Abstract
Most seed-feeding wasps are considered univoltine in regions that exhibit distinct seasonality, such as temperate regions, because they usually oviposit into seeds before the protective tissues surrounding the seeds harden during seed development. However, one species belonging to the genus Macrodasyceras was reported to be partially bivoltine, although in a temperate region, this could lead to more intense seed utilization than that seen in univoltine species. Such life cycle of seed-feeding wasps would be related to the extent of predispersal seed damage in their host plant species. In this study, we investigated the life cycle of the seed-feeding wasp Macrodasyceras japonicum and its seasonal seed utilization patterns of several fleshy fruited Ilex species in a warm-temperate region of Japan. The observation of periodically sampled fruits and oviposition behavior using reared adults showed that M. japonicum produced at least two generations per year within the seeds of multiple Ilex species and overwintered as adults outside of the fruits. The extent of seed damage caused by the M. japonicum association amounted to ca. 80% in I. pedunculosa, whose seed development phenology was most synchronized with the life cycle of M. japonicum among the host Ilex species. Adults overwintered outside of fruits, likely to avoid being eaten by birds, but risked a large reduction in population size during the winter. However, M. japonicum may overcome this disadvantage through multivoltinism and the ability to use multiple species, which might, in turn, lead to severe predispersal seed damage in the primary host plant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed plants allocate considerable resources to seeds during development because substantial amounts of nutrients are necessary for seed growth and subsequent seedling germination (Esau 1977; Linkies et al. 2010). To defend against predation pressure on seeds by a wide range of animals, various types of physical (e.g., hardness and thickness of seed-enclosing structures) and/or chemical (e.g., tannins) protection strategies have evolved in seed plants (Tiansawat et al. 2014; Wang et al. 2018). However, there are insects belonging to Hymenoptera, Coleoptera, Lepidoptera, and Diptera, which can predate the predispersal seeds of plant species confined almost exclusively to a single family or genus (c.f., Crawley 1992; Boivin et al. 2019). The life cycles of such seed-feeding insects are synchronized with the seed and fruit development of the host plant (Harman 1999; Barat et al. 2007; Hirayama et al. 2018). This is because the morphological and physiological properties of their seeds and fruits, including their physical and chemical properties, change dramatically during development (Esau 1977; Linkies et al. 2010).
Among seed-feeding insects, a number of chalcid wasps (Hymenoptera: Chalcidoidea) exploit the potential resources of immature seeds during larval development (Takagi et al. 2010; Boivin et al. 2015; Jansen-González et al. 2020). Previous studies have shown that seed-feeding chalcid wasps oviposit into seeds at an appropriate time, usually synchronizing with resource accumulation within the seeds and before the protective tissues surrounding the seeds harden during seed development in both gymnosperms (Boivin et al. 2015) and angiosperms (Jansen-González et al. 2020). Plant phenologies, such as flowering, fruiting, and seed development, tend to be synchronized in a relatively short period of time among individuals in regions with discrete seasonality, such as temperate regions (Satake et al. 2022). Therefore, most seed-feeding wasps are considered univoltine species in such regions (Boivin et al. 2019). According to Boivin et al. (2015), half of the species of the genus Megastigmus (Hymenoptera: Megastigmidae), which included more than 125 species described mainly from the Holarctic and Australasian regions (Grissell 1999), are seed feeders that are associated with both gymnosperms and angiosperms, and all of the seed feeders are considered univoltine.
Macrodasyceras (Hymenoptera: Megastigmidae), as defined by Kamijo (1962), is a genus of seed-feeding chalcid wasps associated with species of Ilex (Aquifoliaceae) distributed in East Asia (Kamijo 1981; Grissell & Desjardins 2002). Macrodasyceras hirsutum Kamijo was recorded in the seeds of Ilex integra Thunb. (Kamijo 1981) and Ilex latifolia Thunb. (Takagi et al. 2020). Macrodasyceras japonicum (Ashmead) was found in the seeds of Ilex chinensis Sims, Ilex serrata Thunb., Ilex pedunculosa Miq., and Ilex rotunda Thunb. (Matsuo and Takagi 2016), although a recent study clarified that the wasps emerging from I. rotunda differed from M. japonicum based on morphology and molecular sequencing (Macrodasyceras sp.) (Matsuo et al. in preparation). Takagi et al. (2010) reported that M. hirsutum is partially bivoltine in a warm-temperate region of Japan; M. hirsutum overwinters as larvae, emerges as adults, and deposits eggs into fertilized seeds from May to June. Some of the M. hirsutum offsprings that are oviposited from May to June emerge as adults in August to deposit their eggs into the remaining seeds. Such life cycle could lead to more intense seed utilization than that seen in univoltine species (Numata and Shintani 2023) and, in turn, negatively influence sound seed production in host plant species, contributing to plant recruitment and fitness (Crawley 1992; van Klinken 2005).
