Abstract
Dead-end trap crops are plants used in pest management that are highly attractive to egg-laying adults but do not support the survival of the developing offspring. For the diamondback moth (DBM; Plutella xylostella), Barbarea vulgaris and B. verna (upland cress) are proven dead-end trap crops but the evidence for Lepidium sativum has been contradictory with one study claiming dead-end effects but others showing that it is a suitable host. Since glucosinolate and saponin levels, which, respectively, stimulate oviposition and act as deterrents, vary with plant age in Barbarea spp., the goal of the present study was to investigate the effects of plant age on the attractiveness and dead-end properties of upland cress and two cultivars of L. sativum (garden cress and broadleaf cress). When given the opportunity to lay eggs on the putative dead-end trap crops or cabbage (Brassica oleracea), DBM did not preferentially lay eggs on garden cress and upland cress until the plants were 5 weeks or older, while broadleaf cress was attractive at all ages. Egg-to-adult survival and growth rate on garden cress and broadleaf cress was as high or higher as on cabbage, regardless of plant age. Upland cress did not reduce survival of DBM at 2 and 5 weeks old but did at 10 weeks old. We confirm that plant age is critical to the effectiveness of upland cress as a dead-end trap crop and conclude that garden cress and broadleaf cress can be suitable trap crops but exhibit no dead-end properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trap cropping is a cultural control tactic that leverages the sensory capabilities of the pest to find a suitable host plant. Plants that are more favorable than the focal crop for oviposition are planted near the cash crop to divert egg-laying away from the cash crop (Hokkanen and Jokioinen 1991; Holden et al. 2012). However, because trap crops are suitable host plants for the pest, they can often act as nurseries that increase the pest population. Additionally, trap crops that are preferred oviposition sites are not necessarily preferred by feeding juveniles. In such a case, mobile juveniles may move from the trap crop onto the cash crop (Holden et al. 2012). To minimize these negative effects, the trap crops are often treated with pesticides or tilled to remove the pests before they move to the cash crop and complete their development (Dogramaci et al. 2004). A more sustainable method for killing juveniles on the trap crop is to use such a crop that is highly attractive to ovipositing adults but do not support the survival of the developing offspring (Shelton and Nault 2004). Such attractive-but-deadly plants used for pest management are termed dead-end trap crops (Shelton and Nault 2004).
The diamondback moth, Plutella xylostella, is a globally distributed pest of plants in the family Brassicaceae (Talekar and Shelton 1993). Oviposition and feeding in DBM is stimulated by glucosinolates, which are defensive secondary metabolites specific to plants in the order Brassicales. When plant tissue is damaged by insect feeding, glucosinolates are hydrolyzed by a myrosinase enzyme to produce toxic chemicals called isothiocyanates (Hopkins et al. 2009). To withstand this plant defense, DBM have evolved glucosinolate-sulfatase detoxification enzymes that remove the sulfates from glucosinolates, which prevents hydrolysis by the myrosinases and lead to the production of non-toxic breakdown products (Ratzka et al. 2002). Thus, instead of being deterred, DBM are attracted to high concentrations of glucosinolates and use them as cues for host plant recognition (Ratzka et al. 2002; Renwick et al. 2006; Städler and Reifenrath 2008). Saponins are another defensive secondary metabolite in some brassicas (Hussain et al. 2019). They are triterpenoid compounds that are widely distributed in plants (Hussain et al. 2019). Saponins can deter feeding, disrupt digestion by interacting with proteinases in insect guts, and can be cytotoxic to lepidopteran cells by penetrating cell membranes and inducing apoptosis (Hussain et al. 2019). Two saponins are recognized as strong feeding deterrents for DBM, leading to the starvation and death of early instar larvae: triterpenoid saponins 3-O-b-cellobiosylhederagenin (saponin I) and 3-O-b-cellobiosyloleanolic acid (saponin II) (Shinoda et al. 2002; Agerbirk et al. 2003; Badenes-Perez et al. 2014b).
Plants containing both glucosinolates, which attract adults for oviposition, and deterrent saponins, which cause larval mortality, are considered dead-end trap crops for DBM. In laboratory studies in Europe and North America, several plants have been shown to have these attractive-but-lethal properties, including wintercress (Barbarea vulgaris; Agerbirk et al. 2003; Shelton and Nault 2004; Newman et al. 2016), upland cress (B. verna; Badenes-Pérez et al. 2011; 2014b), and garden cress (Lepidium sativum; Newman et al. 2016). In these studies, wintercress, upland cress, and garden cress were highly preferred as oviposition sites but none of the DBM larvae that hatched from eggs on those plants survived to adulthood. Wintercress contains both saponin I and II while upland cress contains only saponin I (Badenes-Pérez et al. 2011).
A key factor in the effectiveness of wintercress and upland cress as dead-end trap crops is plant age. Saponins I and II in wintercress are higher in 8-week-old plants than 4-week-old plants (Badenes-Perez et al. 2014a). Moreover, the true leaves of wintercress and upland cress contain saponins but not the cotyledons, demonstrating variations in saponin content among plant parts (Badenes-Perez et al. 2014a). Glucosinolates are also present in higher concentrations in younger plants than in older plants and in younger leaves than older leaves, such that the concentrations of the feeding/oviposition stimulant are positively correlated with concentrations of the deterrent/toxic saponins. While wintercress and upland cress are well-studied dead-end trap crops, the dead-end properties of garden cress has only been mentioned in one study (Newman et al. 2016). In two other studies, garden cress was shown to be a suitable host plant for DBM larval survival and development (Joshi 1998; Dabhi et al. 2009).
