Abstract
Potato apical leaf curl disease is an emerging geminiviral disease in tropics and subtropics. It was reported for the first time in the year 1999 in northern plains of India but quickly spread to almost all potato growing regions of the country largely due to prevalence of warmer weather during early crop growth, thereby favoring whitefly vector. The problem of apical leaf curl disease in India became more severe due to lack of seed indexing for this virus in conventional seed production scheme. Although it accounts for major yield loss, there is no conventional source of resistance available in potato against Tomato Leaf Curl New Delhi Virus-Potato (ToLCNDV-Potato) that causes this disease in potato. In the present study, we have investigated the potential use of RNAi for obtaining resistance against this DNA virus in potato. The replication-associated protein gene (AC1) of the virus was used to obtain pathogen-derived resistance. The AC1 gene was PCR amplified from field-infected potato leaves, cloned and sequenced (JN393309). It showed 93% sequence similarity with the AC1 gene of Tomato Leaf Curl Virus-New Delhi (TOLCV-NDe; DQ169056) virus. Transgenic plants encoding the AC1 gene in three different orientations, viz. sense, antisense and hairpin loop, were raised. Transgenic lines when challenge inoculated with ToLCNDV-Potato showed different levels of resistance for all three constructs. Transgene integration and copy number in selected transgenic lines were determined by qPCR and further confirmed by Southern blot analysis. Though a reduction in viral titer was observed in transgenic lines encoding either antisense or hairpin loop constructs of AC1 gene, the latter transgenics showed most significant results as shown by reduction in the level of symptom expression in glasshouse screening as well as real-time data of in vivo virus concentration. In fact, we obtained a few totally asymptomatic transgenic lines with hairpin loop strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, geminiviruses have emerged as serious threats to many crops including potato in the tropics and subtropics. Tomato Leaf Curl New Delhi Virus-Potato (ToLCNDV-Potato) is one such example that has been reported for the first time from northern states of India in the year 1999. Affected plants become severely stunted with apical leaf curl, crinkled leaves, and conspicuous mosaic. Garg et al. (2001) detected a begomovirus associated with this disease by immune-electron microscopy (IEM) using polyclonal antiserum of Indian Cassava Mosaic Virus (ICMV). Comparison of the complete nucleotide sequence of DNA-A revealed that it has 93% identity with that of ToLCNDV isolates but < 75% identity with other Tomato Leaf Curl Virus isolates and Potato Yellow Mosaic Virus. ToLCNDV-Potato belongs to the genus Begomovirus in the family Geminiviridae and possesses circular single-stranded DNA genome packed in geminate particles. This whitefly-transmitted begomovirus has emerged as a devastating pathogen of potato in the last decade. Though potato genotypes with multiple disease resistance have been identified in India using marker-assisted selection (Sharma et al. 2014), no specific source of resistance in potato has yet been reported for ToLCNDV-Potato. RNA interference (RNAi), which is an evolutionarily conserved surveillance system of living organisms (Cogoni and Macino 2000), can be effectively used for conferring resistance to this emerging virus problem of potato. RNAi occurs in most of the eukaryotes as a potent gene regulation phenomenon. In plants, RNAi is used as a generally applicable antiviral strategy. RNAi induced by double-stranded RNA molecules such as short hairpins, short interfering RNAs and long-distance dsRNA has been developed as standard tools in gene function studies and as antiviral strategies.
Unlike RNA viruses, the genomes of single-stranded plant DNA viruses do not encode polymerases. Instead, their replication requires interaction between a viral replication-associated protein (Rep) and host polymerases. Geminiviral Rep protein genes have been widely exploited to confer virus resistance in plants. The rep gene of African Cassava Mosaic Virus (ACMV) inhibited virus replication in cassava protoplasts and induced virus resistance in plants (Hong and Stanley 1995). A construct, having a deletion at the C-terminus, and encoding only the first 129 amino acids of the replicase protein, induced resistance in a strictly sequence-specific manner to the closely related Tomato Yellow Leaf Curl Virus (TYLCV) in tomato (Antignus et al. 2004). Small, non-coding (nc) RNAs comprising small interfering (si) RNAs and micro (mi) RNAs are also gaining wide attention for their role in the regulation of gene expression. The discovery that double-stranded (ds) RNA is the initiator of this regulatory phenomenon (Fire et al. 1998) with small ncRNAs of 21 nt functioning as ultimate effector molecules (Elbashir et al. 2001) laid the foundation for the manipulation of ncRNAs to selectively down regulate any coding region of interest. Their application in the area of development of virus-resistant plant genotypes has shown promising outcomes.