In this study, we targeted a seed-feeding chalcid wasp belonging to the Macrodasyceras genus, M. japonicum. According to Kamijo (1962), all the specimens of M. japonicum were collected in winter, suggesting that M. japonicum overwinters as adults. However, the life cycle of M. japonicum remains unclear, as does whether M. japonicum also overwinters as pupae, larvae, or eggs. We investigated the life cycles and seed utilization patterns of several Ilex species in a warm-temperate region of Japan, namely I. pedunculosa, I. chinensis, I. serrata, and Ilex macropoda Miq. First, we conducted seasonal sampling of fruits of the Ilex species to determine the phenological changes in fruit and seed structures related to M. japonicum utilization and to identify signs of insect damage within the seeds based on the appearance and genetic analysis of the organisms within the seeds. Based on these findings, we evaluated the seasonal association patterns of M. japonicum within the seeds in relation to seed and fruit development of the host plant species and analyzed the effects of M. japonicum association on seed damage in the host species. Second, we reared the adults and observed their oviposition behavior on fruits to confirm the life cycle of M. japonicum. Finally, we discuss the possible mechanisms affecting the life cycle of M. japonicum and how this life cycle is related to seed utilization patterns, that is, predispersal seed damage, in the host plant species.
Materials and methods
Sampling fruits on the tree canopy
We selected several trees of I. macropoda, I. pedunculosa, I. chinensis, and I. serrata in Kyoto, Japan (Table S1). Kyoto is located in a temperate region; it is hot and humid in summer and moderately cold in winter, during which it snows a few times each year. Based on data (collected between 1991 and 2020) from the Kyoto local meteorological observatory (35.0154°N, 135.7328°E, 41 m a.s.l.), the mean annual temperature and precipitation were 16.2 °C and 1522.9 mm, respectively. The data also indicated that mean temperature and precipitation were 26.4 °C and 577.0 mm during summer (June to August) and 5.8 °C and 176.0 mm during winter (December to February). All Ilex species are dioecious, and the ovaries of female trees develop into spherical, fleshy, red fruits. Each fruit usually contains four to six ovules, which develop into endospermic seeds covered with hard endocarps (Fig. S1). The flowering and fruiting periods are synchronous among individuals within the species, although these periods are slightly different or sometimes overlap among species (Table S1).
We sampled twigs with fruits to collect approximately 5–30 fruits from each tree at approximately 2-week intervals in 2021 for all the targeted Ilex species and in 2022 for I. macropoda and I. pedunculosa until December or until we could not collect fruits from the canopy (Tables S2 and S3). On each sampling day, we dissected 2–10 fruits and seeds under a stereomicroscope (S9D, Leica Microsystems GmbH, Wetzlar, Germany) and classified the seeds into the following categories: (I) seeds with underdeveloped endosperm, (II) undeveloped seeds, (III) undamaged and developing seeds, and (IV) seeds with signs of insect damage (see Fig. S2). In category IV, we observed the feeding traces, sizes and shapes of holes, and/or the presence of other organisms inside. The seeds in category IV were further classified according to the organism appearance and genetic analysis, as mentioned in the next section.
To investigate how the fruit and seed structures developed, we cut 1–5 flesh fruits, sampled in the abovementioned procedure, per individual horizontally in half until the fruits reached ripening (Tables S2 and S3) and photographed them under a stereomicroscope. Using ImageJ 1.5.3 (Rasband 1997–2018), we measured the seed width and the thicknesses of the pericarp and the endocarp surrounding the seeds (ovules) for all fertilized seeds based on the photographs (Fig. S1). We defined the width of the seed (ovule) surrounded by the endocarp as the seed width. The thicknesses of the pericarp of I. chinensis and the endocarps of I. macropoda and I. chinensis varied greatly within the fruit, with thick and thin layers, which may have affected ovipositor insertion by M. japonicum. We measured both the thickest and thinnest parts of the layers surrounding each of the fertilized seeds. We could not cut fruits horizontally when they became ripe. Therefore, the period during which these measurements were conducted was shorter than the sampling period for each species.
All fruits that remained after the aforementioned observations and additional sampled fruits within the study sites were kept until adult emergence within plastic Petri dishes (9 cm diameter, 2 cm height) laid with moistened wiping papers to identify the species and/or to rear to confirm the life cycle and investigate the oviposition behavior of M. japonicum, as described in the following sections.
Identification of organisms within the seeds and detailed classification of seed states
When we found organisms within the dissected seeds, we sorted them into (a) eggs, (b) small-sized larvae of wasps (< 0.6 mm), (c) medium-sized larvae of wasps (≥ 0.6 mm and < 2 mm), (d) large-sized larvae of wasps (≥ 2 mm), (e) hairy larvae of wasps, (f) parasitic larvae of wasps, (g) pupae of wasps, (h) adult wasps, (i) lepidopteran larvae, and (j) dipteran larvae (Figs. 1 and S2). In (a)–(d), (g), and (h), we usually found one organism within one seed. We also distinguished whether the organisms were living or dead. Initially, we could not discriminate the species of eggs, larvae, or pupae of wasps based only on stereomicroscopic observation. Therefore, we stored the organisms at − 30 °C in plastic tubes with 99.5% ethanol for use in the genetic analyses, as mentioned below.