The objective of our study was to investigate whether garden cress is a dead-end trap crop by testing oviposition preference and egg-to-adult survival of DBM on different plant ages. There are numerous cultivars of L. sativum, including curly and broad leaf varieties (Sabaghnia et al. 2015). Garden cress is the most used name for L. sativum, when the characteristics include curly, small leaves. Another common cultivar of L. sativum has larger, broader leaves, and is called broadleaf cress or Dutch broad leaf cress. Here, we refer to these two morphological types as cultivars of L. sativum. Seeds of both cultivars are readily available for purchase in North America.
We conducted our study in Hawaii, where DBM populations have been isolated from continental populations since their introduction in 1907, which has resulted in some genetic variation (Chang et al. 1997). Therefore, it was possible that our DBM populations could have different responses to the dead-end trap crops. Hence, we included upland cress in our experiments for comparison of the efficacy of garden cress and broadleaf cress to a known dead-end trap crop of DBM. We assessed DBM oviposition preference, survival, and development on the trap crops relative to the DBM’s responses to head cabbage, because head cabbage is the top volume-producing vegetable crop in Hawaii (USDA 2020). We did not include wintercress in our study, even though it is the most well studied dead-end trap crop, because it is considered to be high risk for becoming invasive in Hawaii and the Pacific Islands (PIER 2018).
Materials and methods
Insects
DBM pupae were collected from a commercial farm in Waianae, Oahu in April 2020 and maintained on head cabbage (Br. oleracea cv. K-K Cross) at 24 °C at the University of Hawai`i at Mānoa. Pupae were housed in a 3.8 L plastic jar (approx. 50 pupae per jar) and supplied with 10% sugar solution. DBM eggs were collected on paper towels by covering the jar opening with the paper towel and placing cabbage leaves on top of the paper towel to stimulate egg-laying. Eggs were collected daily to synchronize hatching to a 24 h period. A second field population was collected from Kekaha, Kauai in April 2021 and used for only the 5-week-old plant survival experiment. We included the Kauai population because the Oahu population is known to be highly resistant to numerous insecticides, and therefore, we wanted to ensure that their potential resistance to the dead-end trap crops was not population-specific.
Plants
Seeds of upland cress (Ba. verna) and garden cress (L. sativum) were purchased from Stoke Seeds (Holland, MI, USA), seeds of broadleaf cress (L. sativum) were purchased from Adaptive Seeds (Sweet Home, OR, USA), and seeds of head cabbage (Br. oleracea var. K-K Cross) were purchased from Holmes Seed Company (Canton, OH, USA). All plants for experiments were grown in an air-conditioned glass greenhouse with natural sunlight (approx. 12 h per day). The daily temperature in the greenhouse fluctuated from approximately 20 to 30 °C. All plants were grown in 8 × 8 × 9.5 cm (L × W × H) dark green plastic pots in potting mix (Sunshine Mix #4; Sungro Horticulture, Agawam, MA, USA) and fertilized directly at sowing with Osmocote Plus (15-9-12, N-P-K; The Scotts Miracle-Gro Company LLC, Marysville, OH, USA). Plants were transferred to larger round plastic pots (15 × 14 cm; DIA × H) at 3-week-old for experiments conducted on 5- and 10-week-old plants. All experiments involving live potted plants were conducted in the greenhouse. Additional growing conditions, such as plant age and number of plants per pot, are described below for each experiment.
Oviposition preference
Oviposition preference was assessed in two-choice experiments. Four ages of trap crop plants were used: 2-week-old (13–18 d), 3.5-week-old (24 d), 5-week-old (35–38 d), and 10-week-old (68–70 d). Two-, 3.5-, and 5-week-old trap crop plants were each placed in a cage with a same-aged cabbage plant. Ten-week-old trap crop plants were placed in a cage with 7-week-old cabbage plants. Choice experiments for 2- and 3.5-week-old plants were conducted in 30 × 30 × 30 cm mesh cages and experiments for 5- and 10-week-old plants were conducted in 40 × 40 × 60 cm mesh cages. Each pot of 2-, 3.5-, and 5-week-old trap crop plants contained a cluster of three plants to produce approximately the same leaf surface area as one cabbage plant. The above-ground surface area of the 5-week-old plants were measured using ImageJ after eggs were counted because it was harder to visually assess whether the surface areas of these older cabbage and trap crops were similar. Pots for 10-week-old plants contained only one plant. The trap crop pot and cabbage pot were placed at opposite sides of the cage, such that the leaves of the two plants that were closest to each other were at least 8 cm apart. The position of the pots in the cages and the placement of the cages were randomized to control for directional bias. One newly emerged male and female DBM (< 24 h after emergence from pupa) were placed into the enclosure to mate and lay eggs. Moths were supplied with 10% sugar solution. For 2 and 3.5-week-old plants, the plants were checked daily for eggs and the number of eggs on each plant recorded on the first day that eggs were observed. For 5- and 10-week-old plants, the high amount of leaves made it difficult to determine the first day of egg-laying. Therefore, eggs on these older plants were counted 48 h after the moths were introduced. Ten-week-old garden cress was excluded from this experiment because it bolted at approximately 6 weeks old and the leaf quality declined rapidly thereafter. All plants were destructively searched to ensure all eggs were counted. The proportion of eggs laid on each plant was calculated by dividing the number of eggs laid on the trap crop by the sum of the eggs laid on the cabbage and trap crop.
