Abstract
Crop improvement is very essential to meet the increasing global food demands and enhance food nutrition. Conventional crop-breeding methods have certain limitations such as taking lot of time and resources, and causing biosafety concerns. These limitations could be overcome by the recently emerged-genome editing technologies that can precisely modify DNA sequences at the genomic level using sequence-specific nucleases (SSNs). Among the artificially engineered SSNs, the CRISPR/Cas9 is the most recently developed targeted genome modification system and seems to be more efficient, inexpensive, easy, user-friendly and rapidly adopted genome-editing tool. Large-scale genome editing has not only improved the yield and quality but also has enhanced the disease resistance ability in several model and other major crops. Increasing case studies suggest that genome editing is an efficient, precise and powerful technology that can accelerate basic and applied research towards crop improvement. In this review, we briefly overviewed the structure and mechanism of genome editing tools and then emphatically reviewed the advances in the application of genome editing tools for crop improvement, including the most recent case studies with CRISPR/Cpf1 and base-editing technologies. We have also discussed the future prospects towards the improvement of agronomic traits in crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid growth in population, climate change and environmental hazards are certain important issues that have threatened the global food security (Sundström et al. 2014). Increasing agricultural productivity is crucial for attaining food sufficiency. Hence, developing high yield, disease resistant and climate resilient crops is very essential to enhance the productivity of crops. All these years, conventional breeding methods like hybridization and mutation breeding have played a vital role in increasing crop productivity. But the gradual declination in natural genetic diversity in crop plants has hugely affected the crop production (Govindaraj et al. 2015). In last few decades, molecular breeding technology such as making use of transgenic or genetically modified (GM) crops has held great promise in overcoming the problems of conventional breeding approach and has strengthened the agricultural productivity. However, it is still labour-intensive and time consuming. Moreover, public acceptance of these GM crops is a great challenging due to social, environmental and political issues (Lusser et al. 2012).
Recently, genome editing has emerged as a novel technology that make use of sequence-specific nucleases (SSNs) to introduce targeted mutations in crops with high efficiency and precision and has been successful in overcoming the limitations of conventional breeding approach (Georges and Ray 2017). The artificially engineered SSNs such as zinc finger nucleases (ZFNs), transcriptional activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)-associated endonuclease Cas9 (CRISPR/Cas9) have proven to be highly efficient in targeted mutagenesis in a wide range of crops and model plants (Schiml and Puchta 2016; Miglani 2017). The engineered nucleases can create a double-stranded breaks (DSBs) in the target region of the DNA, which is subsequently repaired by cell’s own natural repair mechanism of homologous recombination (HR) or non-homologous end joining (NHEJ). The non-homologous end joining pathway is basically error prone, as it creates random mutations leading to frameshift mutations and target gene knockout, whereas in HR pathway the repair mechanism is more precise where a donor DNA containing sequences homologous to those flanking the DSBs site leading to gene replacement or foreign gene cassette knock in as intended (Voytas and Gao 2014; Baltes et al. 2015).
Although HR is more precise as compared to NHEJ, the frequency of illegitimate recombination is higher in NHEJ as compared to homologous recombination (Steinert et al. 2016). Hence, the use of gene replacement or gene targeting via HR is very limited in comparison to NHEJ. Gene knock-outs and loss-of-function mutations help in understanding gene functions, but their applications in crop improvement are still limited as many of the important traits are conferred by the random point mutations, indels or insertion of a new gene (Lawrenson et al. 2015). Recently, CRISPR–Cpf1 (CRISPR from Prevotella and Francisella 1) is identified as a new tool for efficient genome editing, with higher efficiency, specificity, and potentially wider applications than CRISPR–Cas9 (Zaidi et al. 2017). Most recently, Base editing has emerged as an advanced approach which enables conversion of G–C base pairs to A–C base pairs without the requirement of a DSB or donor template (Komor et al. 2016; Shimatani et al. 2017). These advances in the field of genome editing can help in creating novel traits with high efficiency and precision and accelerate crop improvement.
There are several informative reviews on genome editing tools and their applications in crop improvement (Abdallah et al. 2015; Kumar et al. 2015; Petolino et al. 2016; Song et al. 2016; Khandagale and Nadaf 2016; Miglani 2017; Sharma et al. 2017). A thorough review of SDN-1 (site-directed nuclease-1) by (van de Wiel 2017) has mentioned the improvements and new variants of SDN-1, which is the generation of small deletions or insertions (indels) at a desired target by genome editing tools. In this review, we emphatically reviewed the advances in application of genome editing tools for crop improvement, including the most recent case studies with CRISPR/Cpf1 and base-editing technologies. We also address the challenges and future prospects of the genome editing tools towards improvement of crops.