Variation in the state of a living wasp within the seed. a Egg of Macrodasyceras japonicum*. b Small-sized larva of M. japonicum* (< 0.6 mm). c Medium-sized larva of M. japonicum* (≥ 0.6 mm, < 2.0 mm). d Large-sized larva of M. japonicum* (≥ 2.0 mm). e Pupa of M. japonicum*. f Female adult of M. japonicum*. g Male adult of M. japonicum*. h Larva parasitizing M. japonicum (Sycophila sp*). i Pupa of Sycophila sp*. j Adult of Sycophila sp*. k Hairy larva (Eupelmus sp*.). *Species names are based on morphological observation and genetic analyses. Scale bar: 0.5 mm in A, B, C, H, and I and 1.0 mm in the other images
To identify wasp species, we first classified the emerged adults into M. japonicum, Sycophila sp., and Eupelmus sp. based on morphological observations using the following publications: Kamijo (1962), Matsuo and Takagi (2016), Gibson (1995), and Burks (1971). Then, to determine the species of wasps within the seeds based on the appearance of the organisms and the sequence similarity of the mitochondrial gene cytochrome c oxidase subunit I (COI), we extracted total DNA from the identified adults of each species and from one to several samples in each of the immature stages (a)–(g) using a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol (Table S4). Partial sequences of COI were amplified using the primers COI_pF2 (Simon et al. 1994) and COI_2437d (Kaartinen et al. 2010). Purification and sequencing were performed as described by Nakabayashi and Ohshima (2024), and all the determined sequences were deposited in the DNA Data Bank of Japan (DDBJ; http://www.ddbj.nig.ac.jp/) under the accession numbers listed in Table S4. The sequences from the immature samples were clustered with the sequences of the identified adults of each species using the neighbor-joining (NJ) method based on the Kimura 2-parameter distance model (K2P) with MEGAX (Kumar et al. 2018).
The three identified species of adults were clearly distinguished by the inferred NJ tree, and each species formed a monophyletic group supported by 100% bootstrap values. The sequenced immature samples comprised any one of monophyletic groups, which were consistent with the differences in the appearances of the organisms (a)–(g) (Fig. S3). Therefore, we classified the seed states within fruits as follows:
-
(1)
Seeds with underdeveloped endosperm (e.g., Fig. S2a).
-
(2)
Undeveloped seeds (e.g., Fig. S2b).
-
(3)
Undamaged and developing seeds (e.g., Fig. S2c).
-
(4)
Seeds with living M. japonicum inside (e.g., Fig. S2d, e, f).
-
(5)
Seeds with dead M. japonicum inside (e.g., Fig. S2h).
-
(6)
Seeds with an exit hole made by emerged M. japonicum (e.g., Fig. S2g).
-
(7)
Seeds with Sycophila sp. or Eupelmus sp. parasitizing M. japonicum (e.g., Fig. S2i, j, k, l).
-
(8)
Seeds damaged by lepidopteran or dipteran larvae (e.g., Fig. S2m, n).
Rearing of adults and observation of their oviposition behavior
To confirm the life cycle of M. japonicum and the oviposition behavior of the overwintered females on the fruits, we reared adults that emerged from our sampled fruits via the abovementioned rearing procedure. We reared them, individually or in female–male pairs, in plastic Petri dishes containing a drop of honey and moistened wiping paper in a non-air-conditioned room, to obtain both mated and unmated adults. The temperature in the non-air-conditioned room changed similarly in the field and dropped to 4 °C during winter.
Beginning in late June 2022, when we began to find M. japonicum eggs within host plant seeds under natural conditions, five fruits of each tested Ilex species were provided to each overwintered female, whether they were mated or unmated. We set the female and the fruits attached to twigs inserted into wet floral foam within a plastic case (12 cm diameter, 8 cm height) containing a drop of honey (Table S5). We observed the oviposition behavior of the overwintered females and how the ovipositor was inserted into the fruit and seed. We selected I. pedunculosa and I. macropoda as test plants because predispersal damage to the seeds caused by M. japonicum was high in I. pedunculosa and was rarely observed in I. macropoda. We replaced the tested fruits every 3–6 days and observed the oviposition behavior until the female died.
The fruits were photographed when M. japonicum displayed oviposition behavior to determine the insertion position of the ovipositor, and the fruits were stored in FAA. The fruits were then rinsed with tap water for approximately 12 h and placed in 50% alcohol. Longitudinal sections of the fruits (60 µm thickness) were serially cut using a sliding microtome (TU-213, Yamato Kohki Industrial Co., Ltd., Saitama, Japan) and stained with 0.06% safranin solution and 0.33–0.50% Fast Green solution to reveal the insertion pattern of the ovipositor into the fruit and seed. Because the fruits and seeds were easy to cut using a sliding microtome because of their moderate hardness, they were not embedded in resin or paraffin. The stained sections were mounted on microscope slides, and images were captured using an all-in-one fluorescence microscope (BZ-X800, Keyence, Osaka, Japan) and a stereomicroscope (STZ-168, Shimadzu RIKA Co., Tokyo, Japan) fitted with a digital camera.
Winter observation of fallen fruits and bird-dispersed seeds under the canopy
Three seed traps (0.25 m2 surface area with a circular mouth) made of polyethylene cloth (mesh size, 1 mm) were set under the crown of six I. pedunculosa trees in the same study site where we sampled I. pedunculosa fruits on the canopy. The contents of the traps were collected twice a month from May 2021 to April 2023. To assess whether some fallen fruits or bird-dispersed seeds contained living eggs, larvae, pupae, or adults of M. japonicum during the winter, we targeted fallen mature fruits and bird-dispersed seeds, irrespective of whether there were exit holes made by wasps or no signs of exit holes from the seed traps from November to April. We dissected all of the fruits that had no signs of exit holes and the bird-dispersed seeds for observation under a stereomicroscope.