Survival and development on intact plants
The ability of DBM larvae to complete their lifecycle on the putative dead-end trap crops was tested. Two-, 5-, and 10-week-old plants were grown as described for the oviposition experiment, including the same numbers of plants per pot. To assess survival and development on 2-week-old plants, a small piece of paper towel containing 10 eggs was placed on the leaves of a cluster of 13-d old plants in each pot and the eggs hatched 2 days later. A 13 × 40 cm (DIA × H) clear plexiglass tube enclosure was placed over each 8 × 8 × 9.5 cm (L × W × H) plastic pot, which contained the trap crop plants or cabbage plant, to fully enclose the plants. The plexiglass tube enclosure was covered with fine mesh on top and there were six 8 cm diameter windows with mesh on the sides for ventilation. The plants were watered as needed and maintained in the greenhouse. Since 2-week-old plants were too small to support complete larval development, all plants were destructively sampled 6 days after egg-hatch to collect and weigh all surviving larvae to the nearest 0.01 mg. At this point, the plants were close to being completely defoliated. There were five replicate enclosures of cabbage, broadleaf cress, garden cress, and upland cress. One 2-week-old garden cress pot was accidentally dropped and the plants broken, thus it was excluded from analyses. All experimental plants with larvae were maintained under greenhouse conditions as described above.
For 5-week-old plants, five eggs on a piece of paper towel were placed on the cluster of trap crops or a single cabbage plant in each pot. The 13 × 40 cm (DIA × H) clear plexiglass tube enclosure was fitted in each round plastic pot (15 × 14 cm; DIA × H) and pressed into the soil to fully enclose the plant. Since searching for the larvae and pupae could be disruptive to their development and survival, the DBM were not collected until all of the surviving larvae developed into moths. Thus, the total number of moths that emerged in each plexiglass cage, live and dead, were recorded 30 d after eggs were introduced. The plants were also inspected to ensure there were no larvae or pupae remaining. The forewing length of each DBM that survived to the adult stage was measured as a proxy for adult size using a Dino-Lite Edge digital microscope and DinoCapture 2.0 software (Dunwell Tech, Inc., Torrance, CA, USA). The measurement was taken from the base of the head to the most distal point on the forewing. Both the Oahu and Kauai DBM populations were tested on 5-week-old plants with five replicate enclosures of cabbage, broadleaf cress, garden cress, and upland cress for each DBM population. All experimental plants with larvae were maintained under greenhouse conditions as described above.
Ten-week-old trap crops were tested against 7-week-old cabbages. One broadleaf cress, upland cress, or cabbage plant was grown per pot (15 × 14 cm; DIA × H). Garden cress was excluded from this experiment because it bolted at approximately 6 weeks old and the leaf quality declined rapidly thereafter. Ten eggs on a piece of paper towel, was placed on each plant. Since the plants were too large for the plexiglass enclosure, each potted plant was fully enclosed in a fine mesh bag. There were five replicate plant pots per plant treatment. The bags were opened 10 d after the eggs hatched and the numbers and developmental stage of surviving DBM on each plant were recorded.
The experiment described above for 10-week-old plants did not allow enough time for larvae to develop to pupation on upland cress and cabbage. Therefore, we repeated the experiment with ten 10-week-old upland cress plants and ten 7-week-old cabbage plants. Ten potted upland cress and 10 potted cabbage plants were each fully enclosed in a mesh bag with 10 eggs on each plant and left for 30 d in the greenhouse. The plants were destructively searched to find all moths, pupae, and larvae, though only moths were found.
Since placing eggs on random parts of the plants could have biased larval survival, we assessed survival of larvae from eggs that were laid directly on the plants, such that we accounted for maternal choice in addition to neonate choice. We placed a mating pair of DBM in a 40 × 40 × 60 cm cage with sugar solution and one pot of either 10-week-old broadleaf cress, 10-week-old upland cress, or 7-week-old cabbage. The DBM were allowed to oviposit on the plants for 72 h. The moths were then removed from the cages and each plant was carefully searched and the numbers of eggs were counted. We allowed the eggs to hatch on their respective plants and counted the numbers of live larvae 8 d later, which was approximately 6 d after egg-hatch. Therefore, the eggs hatched where the female DBM chose to lay her eggs. Six replicate cages were set up for each plant treatment.