Genome editing tools: an overview
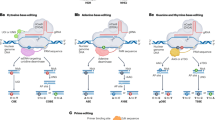
All genome editing tools rely on the SSNs which introduce double-stranded breaks (DSBs) at the specific target locus (Carroll 2014). The breaks are then subsequently repaired by cell’s own endogenous repair mechanisms either by non-homologous end joining (NHEJ) or homologous recombination (HR) (Fig. 1) (Sander and Joung 2014). These repair mechanisms introduce point mutations in the target DNA sequences, thereby altering the reading frames and generating gene knockouts, insertions, replacements and chromosomal rearrangements (Chen and Gao 2014).
The basic mechanism of genome editing systems: the sequence-specific nucleases (ZFNs, TALENs and CRISPR/Cas9) create a double-stranded breaks (DSBs) at the target site which is subsequently repaired either by non-homologous end joining method (NHEJ) or homologous recombination (HR) by cellular system which results in gene disruption by indels or gene addition or replacement, respectively
Mega nucleases
Mega nuclease enzymes were the first to be used for genome editing purposes (Paques and Duchateau 2007). The mega nucleases are mainly classified into four families: LAGLIDADG, His-Cys box, GIY-YIG, and the HNH family and the LAGLIDADG family consists of popular mega nucleases I-CreI and I-SceI. They usually recognise and cut large DNA sequences (12–40 bps). Initially developed for the animal and human system, it has also been used for genome editing purposes in plants, including Arabidopsis thaliana and Zea mays (Gao et al. 2010; Antunes et al. 2012). A modified mega nuclease, derived from the I-CreI homing nuclease was used to target MS26 gene of maize and create plants that were male sterile when homozygous for the gene knockout (Djukanovic et al. 2013). However, the overlapping nature of the DNA-binding and nuclease domains of homing endonucleases makes it difficult to engineer them to target different DNA site(s) (Paques and Duchateau 2007) and their use was also limited due to patent issues.
Zinc finger nucleases (ZFNs)
Zinc finger nucleases are thought to be the first generation-engineered endonucleases that uses designed zinc finger domains to recognise a specific DNA sequence in the genome (Carroll 2014; Lloyd et al. 2005). A typical Cys2-His2 zinc finger protein is composed of 3–6 zinc finger domains (ZFPs) and each is capable of recognising a 3 bp long target DNA sequence. The zinc finger protein is linked to a non-specific nuclease domain named FokI. A pair of ZFNs bind to their respective DNA target sequences and align with each other in reverse fashion. Dimerization of the FokI nuclease domain makes it active and enable it to create DSBs between the recognised sequences (Fig. 1) (Durai et al. 2005; Weeks et al. 2016). The DSBs are usually repaired by error-prone NHEJ pathway.
Sangamo Biosciences is the first company to develop ZFNs (Scott 2005). Although vectors containing corresponding DNA were available commercially from Sigma-Aldrich, the licensing agreements with Sangamo Biosciences and Dow Agro sciences have limited the use of ZFNs in major public funded molecular genetic laboratories. Oligomerized pool engineering, has been developed for use in plant genome modification (Townsend et al. 2009; Zhang et al. 2010). Context-dependent assembly (CoDA), which uses pre-selected two-finger units and bacterial one-hybrid screening of zinc finger libraries has been used as alternative methods to generate ZFNs (Sander et al. 2011; Curtin et al. 2012; Joung et al. 2000; Durai et al. 2005). Certain improvements in the generation of ZFNs can help simplify the editing tool, however, the non-specific binding of the zinc-finger motifs often results in off-target cleavage with ZFNs (Carroll 2011; Voytas 2013).
The use of ZFN for plant genome editing was first time reported in tobacco (Wright et al. 2005) and Arabidopsis (Lloyd et al. 2005). Till date, there are numerous successful reports of genome editing in tobacco, maize, Arabidopsis, soybean, canola and other plants (Shukla et al. 2009; Osakabe et al. 2010; Townsend et al. 2009; Curtin et al. 2011). An endochitinase-50 (CHN50) gene sequence was successfully targeted by ZFNs for site-directed DNA integration and herbicide resistance in tobacco (Cai et al. 2009). The donor plasmid consisting of both ZFNs and a donor DNA construct comprising a pat herbicide resistance gene cassette flanked by short stretches of homology to the CHN50 gene was successfully delivered by Agrobacterium and yielded up to 10% targeted homology-directed transgene integration. Similarly, Shukla et al. (2009) reported the targeted cleavage of the Inositol-1, 3, 4, 5, 6-Pentakisphosphate kinase 1 (IPK1) gene, one of the phytic acid biosynthesis gene in maize. The donor template DNA containing a promoter less PAT gene along with a 2A ‘stutter’ sequence flanked by 815 bp of sequence homologous to IPK1 gene was co-delivered by Agrobacterium. The ABA Insensitive-4 (ABI4) gene, driven by a heat-shock protein promoter in Arabidopsis (Osakabe et al. 2010), Dicer-like genes, DCL4a and DCL4b, under the control of an estrogen-inducible promoter in soybean (Curtin et al. 2011) and Alcohol dehydrogenase and chalcone synthase genes in Arabidopsis (Zhang et al. 2010) were successfully targeted by ZFNs for site targeted mutagenesis. Furthermore, Ainley et al. (2013) reported multiple trait stacking at a specific locus using ZFNs.