In the present study, we have utilized RNAi-mediated resistance against geminivirus exploiting AC1 (Replicase-associated protein) gene for conferring resistance to ToLCNDV-Potato infection in potato. Kufri Badshah and Kufri Pukhraj which are popular high-yielding potato cultivars in the region affected by ToLCNDV-Potato were transformed with constructs carrying the AC1 gene in three different orientations, viz., sense, antisense and hairpin loop. The hairpin loop transgenic lines showed better resistance to the virus than sense and antisense transgenic lines. Some of the transgenic potato lines showed extreme resistance to ToLCNDV-Potato. The results imply that RNAi targeting AC1 gene provides a promising approach for conferring resistance in potato to the whitefly-transmitted geminivirus ToLCNDV-Potato.
Materials and methods
Cloning and sequencing of AC1 gene from infected plants
Replication-associated protein gene (AC1) of the ToLCNDV-Potato was PCR amplified using primer pair PALCVSF and PALCVSR (Table 1) from virus-infected plants maintained in the virology facility of ICAR-Central Potato Research Institute (ICAR-CPRI), Shimla. The amplified fragment was cloned in pDrive PCR cloning vector (M/S Qiagen). The cloned fragment was sequenced from both the sides of the multicloning site (MCS) of the cloning vector using BigDye™ Terminator Cycle sequencing kit (M/S Applied Biosystems, USA) following the manufacturer’s instruction. Analysis of the sequence data was performed using Sequencing Analysis version 3.4 and Sequence Navigator software of M/S Applied Biosystems. Sequence database searches were carried out using the BLAST (Basic Local Alignment Search Tool; Altschul et al. 1997).
RNAi constructs
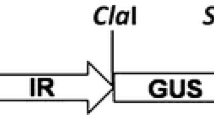
Primers were designed from the AC1 gene sequence thus obtained using Primer3 software for cloning full length 1086-bp AC1 gene in sense orientation, antisense orientation and for the fragments to be used for construction of the hairpin loop transgene. Primer pair PALCVAF and PALCVAR including the sites for SalI and KpnI, respectively (Table 1) was used to amplify a 1086 bp of AC1 gene in antisense orientation. Primer pair PALCVHP1F and PALCVHP1R including the sites for KpnI and SmaI, respectively (Table 1), was used to amplify a 704-bp sense fragment from the AC1 gene for hairpin loop construct. Primer pair PALCVHP2F and PALCVHP2R including the sites for SalI and SmaI, respectively (Table 1), was used to amplify a 470-bp antisense fragment from the AC1 gene for hairpin loop construct. The polymerase chain reaction was performed in a reaction volume of 20 µl containing 1× Taq DNA polymerase buffer with 1.5 mM MgCl2, 200 µM dNTPs, 10 pmole of each primer, 100 ng DNA template and 1 unit Taq DNA polymerase (Bangalore Genei; Table 1). Amplification was performed in an Applied Biosystem Gene AmpR PCR system. Amplified product was separated by gel electrophoresis in 0.8% (w/v) agarose gel with 0.5 µg/ml ethidium bromide. These fragments were cloned using pDrive (Qiagen) PCR cloning vector. Further, they were excised from pDrive using respective restriction enzymes and subcloned in Binary vector pBinAR to generate plasmid pBinGTLC1 for antisense, pBinGTLC2 for hairpin and pBinGTLC3 for sense (Fig. 1). Sequencing of the transgene insertion sites in the above three transformation vectors was done following the already described procedure to confirm their integrity.
Plant transformation and analysis
The binary vectors pBinGTLC(1–3) were mobilized into the Agrobacterium strain EHA105 by freeze–thaw method (Höfgen and Wilimitzer 1988). Transformation of the potato cultivars Kufri Badshah and Kufri Pukhraj was done by co-cultivation of internodal stem cuttings of potato microplants with freshly grown Agrobacterium suspension. Internodal cuttings of Kufri Pukhraj and Kufri Badshah microplants were used as explants for transformation (Naik et al. 1997; Chakrabarti et al. 2000). The regenerated shoots were maintained in the selection medium having antibiotic carbenicillin (200 mg/l) and kanamycin (50 mg/l) for few weeks and then multiplied on MS propagation medium (Chakrabarti et al. 2000). Transgenicity of the regenerated microplants was confirmed by PCR amplification of the nptII selectable marker gene using primers NPTIIF and NPTIIR (Table 1).