Survival and development on excised leaves
Excised leaves from 10-week-old broadleaf cress, 10-week-old upland cress, and 7-week-old cabbage were placed in 30 mL plastic cups. There were four treatment groups, consisting of medium-age leaves from broadleaf cress, medium-age leaves from cabbage, old leaves from upland cress, and medium-age leaves from upland cress. The age of leaves was based on positioning on the plant from the center of the rosette and leaf width. For upland cress, old leaves were on the outside of the plant and leaf width was > 5 cm, while medium-age leaves were closer to the center with a leaf width of 3–4 cm. We did not test young leaves, which have the highest concentrations of glucosinolates and saponins (Badenes-Perez et al. 2014a) because there was not enough young leaves to supply cups with the same amount as medium and old-aged leaves. Only leaves from one plant were used to feed a larva in one cup. Ten cups were set up for cabbage and broadleaf cress and 20 cups each for old and medium-aged upland cress leaves, with each cup receiving leaves from a different plant. One newly molted third instar larva, which had been reared on cabbage, was weighed to the nearest 0.01 mg and placed in each cup. After 72 h of feeding, the larva was reweighed to calculate weight gain. The larvae were placed back in their respective cups and provided fresh leaves every 2 days from the same plants until death or pupation.
Statistical analysis
To assess the attractiveness and deterrence of the trap crop relative to cabbage for DBM oviposition, we compared the numbers of eggs laid on the trap crop and cabbage using a paired sample t-test (two-tailed). The proportion of DBM larvae survived was analyzed by generalized linear model using a binomial distribution and logit link function. Larval weight and adult forewing length were analyzed by a mixed model with the individual enclosure included as a random effect to account for multiple DBM being measured from the same plant. Tukey HSD tests were performed to compare the weight and size of DBM between plant treatments. Larval weight was log10 transformed to meet the assumptions of normality. Larval weight when reared on excised leaves were analyzed by analysis of covariance (ANCOVA) with sex and initial larval weight included as covariates. Differences in mean weight gain between treatments were compared by Tukey’s Honest Significant Difference test. Time to pupation was analyzed by Cox proportional hazards. All statistical analyses were performed on JMP Pro 17 (SAS Institute, Cary, NC, USA).
Results
Oviposition preference
When 2-week-old trap crops were paired with 2-week-old cabbage, DBM laid significantly more of their eggs on broadleaf cress than on cabbage (t12 = 2.43, p = 0.03; Table 1). However, they exhibited no significant preference for garden cress over cabbage (t12 = − 0.12, p = 0.91) and laid more eggs on cabbage than upland cress (t13 = − 2.62, p = 0.02). When the trap crops and cabbage were 3.5 weeks old, DBM tended to lay higher numbers of eggs on all three of the trap crops than on cabbage, though these differences were not statistically significant (broadleaf cress, t5 = 2.35, p = 0.07; garden cress, t5 = 1.68, p = 0.15; upland cress, t4 = 2.64, p = 0.06). On 5-week-old plants, DBM laid significantly more eggs on all the trap crops than on cabbage (broadleaf cress, t9 = 5.24, p < 0.01; garden cress, t9 = 4.35, p < 0.01; upland cress, t8 = 4.14, p < 0.01; Table 1). When given a choice to lay eggs on 7-week-old cabbage and 10-week-old broadleaf cress or upland cress, DBM showed a significant preference for the two trap crops (broadleaf cress, t5 = 3.52, p = 0.02; upland cress, t5 = 3.52, p = 0.02; Table 1).
Survival and development on intact plants
Two-week-old plants
Survival of DBM larvae for 6 days post egg-hatch on 2-week-old broadleaf cress, garden cress, and upland cress were high (74–85% survival) such that survival was not significantly different between the three trap crops and cabbage (X23 = 0.74, p = 0.86; Table 2). Larval weight also did not differ significantly among plant treatments (F3 = 2.49, p = 0.10; Table 2).
Five-week-old plants
Survival from egg to adult on 5-week-old plants was not affected by host plant treatment, regardless of DBM population (plants, X3 = 1.18, p = 0.76; DBM population, X21 = 0.02, p = 0.89; Table 3). However, the forewings of moths that developed on broadleaf cress and garden cress were significantly longer than those that developed on cabbage (F3 = 6.23, p = 0.002) (Table 3; random effect Wald p-value = 0.49). The forewing length of moths from upland cress did not differ significantly from those on cabbage and the other cresses. This plant effect on forewing length was consistent between DBM populations, though the Kauai population had overall longer forewings than the Oahu population (population, F1 = 7.36, p = 0.01; population by plant interaction, F3 = 0.53, p = 0.66).
Ten-week-old plants
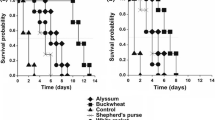
Significantly fewer DBM larvae survived for 11 d on 10-week-old upland cress than on 10-week-old broadleaf cress and 7-week-old cabbage (X22 = 32.39, p < 0.0001; Table 4). Of larvae that survived, the number in each developmental stage at that time point differed significantly by plant (3rd instars: X22 = 10.08, p = 0.007; 4th instars: X22 = 52.39, p < 0.0001; pupae: X22 = 56.63, p < 0.0001). Out of the total 50 eggs placed on 5 broadleaf cress plants, all 30 survivors had already pupated at 11 d post egg-hatch, but on cabbage, the majority of survivors were still in the 4th instar (25 out of 36 survivors; Table 4). Only 9 larvae were found alive on upland cress, with most larvae still in the 3rd instar (6 out of 9 survivors). When the experiment was repeated with 10 upland cress and 10 cabbage plants, and DBM were allowed to complete development (i.e., counted after 30 d), we found that 66 ± 14% of DBM survived from egg-to-adult on cabbage while only 2 ± 0.4% survived to adult on upland cress (X21 = 108.60, p < 0.0001).