Statistical analysis
All wasps were associated with fertile seeds; no wasps at any life stage were found within the undeveloped seeds. In addition, wasps other than M. japonicum, i.e., Sycophila sp. and Eupelmus sp., were identified as parasitoids of M. japonicum based on morphological observations and genetic analyses. Therefore, the ratio of fertilized seeds that were damaged by wasps, either M. japonicum or the parasitoids, was considered to be equal to the extent of seed damage caused by M. japonicum association.
To evaluate the effects of M. japonicum association on the seed damage of the host species, we constructed a generalized linear-mixed model (GLMM) with a logit link and binomial distribution. The response variable was the logit of the probability p of seeds damaged by M. japonicum association within all the fertilized seeds in the fruit (i.e., log(p/(1-p)); the explanatory variables were (1) the host species, (2) the seasonality (in August, in September, and after October), and (3) their interactions. The individual trees were treated as the random effect. We could not include the data before August, when there were no fertilized seeds within I. chinensis fruits. In addition, data for I. macropoda were excluded because there were no wasps within these seeds in 2021 and very few in 2022. After we constructed the GLMM (Table S6), the likelihood ratio test was also conducted to identify the significant contribution of the explanatory variables to the response variable. In the likelihood ratio test, the interaction term (3) was statistically significant (Table S7). Therefore, we carried out Tukey’s multiple comparison tests following the GLMM among the host species and among the seasons, separately. The GLMM was fitted using the ‘lme4’ package of R 4.2 (R Core Team 2022). Likelihood ratio tests were carried out using the ‘Anova’ function in the ‘car’ package of R 4.2 (R Core Team 2022). Multiple comparison tests were carried out using the ‘multcomp’ package of R 4.2 (R Core Team 2022).
Results
Seasonal changes in fruit and seed structures
The pericarp thickness tended to increase gradually until the fruits ripened for all the studied species (Figs. 2 and S4). In contrast, the seed width reached a plateau in late June for I. macropoda, early July for I. pedunculosa, mid-July for I. serrata, and early August for I. chinensis, almost corresponding to a time when the endosperm began to develop within the seed (Figs. 2 and 3). The seed width after reaching this plateau was particularly high in I. pedunculosa at approximately 1.9 mm (Table 1), in 2021 data, compared to those in the other studied species, at approximately 1.1–1.3 mm. Endocarp thickness reached a plateau in late July for I. macropoda, I. pedunculosa, and I. serrata and in late August for I. chinensis. The endocarp of I. macropoda became thick, both in thick and thin layers, reaching approximately 0.38 mm and 0.23 mm, respectively, in 2021 data, compared with those of the other studied species, at approximately 0.05–0.18 mm.
Seasonal changes in seed width, pericarp thickness, and endocarp thickness for Ilex spp. in 2021. The means and standard errors for each sampling date are shown. A later sampling date was indicated if the sampling was conducted for 2–4 days. For the thicknesses of the pericarp of I. chinensis and of the endocarp of I. macropoda and I. chinensis, values for both thin (filled symbols) and thick (open symbols) layers are indicated
Mean number of seeds belonging to each category within one fruit for each sampling date for each of the Ilex species. For I. macropoda and I. pedunculosa, the results are shown for both 2021 and 2022. A later sampling date was indicated if the sampling was conducted for 2–4 days. Gray shading indicates that almost all the fruits were ripe on the given dates
Seasonal association pattern of M. japonicum within the seeds
M. japonicum began to associate within the seeds in late June in I. pedunculosa, in mid-July in I. serrata, and in early August in I. chinensis, almost corresponding to a time when the seed width reached a plateau and the endosperm began to develop within the seed (Fig. 3). For I. macropoda, whose endocarp became thicker compared to those of other Ilex species, very few M. japonicum associations were found between late June and late July in 2022.
Eggs within the seeds were found from late June to early July and early August in I. pedunculosa and in late July in I. serrata (Fig. 4). Similarly, small-sized larvae were mainly found from late June to early July and early August in I. pedunculosa, from late July to early August in I. serrata, and in early August in I. chinensis. The percentage of M. japonicum associations increased, especially in August, for these Ilex species (Fig. 3). After that, larvae and pupae were continuously found until October, and adults within the seeds were mainly found in October for these Ilex species (Fig. 4). Seeds with Sycophila sp. or Eupelmus sp. paratisizing M. japonicum were found approximately beginning in September and increased in October for these species (Fig. 3).
Composition of the life stages of living Macrodasyceras japonicum within seeds for each sampling date for each Ilex species. For I. macropoda and I. pedunculosa, the results are shown for both 2021 and 2022. A later sampling date was indicated if the sampling was conducted for 2–4 days. The gray shading indicates that almost all the fruits were ripe on the given dates. The numbers in parentheses indicate the number of observed living M. japonicum within the seeds
A number of M. japonicum adults were also found within the seeds of I. pedunculosa in late July. In I. pedunculosa, seeds with an exit hole made by emerged M. japonicum adults were found from late July, increasing especially in October, although such seeds in I. chinensis and I. serrata were found mainly from October (Fig. 3). After November, almost no living M. japonicum, including eggs, larvae, pupae, or adults, were found within the seeds on the canopy fruits, although dead M. japonicum larvae, pupae, or adults were found (Fig. 3).
During the winters of 2021 and 2022, we collected a total of 239 fallen mature fruits that had no signs of exit holes made by the wasps under the canopy of I. pedunculosa (Table S8). Of the 239 fruits, 116 had seeds damaged by the wasps. However, there were no living M. japonicum organisms, including no eggs, larvae, pupae, or adults, inside. In addition, no living M. japonicum was found within the bird-dispersed seeds, although we dissected a total of 482 bird-dispersed seeds and found a total of 355 bird-dispersed seeds damaged by the wasps during the 2-year period.