Screening of transgenic lines by challenge inoculation
Forty nine transgenic lines of Kufri Badshah and 33 lines of Kufri Pukhraj having normal phenotype and good vigour were planted in earthen pots inside a glasshouse in replication of three for each line. Two plants of each transgenic line were inoculated with virus via grafting with ToLCNDV-Potato infected leaf from plant of same age (Fig. 2B) and the third plant was kept as control. Tubers obtained from plants which were asymptomatic for the infection or showed significant reduction in the degree of infection were planted next season and checked similarly for second year evaluation. The plants were maintained at 30 °C with 14/10-h photoperiod for buildup of virus concentration and symptom development. Symptom development was observed after 15, 30, 45 and 60 days of planting. The viral load was quantified through qPCR.
Determination of viral load through real-time PCR
Genomic DNA from the AC1 transgenics lines was extracted and purified with Genelute plant DNA extraction kit (Sigma). DNA quality was checked spectrophotometrically with a NanoDrop 2000 (Thermoscientific) spectrophotometer and analyzed by means of 0.8% agarose gel electrophoresis in 1× Tris–borate–EDTA buffer and stained with ethidium bromide. The oligonucleotide primers and TaqMan probes were designed with Primer Express 2.0 software (Applied Biosystems). The PALCV_CP_F: ACCGTCGTCCTACAGGATCTC and PALCV_CP_R: GCTCGGTTCATTGTCAAACATGT primer pair combined with the PALCV_CP_M: FAM CTTCCCCAAAATCTTG were designed to detect a 99-bp coat protein specific fragment for quantitation of ToLCNDV-Potato viral concentration in inoculated plants using qPCR. The primer probe was FAM labeled with non-fluorescent quencher at the 3ʹ end of the probe. The qPCR was carried out in the Applied Biosystems StepOnePlus™ thermal cycler. Reaction components were assembled in 96-well plate (microAmp™ optical 96-well reaction plate). Dilution series of a plasmid harboring the transgene was prepared containing 107, 106, 105, 104, and 103 copies/µl. qPCR data of these samples were used to generate a standard curve of the log of template quantity versus Ct cycle. All the samples were analyzed in triplicates within the same plate. The qPCR reaction (20 μl) contained, 10 μl 2× Taqman universal mastermix, 2 μl 20× primer probe mix, 4.2 ng of genomic DNA. PCR cycle included initial denaturation at 94 °C for 5 min followed by 40 cycles of 94 °C for 15 s and 60 °C for 60 s. The viral counts (x) in the reaction volume was calculated based on the formula x = 10(y − 41.93)/(− 3.317) where y denotes the mean Ct value of the sample. This formula was derived from real-time PCR standard curve obtained from serial dilution of cloned plasmid. The virus count per ng of genomic DNA (A) was derived by dividing x with 4.2 ng which was the amount of genomic DNA used as template in the real-time PCR reaction.
Copy number estimation of the transgene
Transgene copy numbers of six promising AC1 transgenic lines which showed highly resistant phenotype in the glasshouse during second year of evaluation were estimated. Initially copy number of EF1-α, a house keeping gene was determined taking urease gene as a reference (internal control) for both the cultivars Kufri Pukhraj and Kufri Badshah using SYBR Green chemistry. The oligonucleotide primers for EF1-α as designed and reported by Nicot et al. (2005), i.e. EF1-F (ATTGGAAACGGATATGCTCCA) and EF1-R (TCCTTACCTGAACGCCTGTCA) and for urease gene UreF (GACCTGTTTGCTGAAATTGAGA) and UreR (GAACTTTTCCACCCCCAAAC) as reported by Bradeen et al. (2009) were used. All primers were synthesized by M/S Integrated DNA Technologies. EF1-α and urease primers in the PCR reactions were used at a concentration of 400 and 300 nM, respectively.
Primer probe for TaqMan assay for copy number estimation of AC1 gene were designed by Applied Biosystem. The probe was FAM labeled for EF1-α and NED labeled for AC1 gene with non-fluorescent quencher at 3ʹ end of the probe. All samples were analyzed in triplicates within the same plate. The PCR reaction (20 μl) contained 10 μl 2× Taqman universal mastermix, 2 μl 20× primer probe mix, and 4.2 ng of genomic DNA. PCR cycle included initial denaturation at 94 °C for 5 min followed by 40 cycles of 94 °C for 15 s and 60 °C for 60 s.