When female moths were allowed to deposit their eggs directly on the plants, rather than on paper towels, the survival of those offspring from egg for 8 d (approximately 6 d into the larval stage; mean survival ± SE) was 86.6 ± 6.2% for broadleaf cress, 85.7 ± 2.5% for cabbage, and 13.8 ± 2.5% for upland cress (F2,15 = 103.39, p < 0.0001). The moths laid significantly more eggs on broadleaf cress (61.5 ± 8.4 eggs) and upland cress (53.8 ± 9.8 eggs) than on cabbage (24.0 ± 3.5 eggs) (F2,15 = 6.57, p = 0.009).
Survival and development on excised leaves
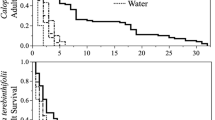
On excised leaves from 10-week-old broadleaf cress, 10-week-old upland cress, and 7-week-old cabbage, survival of 3rd instar larvae over a 72 h feeding period was significantly lower on medium-age upland cress leaves compared to cabbage, broadleaf cress, and old upland cress leaves (X23 = 32.79, p < 0.0001; Table 5). Larval weight gain after 72 h was significantly higher on broadleaf cress than cabbage and significantly lower on both old and medium-aged upland cress than cabbage (F3,40 = 27.91, p < 0.0001; Table 5). There were no significant effects of initial larval weight (F1,40 = 1.23, p = 0.27) and sex (F1,40 = 0.02, p = 0.88) on the amount of weight gain. Only one larva out of 20 survived to adult emergence on medium-age upland cress leaves and only eight larvae out of 20 survived to adult emergence on old upland cress leaves, which was significantly lower than survival on cabbage and broadleaf cress (X23 = 25.94, p < 0.0001; Table 5). Since only one larva reached adulthood on medium-age upland cress, it was not included in statistical analyses for comparing development time and pupal weight. Development time from 3rd instar to pupation did not significantly differ among old upland cress leaves, broadleaf cress and cabbage (X22 = 1.61, p = 0.45), though larvae did appear to develop faster on broadleaf cress than the other plants (Table 5). Sex and initial weight did not affect development time (sex: X21 = 0.06, p = 0.80; initial weight: X21 = 0.16, p = 0.69). Larvae that developed on old upland cress leaves produced smaller pupae than those that developed on cabbage and broadleaf cress (F2,22 = 33.54, p < 0.0001; Table 5). Female pupae were bigger than male pupae (covariate, sex: F1,22 = 20.22, p = 0.0002).
Discussion
Previous studies demonstrated that both upland cress and garden cress are highly preferred for oviposition but subsequent DBM larvae achieved 0% survival to adulthood (Badenes-Pérez et al. 2011, 2014b; Newman et al. 2016). As expected, we found an age-dependent effect of upland cress as a dead-end trap crop for DBM. Larval survival on upland cress was as high as on cabbage when the plants were 2 and 5 weeks old, because young upland cress contain low amounts of saponins (Badenes-Perez et al. 2014a). Upland cress has significantly higher amounts of saponin at 8-week-old (Badenes-Perez et al. 2014a), and correspondingly we found significantly reduced growth rate and survival of DBM larvae on 10-week-old plants. In contrast, both broadleaf cress and garden cress exhibited no dead-end properties. Larval survival was as high as on cabbage at all ages of the plant tested (2 and 5 weeks old for garden cress and 2, 5, and 10 weeks old for broadleaf cress). This was in contrast to the complete mortality of DBM on 4- to 6-week-old garden cress reported by Newman et al. (2016). Plants in the Brassicaceae family are known to exhibit genotypic variation in glucosinolate and saponin content (Padilla et al. 2007; Verkerk et al. 2010; Badenes-Perez et al. 2014b). In the most well-studied dead-end trap crop wintercress, there are varieties that are effective dead-end hosts, and others that support DBM development. DBM do not survive on the G-type (glabrous) varieties, but do survive on the P-type (pubescent) as the P-type lacks saponin I and II (Badenes-Perez et al. 2014a). Whether genotypic variation is the reason for the contradicting results for garden cress and broadleaf cress’ effects on DBM survival is unknown. It is possible that isolation of DBM in Hawaii for over 100 years has resulted in evolution of their abilities to detoxify or habituate to certain plant secondary metabolites. Outside of Brassicaceae farms, DBM in Hawaii may be adapted to utilizing different Brassicaceae weeds as alternate host plants, especially since the diversity of Brassicaceae weeds in Hawaii is lower than many parts of the world (Haselwood et al. 1983). We previously showed that DBM in Hawaii have a high preference for ovipositing on and exhibit high survival on a wild Lepidium spp., L. virginicum (Pugh et al. 2022). Regardless, considering our results and the findings from two studies from India demonstrating L. sativum to be a suitable host plant for DBM (Joshi 1998; Dabhi et al. 2009), we would not recommend the use of garden cress and broadleaf cress as dead-end trap crops.
