lead smelting slag obtained from the SKS method. This new type of bottom-blown reduction smelting furnace has high reducing and thermal efficiency along with a low flue gas volume. The experimental results showed that the recovery rate of the lead reached up to 98% and that greater than 85% zinc entered into the reduction slag under the optimum conditions of coal ratio, 3.2–3.5%; reduction temperature, 1200–1225°C; time, 30–60 min; CaO/SiO2 ratio 0.17; and FeO/SiO2 ratio 1.66.
By using the novel reduction furnace, the average recovery rate of lead was above 96.0% during pilot-scale test under the condition of reduction temperature 1100–1200°C, coal ratios 5.5–7.5%, reduction time 90–150 min, CaO/SiO2 ratio 0.35–0.45, and FeO/SiO2 ratio 1.4–1.55. The Pb content in the reduced slag was less than 2.0% Pb with more than 14% Zn. Compared with the blast furnace method, the energy consumption was reduced by 250 kg of standard coal per ton crude Pb output during this process.
References
X.F. Zhu, L. Li, X.J. Sun, D.N. Yang, L.X. Gao, J.W. Liu, R. Vasant Kumar, and J.K. Yang, Hydrometallurgy 117, 24 (2012).
A.G. Matyas and P.J. Mackey, JOM 28, 10 (1976).
G.P. Ye, Nonferrous Metals (Extractive Metallurgy) 4, 20 (2000).
D.Y. Xu, Nonferrous Metals (Extractive Metallurgy) 6, 11 (2006).
P.E. Queneau, JOM 41, 30 (1989).
P.E. Queneau and A. Siegmund, JOM 48, 38 (1996).
L. Chen, T. Yang, S. Bin, W. Liu, D. Zhang, W. Bin, and L. Zhang, JOM 66, 1664 (2014).
K. Ramus and P. Hawkins, J. Power Sources 42, 299 (1993).
R.B.M. Brew, C.R. Fountain, and J. Pritchard, ISASMELT for Second Lead Smelting, Lead 90: 10th International Lead Conference (The Lead Development Association). 170 (1991).
L. Zhang, G.M. Lin, W.D. Bin, Y.X. Li, and X.B. Li, China Nonferrous Metall. 2, 12 (2012).
Acknowledgements
The authors would like to express sincere thanks to the National High Technology Research and Development Program of China in supporting this research project (2011AA061002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Zhan, J., Fan, Y. et al. Research and Industrial Application of a Process for Direct Reduction of Molten High-Lead Smelting Slag. JOM 69, 784–789 (2017). https://doi.org/10.1007/s11837-016-2236-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-016-2236-z