Southern blot analysis
Genomic DNA was isolated from the in vitro plants of the selected trangenic lines using CTAB method and then quantified spectrophotometrically. Approximately 15 µg of genomic DNA was digested with the restriction enzyme HindIII for 12 h, which cut once in the T-DNA region (Fig. 1). The digested DNA was electrophoresed in 0.8% agarose gel at 35 V for 18 h and transferred onto a positively charged Nylon membrane (Amersham, GE Healthcare, USA) by capillary transfer in 10× SSC solutions following standard procedure (Sambrook et al. 1989) and hybridization was carried out using nptII gene fragment as probe. The DNA fragment was labeled with α[32P]-dCTP using random primer DNA labeling kit (Amersham, GE Healthcare, UK). Hybridization was done at 65 °C for 18 h. The filter was washed at room temperature with 2× SSC and 0.1% SDS, followed by 1× SSC and 0.1% SDS, for 10 min each (Sambrook et al. 1989) and the image was captured and analyzed using a phosphoimager (Bio-Rad System, USA).
Northern blot analysis
Total RNA was recovered from leaves of ToLCNDV-Potato-challenged transgenic and non-transgenic plants using TRIzol reagent (Invitrogen, USA). Small RNAs from 15 μg total RNA samples were separated on 15% polyacrylamide gels. RNAs were transferred to Hybond-N+ membrane (Amersham Bioscience, USA) using the semi-dry blot method, and the blot was hybridized with α[32P]-dCTP-labeled probe obtained from PCR amplification of the Rep gene from plasmid DNA using a random primer DNA labeling kit (Amersham, UK). Hybridization was performed at 65 °C for 18–20 h. The filter was washed at room temperature in 2× SSC and 0.1% SDS, followed by 1× SSC and 0.1% SDS for 10 min each (Sambrook et al. 1989). The image was captured and analyzed using a phosphoimager (Bio-Rad System, USA).
Results
Constructs and transformation
To check the effectiveness of RNAi in generating transgenic potato variety resistant to ToLCNDV-Potato by silencing AC1 gene of the virus, three constructs were generated, viz. sense, antisense, and hairpin loop. In these constructs, AC1 gene was directionally cloned in various orientations (Fig. 1). The clones were confirmed by restriction digestion and sequencing of the junction site of the binary vector backbone. Transgenic plants were raised for each of the three constructs in two popular cultivars Kufri Badshah and Kufri Pukhraj by Agrobacterium-mediated transformation. All the transgenic plants generated were primary transformants resulting from independent transformation events. The organogenic calli started developing at the ends of internodal cuttings after 4–6 weeks thus forming dumble-shaped structure. Plants which showed profuse rooting in 100 mg/l of kanamycin-amended medium were selected initially for screening. We got different transformation frequency for the different constructs (Table 2). Molecular analysis for 686-bp fragment of nptII transgene confirmed the transgenic plants in both the varieties. Forty nine transgenic lines of Kufri Badshah (11 antisense, 31 hairpin loop, and 7 sense) and 33 lines of Kufri Pukhraj (21 antisense, 6 hairpin loop, and 6 sense) were selected for glasshouse evaluation.
Glass house evaluation of transgenic lines
Thirteen transgenic lines with AC1 sense construct (7 of Kufri Badshah and 6 of Kufri Pukhraj) failed to give any detectable resistance to ToLCNDV-Potato. However, five lines of Kufri Badshah encoding AC1 gene in antisense orientation and six lines encoding hairpin loop construct of the same gene showed high to moderate level of resistance. Similarly, six lines of Kufri Pukhraj encoding antisense AC1 and five lines encoding hairpin loop construct of the same gene showed resistance to ToLCNDV-Potato virus. No visible phenotypic change was observed among transgenic lines encoding AC1 gene in either antisense, hairpin loop or sense orientation.
Evaluation of promising transgenic plants for the second year
Tubers harvested from the 22 promising lines along with non-transgenic control of Kufri Badshah and Kufri Pukhraj were re-evaluated through grafting for the second year (Fig. 2b). Transgenic plants showed varied level of resistance against ToLCNDV-Potato compared to non-transgenic control plants. Transgenic event GTLC2-127 of Kufri Badshah encoding hairpin loop construct of AC1 showed complete resistance (Fig. 2a), and four other events showed moderate level of resistance to ToLCNDV-Potato (Table 3.). Similarly, three of the transgenic lines of Kufri Pukhraj encoding hairpin loop construct showed complete resistance, whereas three lines were moderately resistant to the virus (Table 3). These results showed varied levels of resistance to ToLCNDV-Potato among the constructs tested. Out of three different constructs tested, transgenic plants obtained by AC1 hairpin loop approach showed highest degree of resistance/tolerance followed by antisense strategy.