Adaptation of DBM to dead-end trap crops has been a significant concern for their long-term use in DBM management (Badenes-Perez et al. 2006). Thus, one concern from our study was that a small number of DBM larvae survived to adulthood on 10-week-old upland cress. Some dead-end trap crops, such as wintercress, stimulates increased oogenesis, which could accelerate selection for wintercress-resistant DBM (Badenes-Perez et al. 2006). We similarly found that when DBM were allowed to lay eggs on 10-week-old upland cress, 10-week-old broadleaf cress, or 7-week-old cabbage in the survival experiment, the moths laid more than double the number of eggs on the cresses than on cabbage during the first 72 h after introduction to the cages. Additionally, larvae exhibited higher survival on excised old leaves from upland cress than medium-aged leaves. DBM are known to lay more eggs on younger leaves of wintercress, which have higher concentrations of glucosinolates (Badenes-Perez et al. 2014a). Since the younger leaves also contain more saponins and other toxic/deterrent allelochemicals, larvae that hatch will die from eating those young leaves (Badenes-Perez et al. 2014a). On intact upland cress plants, we observed that more of the surviving DBM larvae were on older leaves and rarely on younger leaves, though we found more eggs on young and medium-aged leaves than old leaves (observation; data not collected). This suggests that some DBM larvae may exhibit behavioral resistance by avoiding younger leaves with higher amounts of saponins and other toxic/deterrent allelochemicals or that the eggs of those surviving larvae were laid on the old leaves and they chose to remain on those leaves. Additionally, the DBM in our study was collected from intense cabbage growing areas with high populations and were reared in the lab in high densities (Armstrong et al. 2024). Since DBM from higher density populations tend to have broader host plant ranges (Bigger and Fox 1997), it is possible that these populations were more tolerant of saponins and other chemical defenses. While we only tested one population of DBM on 10-week-old plants, DBM populations in Hawaii have significant gene flow, meaning that DBM from different islands readily interbreed and are genetically very similar to each other (Caprio and Tabashnik 1992). It should be noted that since the Hawaii population does exhibit greater genetic differentiation from mainland populations (Caprio and Tabashnik 1992), it is possible that some of the survival, development, and preferences on the three cresses we tested could be influenced by geographic isolation.
Environmental conditions in our greenhouse could have also affected larval survival on upland cress. Saponin quantity and quality can vary with changes in light, humidity, temperature, and soil fertility, and can also be induced to higher levels when damaged by herbivore feeding and by pathogen infection (Szakiel et al. 2011; Badenes-Perez et al. 2014a; Hussain et al. 2019). For example, wintercress grown in the greenhouse or collected in the summer months were resistant to DBM larval feeding but those collected in late fall were less resistant (Agerbirk et al. 2003). Seeds from different sources can also produce plants with varying properties (e.g., Zhang et al. 1967; Bedane et al. 2020). Our upland cress seeds were purchased from a different source than other studies (Badenes-Pérez et al. 2011, 2014b).
As a trap crop, all three cresses were highly attractive to ovipositing females, but this was also affected by plant age. Garden cress was not preferred over cabbage for oviposition at 2- and 3.5-week-old but highly attractive at 5-week-old, whereas broadleaf cress was preferred over cabbage at all ages. DBM response to upland cress was similar to their response to garden cress such that upland cress was not preferred over cabbage until the plants were 5 weeks old. The reduced attractiveness of garden cress and upland cress at younger ages could be because younger cabbage have higher concentrations of glucosinolates (Rangkadilok et al. 2002) and lower toughness (Raupp et al. 1988; King et al. 1998) than older cabbage (prior to flowering). Thus, if the young garden cress and upland cress were tested against older cabbage plants, they may have acted as an effective trap crop.
Not only did DBM survive on garden cress and broadleaf cress, but they also tended to grow faster and achieved larger adult size than DBM that developed on cabbage. This was also the case for DBM developing on 2- and 5-week-old upland cress. Larger adult size is usually predictive of higher fitness in insects (Awmack and Leather 2002). Longer forewing length in female DBM is correlated with higher body mass, fecundity, and flight capacity (Shirai 1993; Begum et al. 1996; Campos et al. 2004), and for male DBM, longer forewing length is associated with larger testis as well as higher body mass and flight capacity (Hiroyoshi et al. 2021). Plant species has previously been shown to influence DBM fitness measures. For example, DBM had longer lifespan, larger body size, and increased flying ability when reared on two wild crucifers, Indian marshcress (Rorippa indica) and Virginia pepperweed (L. virginicum), than on cabbage (Begum et al. 1996).
In conclusion, our results indicate that garden cress and broadleaf cress cultivars of L. sativum are not suitable as dead-end trap crops for DBM. Since DBM development can be enhanced on these cultivars, they could act as DBM nurseries if not properly managed. We also confirmed that upland cress could serve as a dead-end trap crop as it ages. However, since it supports DBM development at younger plant ages (< 8 weeks old), more research is needed to develop a proper deployment strategy, which considers the right developmental timing for the trap crop and cash crop.
Data availability
The data that support the findings of this study are available from the author upon reasonable request.