Rearing of M. japonicum adults and their oviposition behavior
The adult males that emerged mainly in autumn died by March under rearing conditions in a non-air-conditioned room (Table S9). In contrast, 23 out of 70 females that emerged in autumn, both mated and unmated, survived until late June, when eggs and small-sized larvae began to be found within the seeds of I. pedunculosa at the study site. A number of adults also emerged from late July to early August from immature I. pedunculosa fruits sampled on 25 July in 2022 (Table S10), which were the offspring females of the overwintered females (i.e., first-generation females). They survived until at most early September.
The overwintered adult female M. japonicum individuals reared from both I. pedunculosa and I. chinensis displayed oviposition behavior on the immature fruits of both I. pedunculosa and I. macropoda until mid-July and then died (Table S5). Based on the observations of the longitudinal sections of the fruits on which M. japonicum displayed oviposition behavior, ovipositor scars were randomly distributed within the fruits (Fig. 5). The scars penetrated the endocarp of I. pedunculosa but not the endocarp of I. macropoda (Fig. 5).
The oviposition behavior of Macrodasyceras japonicum (a) on the fruit of Ilex pedunculosa and (d) on the fruit of I. macropoda. Longitudinal sections of fruits on which M. japonicum displayed oviposition behavior for I. pedunculosa (b, c), and for I. macropoda (e, f). Arrows indicate the ovipositor scars within the fruits. Curly brackets indicate the endocarps within the fruits. All scale bars = 1 mm
Extent of seed damage caused by M. japonicum
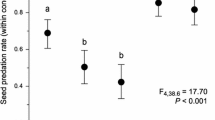
The ratio of seed damage caused by M. japonicum associations tended to be higher in I. pedunculosa than in I. chinensis or I. serrata, although a statistically significant difference was observed only between I. pedunculosa and I. serrata (Fig. 6 and Table 2). For I. pedunculosa, the estimated ratio of seed damage caused by M. japonicum was approximately 0.8 in all seasons in 2021, and the mean rate was also approximately 0.8 in all seasons in 2022.
There was no significant difference among the seasons, i.e., in August, in September, and after October, in the ratio of seed damage caused by M. japonicum association (Table 3). However, this indicated that the actual number of seeds damaged by M. japonicum association would increase over time because immature fruits with exit holes made by the wasps gradually dropped (M. Nishimon, unpublished data).
Discussion
Life cycle of Macrodasyceras japonicum
Adult emergence of M. japonicum occurred mainly from October to November from sampled fruits of I. pedunculosa, I. chinensis, and I. serrata. Under rearing conditions, one-third of the adult females that emerged in autumn lived until late June, when we began to find eggs within the seeds under natural conditions. These overwintered females showed oviposition behavior on the immature fruits of Ilex species until the middle of July and all then died by mid-July.
Under natural conditions, a number of adults were also found in late July within the seeds of the sampled fruits of I. pedunculosa. In addition, a number of adults emerged from the sampled fruits of I. pedunculosa from late July to early August. Larval development of wasps may depend on environmental temperature; Nguyen et al. (2022) indicated that the postdiapause period of larvae until adult emergence was well predicted by the accumulated temperature for apricot seed wasp, Eurytoma maslovskii (Hymenoptera: Eurytomidae). Accordingly, newly hatched larvae from eggs oviposited by the overwintered females of M. japonicum could develop into adults within approximately one month of the season when the environmental temperature is high. Under natural conditions, we began to find seeds and fruits with exit holes made by emerged M. japonicum adults from late July, mainly in I. pedunculosa, and found a number of eggs and small-sized larvae from late July to early August within the seeds of the three host plant species. This indicated that the offspring females of the overwintered females (i.e., first-generation females) oviposited on multiple host species from late July to early August. After that, the first-generation adults that emerged from late July to early August died by early September under rearing conditions, and we continuously found living M. japonicum (larvae and pupae) within the seeds of the sampled fruits of three host species until October. These results implied that M. japonicum produces at least two generations per year within the host plant species at the study site.
Most seed-feeding wasps have been reported to be univoltine in regions that exhibit distinct seasonality, such as temperate regions (Boivin et al. 2019). This could be because host plant phenologies, including seed development, tend to be synchronized in a relatively short period of time among individuals during the year there (Satake et al. 2022). Boivin et al. (2015) indicated that Megastigmus schimitscheki (Hymenoptera: Megastigmidae) and Megstigmus pinsapinis (Hymenoptera: Megastigmidae), which use seeds of Cedrus atlantica (Pinaceae), oviposited into seeds in young cones during only a short period within a year because the ability of ovipositor penetration decreased as cone scales of the host species harden and increased in size.
In contrast, Takagi et al. (2010) showed that M. hirsutum is partially bivoltine, although in a warm-temperate region. This implies that the female ovipositors of M. hirsutum would be adapted to penetrate the developed endocarps of the host plant species I. integra because their adults in the first-generation derived from the overwintered generation oviposited into seeds surrounded by the thick and hard developed endocarps in summer. The ovipositors of M. japonicum, similar to those of M. hirsutum, can penetrate a wide range of developing endocarps and pericarps in I. pedunculosa, I. chinensis, and I. serrata, which contributes to the production of multiple generations within the host plants. The oviposition experiment revealed that, however, the endocarps of I. macropoda were too thick for M. japonicum to insert their ovipositors, which resulted in rare M. japonicum associations within I. macropoda seeds.