Determination of viral load
Virus load in the above 22 promising events was estimated using qPCR. The standard curve generated showed a strong linear relationship with a correlation coefficient greater than 0.97, as well as high amplification efficiency (> 99.5%). This standard curve enabled detection of even very low quantity of viral DNA in transgenic plants. Viral count in transgenic Kufri Badshah encoding hairpin loop construct of AC1 varied from 41 to 22,918 per ng of DNA compared to 641,594 in non-transgenic control (Table 4). Similarly, in transgenic Kufri Pukhraj encoding the same hairpin construct, ToLCNDV-Potato quantity varied from 332 to 69,588 per ng of DNA compared to 1,118,000 in non-transgenic control (Table 4). Virus count in antisense transgenic lines of both the varieties showed comparatively higher value than those of hairpin loop transgenic lines (Table 4). Since, hairpin loop constructs of AC2 gene conferred higher level of resistance, four such transgenic events of Kufri Pukhraj (KPLC2 13, 37, 53, and 54) and two events of Kufri Badshah (GTLC2 90 and 127) were selected for transgene copy number estimation by both qPCR and Southern blotting.
Copy number estimation through qPCR
Copy number of the transgene was estimated using the formula: copy number = \({2^{ - \Delta \Delta {C_{\text{t}}}}}\) where ∆∆Ct = ∆Ct (target) − ∆Ct (endogenous control). EF1-α was validated as internal control in Kufri Badshah and Kufri Pukhraj with respect to urease which is a single copy gene in potato and several related solanaceous crops (Witte et al. 2005). The urease gene has earlier been utilized for absolute quantification of Rpi-blb1 gene in RB-transgenic potato lines (Bradeen et al. 2009). The copy number of EF1-α was eight and six copies for Kufri Badshah and Kufri Pukhraj, respectively. Before the estimation of copy number of the AC1 transgene, standard curve for the internal control as well as target gene was validated using serial dilutions. The reaction efficiencies for both the genes were found to be above 0.90. The correlation coefficients of the standard curves were good (0.96–0.999). Reproducibility of the experiment was confirmed by repeating in triplicates, which further validated the accuracy and stability of our experiment. Copy number of the inserted AC1 transgene varied from 8 (GTLC2 127) to 3 (GTLC2 90) per tetraploid genome of potato (Table 5).
Confirmation of transgene integration through Southern hybridization
To further confirm the copy number estimation done by qPCR, Southern blot analysis was performed with the same transgenic lines. The genomic DNA was digested with HindIII as the enzyme cuts once in the T-DNA region and probed with a 693-bp nptII gene fragment. A strong signal and difference in the hybridization pattern of the selected transgenic plants confirmed stable integration of T-DNA in the transgenic lines (Fig. 3). Four out of the five transgenic lines tested showed similar copy numbers both by Southern blotting and qPCR. The transgenic line KPLC2-13 showed six copies of the transgene on qPCR analysis; however, in Southern blot only one band was very prominent and the remaining five bands are not very clear. Therefore, in general Southern blot analysis confirmed the copy number estimation by qPCR.
siRNA expression analysis through northern blot
In northern blot analysis, siRNA molecules specific to AC1 gene of ToLCNDV-Potato were detected in both transgenic and non-transgenic plants. Since the non-transgenic control plants were also inoculated with the virus, the siRNA band observed in control plants was due to plant’s natural virus surveillance mechanism present in the non-transgenic control plants. However, in some of the highly resistant transgenic lines such as GTLC2-127 and KPLC2-54 siRNA expression was more than other transgenic and non-trangenic control plants (Fig. 4.). The production of siRNA molecules in these transgenic lines correlated with resistance to ToLCNDV-Potato.
Discussion
Many researchers have made attempts to confer virus resistance in transgenic plants based on the concept of expression of virus-derived genes or genome fragments (Beachy 1993; Wilson 1993; Baulcombe 1994; Lomonossof 1995; Martínez de Alba et al. 2002; Simon-Mateo and Garcia 2011). In comparison with the successful resistance against RNA viruses, effective resistance against DNA viruses has been rarely obtained. ToLCNDV-Potato is a DNA virus which was reported for the first time in northern plains of India in the year 1999 (Garg et al. 2001). In silico analysis of cloned nucleotide sequence of AC1 gene in the present experiment revealed 96% homology with Tomato Leaf Curl New Delhi Virus (Y16421) and 93% homology with Papaya Leaf Curl Virus (AY353080). According to sequence homology search tool, this local isolate of ToLCNDV-Potato falls under the genus Begomovirus, and family Geminiviridae as per the nomenclature criterion given by ICTV. No stable source of resistance against ToLCNDV-Potato has yet been obtained in potato although it leads to major yield loss in this crop (Chandel et al. 2010). The work presented here demonstrated the possibility that the resistance against ToLCNDV-Potato infection in potato can be achieved by expressing RNAi constructs against viral mRNAs encoding essential non-structural proteins, providing a new tool to combat apical leaf curl disease. In this study, we targeted AC1 gene due to its importance in being the only geminiviral gene involved in viral replication (Castillo et al. 2003). Three strategies used in the present study compared the efficacy of sense, antisense and hairpin loop constructs in managing ToLCNDV-Potato. Two popular potato cultivars Kufri Badshah and Kufri Pukhraj were chosen as they are among the high-yielding potato varieties grown in the region affected by ToLCNDV-Potato virus. There is no natural resistance reported in these varieties to ToLCNDV-Potato infection, making them highly susceptible to the virus leading to maximum yield loss.