References
Agerbirk N, Olsen CE, Bibby BM et al (2003) A saponin correlated with variable resistance of Barbarea vulgaris to the diamondback moth Plutella xylostella. J Chem Ecol 29:1417–1433. https://doi.org/10.1023/A:1024217504445
Armstrong KM, Uyeda J, Shikano I (2024) Influence of the parasitoid Cotesia vestalis on the distribution of diamondback moth larvae on cabbage plants. Arthropod plant interact
Awmack CSC, Leather SSR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844. https://doi.org/10.1146/annurev.ento.47.091201.145300
Badenes-Perez FR, Nault BA, Shelton AM (2006) Dynamics of diamondback moth oviposition in the presence of a highly preferred non-suitable host. Entomol Exp Appl 120:23–31. https://doi.org/10.1111/j.1570-7458.2006.00416.x
Badenes-Perez FR, Gershenzon J, Heckel DG (2014a) Insect attraction versus plant defense: young leaves high in glucosinolates stimulate oviposition by a specialist herbivore despite poor larval survival due to high saponin content. PLoS ONE 9:39–42. https://doi.org/10.1371/journal.pone.0095766
Badenes-Perez FR, Reichelt M, Gershenzon J, Heckel DG (2014b) Using plant chemistry and insect preference to study the potential of Barbarea (Brassicaceae) as a dead-end trap crop for diamondback moth (Lepidoptera: Plutellidae). Phytochemistry 98:137–144. https://doi.org/10.1016/j.phytochem.2013.11.009
Badenes-Pérez FR, Reichelt M, Gershenzon J, Heckel DG (2011) Phylloplane location of glucosinolates in Barbarea spp. (Brassicaceae) and misleading assessment of host suitability by a specialist herbivore. New Phytol 189:549–556. https://doi.org/10.1111/j.1469-8137.2010.03486.x
Bedane KG, Zühlke S, Spiteller M (2020) Bioactive constituents of Lobostemon fruticosus: anti-inflammatory properties and quantitative analysis of samples from different places in South Africa. South African J Bot 131:174–180. https://doi.org/10.1016/j.sajb.2020.02.016
Begum S, Tsukuda R, Fujisaki K, Nakasuji F (1996) The effects of wild cruciferous host plants on morphology, reproductive performance and flight activity in the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Res Popul Ecol (kyoto) 38:257–263. https://doi.org/10.1007/BF02515735
Bigger DS, Fox LR (1997) High-density populations of diamondback moth have broader host-plant diets. Oecologia 112:179–186. https://doi.org/10.1007/s004420050298
Campos WG, Schoereder JH, Sperber CF (2004) Does the age of the host plant modulate migratory activity of Plutella xylostella? Entomol Sci 7:323–329. https://doi.org/10.1111/j.1479-8298.2004.00080.x
Caprio MA, Tabashnik BE (1992) Allozymes used to estimate gene flow among populations of diamondback moth (Lepidoptera: Plutellidae) in Hawaii. Environ Entomol 21:808–816. https://doi.org/10.1093/ee/21.4.808
Chang WXZ, Tabashnik BE, Artelt B et al (1997) Mitochondrial DNA sequence variation among geographic strains of diamondback moth (Lepidoptera: Plutellidae). Ann Entomol Soc Am 90:590–595. https://doi.org/10.1093/aesa/90.5.590
Dabhi MR, Mehta DM, Patel CC (2009) Life table of diamondback moth, Plutella xylostella L. on cress (Lepidium sativum L.). Int J Agric Environ Biotechnol 2:80–82
Dogramaci M, Shrefler JW, Roberts BW et al (2004) Comparison of management strategies for squash bugs (Hemiptera: Coreidae) in watermelon. J Econ Entomol 97:1999–2005. https://doi.org/10.1093/jee/97.6.1999
Haselwood EL, Motter GG, Hirano RT (1983) Handbook of Hawaiian weeds. Harold L. Lyon Arboretum, Manoa Valley
Hiroyoshi S, Mitsunaga T, Reddy GVP (2021) Effects of temperature, age and stage on testis development in diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Physiol Entomol 46:12359. https://doi.org/10.1111/phen.12359
Hokkanen HMT, Jokioinen F (1991) Trap cropping in pest management. Annu Rev Entomol 36:119–138. https://doi.org/10.1146/annurev.en.36.010191.001003
Holden MH, Ellner SP, Lee DH et al (2012) Designing an effective trap cropping strategy: the effects of attraction, retention and plant spatial distribution. J Appl Ecol 49:715–722. https://doi.org/10.1111/j.1365-2664.2012.02137.x
Hopkins RJ, van Dam NM, van Loon JJ, a, (2009) Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol 54:57–83. https://doi.org/10.1146/annurev.ento.54.110807.090623
Hussain M, Debnath B, Qasim M et al (2019) Role of saponins in plant defense against specialist herbivores. Molecules 24:1–21. https://doi.org/10.3390/molecules24112067
Joshi NR (1998) Bionomics and control of diamondback moth, Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) on cress (Lepidium sativum Linnaeus). Master of Science Thesis. Gujarat Agricultural University
King BH, Crowe ML, Blackmore MD (1998) Effects of leaf age on oviposition and on offspring fitness in the imported willow leaf beetle Plagiodera versicolora (Coleoptera: Chrysomelidae). J Insect Behav 11:23–36. https://doi.org/10.1023/a:1020810415249