Takagi et al. (2012) indicated that M. hirsutum overwintered as larvae within the seeds of the host plant fruits, which remained greenish in color. The previous study suggested that this color manipulation helps M. hirsutum avoid being killed by birds because frugivorous birds usually prefer red to green fruits (Takagi et al. 2012). The fruits of I. pedunculosa, I. chinensis, and I. serrata all turned red upon ripening, irrespective of the M. japonicum association within their seeds. After November, when the fruits of all the host plant species had ripened, almost no living M. japonicum organisms (eggs/larvae/pupae/adults) were found within the seeds in the sampled fruits on the canopy. We also observed no living M. japonicum in either the fallen fruits or the seeds dispersed by birds under the canopy of I. pedunculosa during the winter. During the winter, a living adult female was found on the underside of a leaf of I. pedunculosa, which is an evergreen tree (M. Nishimon, personal observation), and another one was found among the fallen leaves collected on December 21, 2023, from a litterfall trap set under the canopy of Ilex trees (K. Hirayama, personal observation). In addition, all the specimens of M. japonicum in Kamijo (1962) were collected in winter. Hirayama et al. (2018) clarified the life cycle of Argyresthia assimilis Moriuti (Lepidoptera: Yponomeutidae), which is assumed to be a specialist seed predator of Photinia glabra (Thunb.) Maxim (Rosaceae) and reported that final instar larvae leave their predated fruits to pupate before the fruits ripen, which contributes to the decreased mortality of A. assimilis, because ripe fruits are likely to be eaten by birds. Like A. assimilis, M. japonicum may overwinter as adults outside the fruits to avoid being eaten by frugivorous birds.
Predispersal seed damage caused by M. japonicum association in the host plant species
Previous histological studies have shown that the oviposition timing of predispersal seed-feeding wasps is synchronized with the onset of resource accumulation in seeds (Boivin et al. 2015; Jansen-González et al. 2020). For the observed Ilex species, the endosperm, which serves as an additional source of nutrients for the growing embryo, began to develop close to the time when the seed width reached a plateau. The seed width of I. pedunculosa was the largest among the host plant species and the first to reach a plateau in early July; the seed width of I. serrata reached a plateau in mid-July and that of I. chinensis reached a plateau in early August. We found M. japonicum associations within the seeds at almost the same time, beginning in late June in I. pedunculosa, in mid-July in I. serrata, and in early August in I. chinensis. These results indicated that M. japonicum started oviposition almost in accordance with the timing of nutrient formation within host plant tissues, as indicated in previous studies.
Takagi et al. (2010) indicated that M. hirsutum selectively oviposits on fertilized seeds of I. integra. In addition, McClure et al. (1996) showed that seed- and cone-eating insects lay one egg per cone and suggested that their females probably recognize and avoid previously occupied oviposition sites when cones are abundant. We usually found one M. japonicum within the fertilized seeds of all the host species. Accordingly, female adults of M. japonicum selectively oviposit eggs individually on undamaged fertilized seeds of the host plant species for at least two generations per year until autumn. Although the ratio of seed damage caused by M. japonicum association did not differ significantly among the seasons from August, the number of seeds damaged by M. japonicum association would increase over time, considering that immature fruits with exit holes made by the wasps gradually dropped (M. Nishimon, unpublished data). Focusing on the differences in host species, the extent of seed damage caused by M. japonicum association tended to be greater in I. pedunculosa than in I. chinensis and I. serrata, accounting for approximately 80% of the fertilized seeds of I. pedunculosa in both 2021 and 2022. Because the seed development of I. pedunculosa proceeded earlier than that of the other host species, overwintered female adults of M. japonicum tended to oviposit eggs first within the seeds of I. pedunculosa. For I. pedunculosa, repeated M. japonicum utilization, which is well synchronized with the phenology of I. pedunculosa seed development, leads to a large extent of seed damage caused by M. japonicum.
The synchronization between the seed development phenology of I. pedunculosa and the life cycle of M. japonicum indicated that M. japonicum primarily uses I. pedunculosa, which is widely and abundantly distributed in temperate regions of Japan (Horikawa 1972). In addition, M. japonicum also supplementally used I. chinensis and I. serrata, which showed somewhat delayed phenologies of seed development compared with I. pedunculosa. Previous studies have suggested that seed-feeding insects tend to specialize in a particular host plant species because their life cycle must be well synchronized with the target stage of the host plant during oviposition and larval development within the seeds (Boivin et al. 2019). Accordingly, some seed-feeding insects have a prolonged diapause of more than one year in fluctuating environments, such as the annual variability of seed production in the host plant species (Maeto and Ozaki 2003; Suez et al. 2013; Trandem et al. 2023). However, if seed-feeding insects can use multiple host species, they can survive through a year with low seed production of one host plant species without a prolonged diapause and increase their density in a population (Ims 1990). Although I. chinensis and I. serrata are scatteredly distributed in temperate regions of Japan (Katsuta 1998; Iokawa 2017), M. japonicum could increase its population size more using the multiple Ilex species until autumn than using only one species. Perhaps to avoid being eaten by frugivorous birds, M. japonicum overwinters as adults outside of the fruits, which could lead to a large reduction in population size during the winter. However, M. japonicum may overcome this disadvantage through multivoltinism and the ability to use multiple species, which might, in turn, lead to severe predispersal seed damage in primary host plant species. It is suggested that the life cycle of seed-feeding insects within fleshy fruits may be influenced by fruit–bird interactions, which profoundly affect predispersal seed predation in host plants.