Total number of 49 transgenic lines of Kufri Badshah and 33 transgenic lines of Kufri Pukhraj encoding different forms of the constructs were tested in the glasshouse. We inoculated all the transgenic lines by grafting and observed high degree of resistance to ToLCNDV-Potato virus in about one-third of them. Significant delay in ToLCNDV-Potato infection with antisense constructs was observed, but plants showed disease symptoms after 4–6 weeks of inoculation; hence no complete resistance to virus was obtained using the antisense constructs. However, considerable reduction in viral load was observed in the transgenic lines encoding antisense constructs compared to non-transgenic plants, when viral load was quantified through qPCR. In case of sense construct, no significant level of resistance could be achieved in either Kufri Badshah or Kufri Pukhraj. In general, neither sense nor antisense constructs were able to confer satisfactory level of resistance to the geminivirus ToLCNDV-Potato in two potato varieties tested in this study. On the contrary, one transgenic lines of Kufri Badshah (GTLC2 127) and three lines of Kufri Pukhraj (KPLC2 13, KPLC2 37, and KPLC2 53) encoding hairpin loop construct showed complete resistance (immunity) to the virus. In the present study, exploring the utility of available virus silencing strategies against ToLCNDV-potato, hairpin technology to silence the viral genes showed promising results. Earlier, it was demonstrated in petunia that transgenic lines encoding CHS gene sequence inserted as a inverted repeat, although not highly transcribed, silenced the endogenous CHS effectively (Stam et al. 1997, 2000). It has also been shown that PTGS in plants can be triggered at high efficacy by the presence of an inverted repeat in the transcribed region of the transgene (Hamilton et al. 1998; Chuang and Meyerowitz 2000; Levin et al. 2000; Baulcombe 2002). Complete gene silencing was observed in tobacco plants transformed with constructs that produced RNA capable of duplex formation (Waterhouse et al. 1998; Smith et al. 2000).
Transgenic plants showing high degree of resistance to ToLCNDV-Potato showed AC1 gene copy number varying from 8 to 3 copies through qPCR. Further, we found resistance in transgenic plants to be independent of the copy number of the transgene present which is in agreement with the results obtained by (Aida et al. 2000). Ingelbrect et al. (1999) showed that transgenic plants with different copy numbers can show almost similar resistant phenotype, as well as transgenic plants with same transgene integration pattern did not necessarily display the same phenotype upon inoculation. It has been previously reported that isogenic transgenic lines can display different levels of resistance upon virus inoculation in dicots (Sijen et al. 1996). We found that more transgene dosage leads to higher resistance. Lindbo et al. (1993) reported that combined level of viral and transgene RNA has to surpass a threshold level to induce gene silencing by RNAi or other antiviral stretagies. Hence the transgenic lines with more transgene dosage or copy number will tend to offer greater resistance (Lindbo et al. 1993; Goodwin et al. 1996; Baulcombe 1996). More is the copy number or transgene dosage, more is the siRNA produced which in turn reach the threshold level activating the homology-dependent gene silencing. In support to this, more accumulation of siRNA molecules were observed in highly resistant transgenic lines GTLC2-127 and KPLC2-53 in northern blot analysis which also showed eight and four copies of transgene in southern blot analysis. However, other transgenic lines with five copy numbers accumulated comparatively less siRNA molecules and were comparable with non-transgenic control.