Newman K, You M, Vasseur L (2016) Diamondback moth (Lepidoptera: Plutellidae) exhibits oviposition and larval feeding preferences among crops, wild plants, and ornamentals as host plants. J Econ Entomol 109:644–648. https://doi.org/10.1093/jee/tow002
Padilla G, Cartea ME, Velasco P et al (2007) Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 68:536–545. https://doi.org/10.1016/j.phytochem.2006.11.017
PIER (2018) Institute of Pacific Islands Forestry, Pacific Island Ecosystems at Risk (PIER)—plant threats to Pacific ecosystems. http://www.hear.org/pier/
Pugh M, Kihata N, Uyeda J et al (2022) The effects of a naturalized weed, Lepidium virginicum, on the development and behaviors of the diamondback moth and its natural enemies in Hawaii. Biol Control 173:104994. https://doi.org/10.1016/j.biocontrol.2022.104994
Rangkadilok N, Nicolas ME, Bennett RN et al (2002) Developmental changes of sinigrin and glucoraphanin in three Brassica species (Brassica nigra, Brassica juncea and Brassica oleracea var. italica). Sci Hortic (amsterdam) 96:11–26. https://doi.org/10.1016/S0304-4238(02)00118-8
Ratzka A, Vogel H, Kliebenstein DJ et al (2002) Disarming the mustard oil bomb. Proc Natl Acad Sci USA 99:11223–11228. https://doi.org/10.1073/pnas.172112899
Raupp MJ, Werren JH, Sadof CS (1988) Effects of short-term phenological changes in leaf suitability on the survivorship, growth, and development of gypsy moth (Lepidoptera: Lymantriidae) larvae. Environ Entomol 17:316–319. https://doi.org/10.1093/ee/17.2.316
Renwick JAA, Haribal M, Gouinguené S, Städler E (2006) Isothiocyanates stimulating oviposition by the diamondback moth, Plutella xylostella. J Chem Ecol 32:755–766. https://doi.org/10.1007/s10886-006-9036-9
Sabaghnia N, Ahadnezhad A, Janmohammdi M (2015) Genetic variation in garden cress (Lepidium sativum L.) germplasm as assessed by some morphological traits. Genet Resour Crop Evol 62:733–745. https://doi.org/10.1007/s10722-014-0192-4
Shelton AM, Nault BA (2004) Dead-end trap cropping: a technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot 23:497–503. https://doi.org/10.1016/j.cropro.2003.10.005
Shinoda T, Nagao T, Nakayama M et al (2002) Identification of a triterpenoid saponin from a crucifer, Barbarea vulgaris, as a feeding deterrent to the diamondback moth, Plutella xylostella. J Chem Ecol 28:587–599. https://doi.org/10.1023/A:1014500330510
Shirai Y (1993) Factors influencing flight ability of male adults of the diamondback moth, Plutella xylostella, with special reference to temperature conditions during the larval stage. Appl Entomol Zool 28:291–301. https://doi.org/10.1303/aez.28.291
Städler E, Reifenrath K (2008) Glucosinolates on the leaf surface perceived by insect herbivores: review of ambiguous results and new investigations. Phytochem Rev 8:207–225. https://doi.org/10.1007/s11101-008-9108-2
Szakiel A, Pączkowski C, Henry M (2011) Influence of environmental abiotic factors on the content of saponins in plants. Phytochem Rev 10:471–491. https://doi.org/10.1007/s11101-010-9177-x
Talekar NS, Shelton AM (1993) Biology, ecology, and management of the diamondback moth. Annu Rev Entomol 38:275–301. https://doi.org/10.1146/annurev.en.38.010193.001423
USDA (2020) Hawaii vegetable and melon crops report. NASS, kildare
Verkerk R, Tebbenhoff S, Dekker M (2010) Variation and distribution of glucosinolates in 42 cultivars of Brassica oleracea vegetable crops. Acta Hortic. https://doi.org/10.17660/ActaHortic.2010.856.7
Zhang B, Ge Y, Wei Y (1967) Comparative study on cold resistance of seedlings leaf of Dalbergia odorifera seeds from different places. Angew Chemie Int Ed 6:951–952
Acknowledgements
We would like to thank Mark Wright, Chrissy Mogren, and anonymous reviewers for helpful comments on earlier versions of this manuscript. This work was supported by USDA Specialty Crops Block Grant (SCBG-20-8) managed by the Hawaii Department of Agriculture, and USDA National Institute of Food and Agriculture (NIFA) Capacity Funds and Hatch projects (HAW09051-H to I.S. and HAW09048-H, HAW09053-R, POW-16-964 to K-H.) managed by the College of Tropical Agriculture and Human Resources, University of Hawai‘i at Mānoa.
Funding
This study was funded by Agricultural Marketing Service, SCBG-20-8, Ikkei Shikano, National Institute of Food and Agriculture, HAW09051-H, Ikkei Shikano, HAW09053-R, Koon-Hui Wang,POW-16-964, Koon-Hui Wang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare none.
Additional information
Handling Editor: Francisco Rubén Badenes-Pérez.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Los Reyes, M.P., Wang, KH. & Shikano, I. Age-dependent efficacy of putative dead-end trap crops Barbarea verna and Lepidium sativum on diamondback moth, Plutella xylostella. Arthropod-Plant Interactions (2024). https://doi.org/10.1007/s11829-024-10097-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11829-024-10097-y