Data availability
The data supporting the conclusion are included in this article. The datasets and analysis protocols used during the study are available from the corresponding author on request.
References
Barat M, Tarayre M, Atlan A (2007) Plant phenology and seed predation: interactions between gorses and weevils in Brittany (France). Entomol Exp App 124:167–176. https://doi.org/10.1111/j.1570-7458.2007.00565.x
Boivin T, Gidoin C, von Aderkas P, Safrana J, Candau J-N, Chalon A, Sando M, El Maâtaoui M (2015) Host-parasite interactions from the inside: plant reproductive ontogeny drives specialization in parasitic insects. PLoS ONE 10:e0139634. https://doi.org/10.1371/journal.pone.0139634
Boivin T, Doublet V, Candau JN (2019) The ecology of predispersal insect herbivory on tree reproductive structures in natural forest ecosystems. Insect Sci 26:182–198. https://doi.org/10.1111/1744-7917.12549
Burks BD (1971) A synopsis of the genera of the family Eurytomidae (Hymenoptera: Chalcidoidea). T Am Entomol Soc 97:1–89
Crawley MJ (1992) Seed predators and plant population dynamics. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities. CAB International, Wallingford, pp 157–191
Esau K (1977) Anatomy of Seed Plants, 2nd edn. John Wiley & Sons, New York
Gibson GAP (1995) Parasitic wasps of the subfamily Eupelminae: classification and revision of world genera (Hymenoptera: Chalcidoidea: Eupelmidae). Mem Entomol Int 5:1–421
Grissell EE (1999) An annotated catalog of world Megastigminae (Hymenoptera: Chalcidoidea: Torymidae). Contrib Am Entomol Inst 31:1–92
Grissell EE, Desjardins CA (2002) A revision of Bootania Dalla Torre and recognition of Macrodasyceras Kamijo (Hymenoptera: Torymidae). J Hymenopt Res 11:279–311
Harman HM (1999) The effect of variability in the phenology of the reproductive stages of Scotch broom (Cytisus scoparius) on the synchronization of the life stages of broom seed beetle (Bruchidius villosus) in New Zealand. Biol Control 15:228–234. https://doi.org/10.1006/bcon.1999.0715
Hirayama K, Sasaki M, Ohshima I (2018) First host record of Argyresthia assimilis Moriuti, 1977 (Lepidoptera: Yponomeutidae) and a description of its annual life history. Lepidoptera Sci 69:99–106. https://doi.org/10.18984/lepid.69.3-4_99
Horikawa Y (1972) Atlas of the Japanese Flora I. Gakken Co., Ltd., Tokyo (In Japanese)
Ims RA (1990) The ecology and evolution of reproductive synchrony. Trends Ecol Evol 5:135–140. https://doi.org/10.1016/0169-5347(90)90218-3
Iokawa Y (2017) Aquifoliaceae. In: Oba H, Kadota Y, Murata J, Yonekura K, Kihara H (eds) Wild flowers of Japan, vol 5. Heibonsha, Tokyo, pp 180–185 (In Japanese)
Jansen-González S, Teixeira SP, Pereira RAS (2020) Larval strategy of two species of seed-feeding Chalcidoidea parallels that of Parasitoid Koinobionts. Oecolog Australis 24:903–916. https://doi.org/10.4257/oeco.2020.2404.13
Kaartinen R, Stone GN, Hearn J, Lohse K, Roslin T (2010) Revealing secret liaisons: DNA barcoding changes our understanding of food webs. Ecol Entomol 35:623–638. https://doi.org/10.1111/j.1365-2311.2010.01224.x
Kamijo K (1962) A revision of the species the Megastigminae occurring in Japan. Insecta Matsumurana 25:18–40
Kamijo K (1981) Description of the male and other notes on Macrodasyceras hirsutum (Hymenoptera: Torymidae). Akitsu New Series 38:1–4
Katsuta M (1998) Ilex Linn. In: Katsuta M, Mori T, Yokoyama T (eds) Seeds of Woody Plants in Japan. Japan Forest Tree Breeding Association, Tokyo, pp 228–238 (In Japanese)
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Linkies A, Graeber K, Knight C, Leubner-Metzger G (2010) The evolution of seeds. New Phytol 186:817–831. https://doi.org/10.1111/j.1469-8137.2010.03249.x
Maeto K, Ozaki K (2003) Prolonged diapause of specialist seed-feeders makes predator satiation unstable in masting of Quercus crispula. Oecologia 137:392–398. https://doi.org/10.1007/s00442-003-1381-6
Matsuo K, Takagi E (2016) New host records of Macrodasyceras japonicum (Ashmead, 1904) (Hymenoptera, Torymidae), with notes of its morphology. Jpn J Syst Entomol 22:175–178
McClure M, Quiring DT, Turgeon JJ (1996) Oviposition, temporal distribution and potential impact of Strobilomyia laricis Michelsen and S. viaria (Huckett) (Diptera: Anthomyiidae) on eastern larch, Larix laricina (Du Roi) K. Koch Can Entomol 128:67–78
Nakabayashi Y, Ohshima I (2024) Geographic variation in parasitoid communities and the cause of enemy-free space in a range-expanding myrmecophilous lycaenid butterfly. Biol J Linn Soc. https://doi.org/10.1093/biolinneanblad060. (in Press)
Nguyen HN, Lee IJ, Kim HJ, Hong KJ (2022) Temperature-dependent development of the post-diapause periods of the apricot seed wasp Eurytoma maslovskii (Hymenoptera: Eurytomidae): an implication for spring emergence prediction models. Insects 13:722. https://doi.org/10.3390/insects13080722
Numata H, Shintani Y (2023) Diapause in univoltine and semivoltine life cycles. Annu Rev Entomol 68:257–276. https://doi.org/10.1146/annurev-ento-120220-101047
R Core Team (2022) R: A language and environment for statistical computing. R Foundation Statistical Computing. Vienna, Austria. https://www.R-project.org.