These results suggest that gene-specific post transcriptional gene silencing (PTGS) against ToLCNDV-Potato was induced when AC1 gene was targeted. The final output showed that sufficient gene-specific silencing was achieved with a construct containing both sense and antisense fragment, rather than either sense or antisense construct alone. This result is supported by the similar observations reported by Waterhouse et al. (1998) for rice and in Arabidopsis by Chuang and Meyerowitz (2000). Many other workers have also reported better efficiency of RNAi through transgene that express hpRNA than either of amplicon cassette alone (Smith et al. 2000; Wesley et al. 2001; Brummell et al. 2003; Fukusaki et al. 2004; Ifuku et al. 2003). Results obtained from different transgenic plants showed variation in the degree of silencing for ToLCNDV-Potato, within and among the constructs tested. PTGS by hairpin loop construct was found to be the best method for controlling ToLCNDV-Potato. There lies an important advantage of using hpRNA-mediated silencing from the perspective of food safety, as no virus-derived mRNAs are accumulating in transgenic plants expressing hpRNA constructs thereby reducing the risk of recombination or complementation events. Moreover no viral protein is being produced in these plants circumventing any fears concerning allergic response to novel proteins.
References
Aida R, Kishimoto S, Tanaka Y, Shibata M (2000) Modification of flower colour in torenia (Torenia fournieri Lind.) by genetic transformation. Plant Sci 153:33–42
Altschul FS, Madden LT, Schaffer AS, Zhang J, Zhang Z, Lipman JD (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res 25:3389–3402
Antignus Y, Vunsh R, Lachman O, Pearlsman M, Maslenin L, Hananya U, Rosner A (2004) Truncated Rep gene originated from tomato yellow leaf curl virus-Israel [Mild] confers strain-specific resistance in transgenic tomato. Ann Appl Biol 144:39–44
Baulcombe DC (1994) Replicase-mediated resistance: a novel type of virus resistance in transgenic plants. Trends Microbiol 2:60–63
Baulcombe D (1996) Mechanisms of pathogen-derived resistance to viruses in transgenic plants. Plant Cell 8:1833–1844
Baulcombe D (2002) RNA silencing. Curr Biol 12:R82–R84
Beachy RN (1993) Introduction: transgenic resistance to plant viruses. Semin Virol 4:327–328
Bradeen JM, Iorizzo M, Mollov DS, Raasch J, Kramer LC, Millett BP et al (2009) Higher copy numbers of the potato RB transgene correspond to enhanced transcript and late blight resistance levels. Mol Plant Microbe Interact 22:437–446
Brummell DA, Balint-Kurti PJ, Harpster MH, Palys JM, Oeller PW, Gutterson N (2003) Inverted repeat of a heterologous 3′-untranslated region for high efficiency, high throughput gene silencing. Plant J 33:793–800
Castillo AG, Collinet D, Deret S, Kashoggi A, Bejarano ER (2003) Dual interaction of plant PCNA with geminivirus replication accessory protein (REn) and viral replication protein (Rep). Virology 312:381–394
Chakrabarti SK, Mandaokar AD, Shukla A, Pattanayak D, Naik PS, Sharma RP, Kunlar PA (2000) Bacillus thuringiensis crylAb gene confers resistance to potato against Helicoverpa armigera (Hubner). Potato Res 43:143–152
Chandel RS, Banyal DK, Singh BP, Malik K, Lakra BS (2010) Integrated management of whitefly, Bemisia tabaci (Gennadius) and potato apical leaf curl virus in India. Potato Res J 53:129–139
Chuang CF, Meyerowitz EM (2000) Specific and heritable genetic interference by double stranded RNA in Arabidopsis thaliana. Proc Natl Acad Sci USA 97:4985–4990
Cogoni C, Macino G (2000) Post-transcriptional gene silencing across kingdoms. Curr Opin Genet Dev 10:638–643
Elbashir SM, Lendeckel W, Tuschl T (2001) RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 15:188–200
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Fukusaki EI, Kawasaki K, Kajiyama S, An CI, Suzuki K, Tanaka Y, Kobayashi A (2004) Flower color modulations of Torenia hybrida by downregulation of chalcone synthase genes with RNA interference. J Biotechnol 111:229–240
Garg ID, Khurana SMP, Kumar S (2001) Association of geminivirus with potatoapical leaf curl in India and its a immune electron microscopic detection. J Indian Potato Assoc 28:227–232
Goodwin J, Chapman K, Swaney S, Parks TD, Wernsman EA, Dougherty WG (1996) Genetic and biochemical dissection of transgenic RNA-mediated virus resistance. Plant Cell 8:95–105
Hamilton AJ, Brown S, Yuanhai H, Ishizuka M, Lowe A, Soles AGA, Grierson D (1998) A transgene with repeat DNA causes high frequency, post transcriptional suppression of Acc-oxidase gene expression in tomato. Plant J 15:737–746
Höfgen R, Willmitzer AL (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16:9877
Hong Y, Stanley J (1995) Regulation of African cassava mosaic virus complementary-sense gene expression by N-terminal sequences of the replication-associated protein AC1. J Gen Virol 76:2415–2422
Ifuku K, Yamamoto Y, Sato F (2003) Specific RNA interference in psbP genes encoded by a multigene family in Nicotiana tabacum with a short 3′-untranslated sequence. Biosci Biotechnol Biochem 67:107–113
Ingelbrect IL, Irvine JE, Mirkov ET (1999) Posttranscriptional gene silencing in transgenic sugarcane. Dissection of homology-dependent virus resistance in a monocot that has a complex polyploid genome. Plant Physiol 119:1187–1198
Levin JZ, Framond AJ, Tuttle A, Bauer MW, Heifetz PB (2000) Methods of double stranded RNA-mediated gene inactivation in Arabidopsis and their use to define an essential gene in methionine biosynthesis. Plant Mol Biol 44:759–775
Lindbo JA, Silva-Rosales L, Proebsting WM, Dougherty WG (1993) Induction of a highly specific antiviral state in transgenic plants: implications for regulation of gene expression and virus resistance. Plant Cell 5:1749–1759
Lomonossof GP (1995) Pathogen derived resistance to plant viruses. Annu Rev Phytopathol 33:323–343
Martínez de Alba AE, Flores R, Hernández C (2002) Two chloroplastic viroids induce the accumulation of the small RNAs associated with post-transcriptional gene silencing. J Virol 76:3094–13096
Naik PS, Chakrabarti SK, Sarkar D, Mandaokar A, Chandla VK, Anandkumar P, Sharma RP (1997) An efficient protocol for Agrobacterium tumefaciens mediated transformation and introduction of synthetic Cry1Ab gene into potato. In: National seminar on potato production on strains in low productivity area. OUAT, Bhubaneshwar. Indian Potato Association, Shimla
Nicot N, Hausman FJ, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Sambrook J, Fritschi EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Sharma R, Bhardwaj V, Dalamu D, Kaushik SK, Sharma S, Umamaheshwari R, Baswaraj R, Kumar V, Gebhardt C (2014) Identification of elite potato genotypes possessing multiple disease resistance genes through molecular approaches. Sci Hortic 179:204–211
Sijen T, Wellink J, Hiriart JB, van Kammen A (1996) RNA-mediated virus resistance: role of repeated transgenes and delineation of targeted regions. Plant Cell 8:2277–2294
Simon-Mateo C, Garcia JA (2011) Antiviral strategies in plants based on RNA silencing. Biochim Biophys Acta 1809:722–731
Smith N, Singh S, Wang MB, Stoutjesdijk P, Green A, Waterhouse PM (2000) Total silencing by intron-spliced hairpin RNAs. Nature 407:319–320
Stam M, Mol JNM, Kooter JM (1997) The silence of genes in transgenic plants. Ann Bot 79:3–12
Stam M, de Bruin R, Van Blokland R, van der Hoorn RA, Mol JN, Kooter JM (2000) Distinct features of posttranscriptional gene silencing by antisense transgenes in single copy and inverted T-DNA repeat loci. Plant J 21:27–42
Waterhouse PM, Graham MW, Wang MB (1998) Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc Natl Acad Sci USA 96:13959–13964
Wesley V, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, Robinson SP, Gleave AP, Green AG, Waterhouse PM (2001) Construct design for efficient, effective and high throughput gene silencing in plants. Plant J 27:581–591
Wilson TMA (1993) Strategies to protect crop plants against viruses, pathogen derived resistance blossoms. Pro Natl Acad Sci USA 90:3134–3141
Witte CP, Tiller S, Isidore E, Davies HV, Taylor M (2005) Analysis of two alleles of the urease gene from potato: polymorphisms, expression, and extensive alternative splicing of the corresponding mRNA. J Expl Bot 56:91–99
Acknowledgements
The authors acknowledge the financial grant received from Indian Council of Agricultural Research, New Delhi, India. We are also thankful to Drs. S. K. Pandey and B. P. Singh, Former Directors, ICAR-CPRI, Shimla, for providing laboratory facilities for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Garima Tomar, S. K. Chakrabarti, Nitya Nand Sharma, A. Jeevalatha, S. Sundaresha, Kanika Vyas, and Wamik Azmi declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Tomar, G., Chakrabarti, S.K., Sharma, N.N. et al. RNAi-based transgene conferred extreme resistance to the geminivirus causing apical leaf curl disease in potato. Plant Biotechnol Rep 12, 195–205 (2018). https://doi.org/10.1007/s11816-018-0485-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-018-0485-8