Rasband WS (1997–2018) Image J, U.S. National Institute of Health, Bethesda, Maryland, USA. http://imagej.nih.gov/ij.
Satake A, Nagahama A, Sasaki E (2022) A cross-scale approach to unravel the molecular basis of plant phenology in temperate and tropical climates. New Phytol 233:2340–2353. https://doi.org/10.1111/nph.17897
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87:651–701. https://doi.org/10.1093/aesa/87.6.651
Suez M, Gidoin C, Lefèvre F, Candau J-N, Chalon A, Boivin T (2013) Temporal population genetic of time travelling insects: a long term study in a seed-specialized wasp. PLoS ONE 8:e70818. https://doi.org/10.1371/journal.pone.0070818
Takagi E, Iguchi K, Suzuki M, Togashi K (2010) Selective oviposition in fertilized seed of Ilex integra by the wasp Macrodasyceras hirsutum (Hymenoptera: Torymidae). Eur J Entomol 107:197–202. https://doi.org/10.14411/eje.2010.026
Takagi E, Iguchi K, Suzuki M, Togashi K (2012) A seed parasitoid wasp prevents berries from changing their colour, reducing their attractiveness to frugivorous birds. Ecol Entomol 37:99–107. https://doi.org/10.1111/j.1365-2311.2011.01340.x
Takagi E, Matsuo K, Suzuki M, Adachi Y, Togashi K (2020) Natural occurrence of oviposition and adult emergence of the seed parasitoid wasp Macrodasyceras hirsutum Kamijo (Hymenoptera, Torymidae) on Ilex latifolia Thunberg in Japan. Taiwania 65:541–543. https://doi.org/10.6165/tai.2020.65.541
Tiansawat P, Davis AS, Berhow MA, Zalamea P-C, Dalling JW (2014) Investment in seed physical defence is associated with species’ light requirement for regeneration and seed persistence: evidence from Macaranga species in Borneo. Plos One 9:e99691. https://doi.org/10.1371/journal.pone.0099691
Trandem N, Westrum K, Hofsvang T, Kobro S (2023) Distribution and prolonged diapause of rowan seed predators Argyresthia conjugella (Lepidoptera: Yponomeutidae) and Megastigmus brevivaudis (Hymenoptera: Torymidae) and their parasitoids in Norway. Forests 14:847. https://doi.org/10.3390/f14040847
van Klinken (2005) Total annual seed loss on a perennial legume through predation by insects: the importance of within-season seed and seed feeder dynamics. Austral Ecol 30:414–425. https://doi.org/10.1111/j.1442-9993.2005.01483.x
Wang B, Phillips JS, Tomlinson KW (2018) Tradeoff between physical and chemical defense in plant seeds is mediated by seed mass. Oikos 127:440–447. https://doi.org/10.1111/oik.04867
Acknowledgements
We thank the staff of the Kamigamo Experimental Station of Kyoto University, Kyoto Botanical Gardens, and Midori-Seisaku-Suishin Office of Kyoto City for their permission to use the trees for our study. We thank Dr. Issei Ohshima for the guidance and support on the genetic analyses and for the discussion about the results. We also thank Dr. Takefumi Ikeda for the guidance on the observation of anatomic structure of fruits and seeds, and Dr. Akihiro Sumida for the help on the statistical analyses. Preliminary work conducted by Ryoma Kawamura help us for the observation of the life history of Macrodasyceras japonicum. This study was supported by the Asahi Glass Foundation, the Research Assistant System provided by the Committee for the Promotion of Gender Equality in Kyoto Prefectural University, and the Japan Society for the Promotion of Science (JSPS), grant numbers 19H00942 and 21K05600.
Funding
Funding was provided by Asahi Glass Foundation and Japan Society for the Promotion of Science (Grant Nos. 19H00942, 21K05600)
Author information
Authors and Affiliations
Contributions
KH conceived the ideas and designed methodology. MN collected the data from fruits in the field and conducted oviposition experiments using rearing adults. MH and KM identified the rearing adults. KH conducted genetic analysis, analyzed the data, and wrote the manuscript. All authors contributed the draft and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical approval
Ethics approval was not required.
Additional information
Handling Editors: Miriama Malcicka and Heikki Hokkanen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nishimon, M., Hisano, M., Matsuo, K. et al. Seasonal patterns of host utilization in the multivoltine seed-feeding wasp, Macrodasyceras japonicum (Hymenoptera: Megastigmidae). Arthropod-Plant Interactions (2024). https://doi.org/10.1007/s11829-024-10083-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11829-024-10083-4