TAL effector nucleases (TALENs)
Transcription activator-like effector (TALE) is a class of secreted proteins of plant pathogenic bacterial genus Xanthomonas, tend to be injected into plant cell through the type III secretion system to activate transcription of specific target genes that promote disease in plants (Boch and Bonas 2010). Each TALE contains a central DNA binding domain consisting of a variable number of 33–35 amino acid repeats. The repeats are nearly identical except for the two variable amino acids at the positions 12 and 13, designated as repeat variable di-residues (RVDs). Each RVD specifically recognizes one nucleotide, and the DNA target sequence of a given TALE is determined by combination of repeat number and composition of the RVDs in the repeats (Boch et al. 2009; Moscou and Bogdanove 2009). TALEN is a chimeric protein composed of a DNA binding domain of TALE and a FokI nuclease domain. Like ZFNs, a pair of TALENs together make a cut in desired DNA region (Weeks et al. 2016). The DSBs are then repaired either by NHEJ or HR pathway. TALENs are more efficient than ZFNs in terms of low off-target effects.
The first successful use of TALENs for gene editing was reported in yeast (Christian et al. 2010; Li et al. 2011). Till date, TALENs have been successfully used for genome editing in almost all major model and crop plants like rice (Shan et al. 2015), wheat (Wang et al. 2014), maize (Char et al. 2015), tomato (Lor et al. 2014), potato (Clasen et al. 2016), barley (Wendt et al. 2013), Arabidopsis (Cermak et al. 2011), tobacco (Zhang et al. 2013) etc. TALEN system was used to induce mutations in the target gene, Acetolactate synthase (ALS), in potato (Nicolia et al. 2015) and tobacco (Zhang et al. 2013). The glossy2 (gl2) of maize and PROCERA genes of tomato have been modified with TALENs to create stable and heritable mutations (Lor et al. 2014; Char et al. 2015). TALEN technology has been efficiently used in creating fragrant rice (Shan et al. 2015), for disease resistance against bacteria blight (Li et al. 2012) and blast disease (Wang et al. 2017a, b). These studies indicate the importance of TALEN system in improving traits of crops.
CRISPR–Cas9 system
The type II CRISPR/Cas9 system from Streptococcus pyogenes is the most recently developed genome editing tool, widely accepted because of its simplicity, efficiency, easy to use and amicability. CRISPR is a bacterial defence mechanism against bacteriophages (Barrangou et al. 2007; Jinek et al. 2012; Cong et al. 2013). CRISPR story began in 1980s with the discovery of 29-nucleotide repeat sequences in the E. coli genome by Ishino and co-workers while working on the iap gene (Ishino et al. 1987). The initial findings stimulated the interest in CRISPR research and then the pace got accelerated with a huge number of publications unravelling different aspects of CRISPR system. The CRISPR/Cas9 system contains mainly three components: the Cas9 endonuclease, a crRNA (CRISPR RNA) and a tracrRNA (trans-activating crRNA). The role of Cas9 endonuclease is to cut the invading phage DNA into small pieces, which get integrated into the CRISPR array as a spacer. Subsequently, crRNA and a complementary tracrRNA are transcribed from the CRISPR array and form a double-stranded RNA structure that recruits Cas proteins for cleavage (Datsenko et al. 2012; Jinek et al. 2012). A protospacer adjacent motif (PAM) sequence (5′-NGG-3′) present in the downstream of the target DNA is a pre-requisite for the binding and cleavage of target DNA (Jinek et al. 2012).
There are two nucleic acid binding grooves namely REC lobe and NUC lobe in the Cas9 endonuclease (Gasiunas et al. 2012; Jinek et al. 2012). The REC lobe is a Cas9-specific functional domain, whereas the NUC lobe consists of three components-RuvC, HNH and PAM-interacting domains (Nishimasu et al. 2014).The homology of RuvC and HNH helps in predicting their nuclease domain structures. The Cas9 enzyme becomes active when it comes in contact with sgRNA at its REC lobe. Then Cas9-sgRNA complex scans the double-stranded DNA for PAM sites, once encountered the proper PAM, the HNH nuclease domain cleaves the RNA–DNA hybrid, while RuvC cleaves the other strand. Subsequently, the DSBs is repaired either by the error-prone NHEJ or highly precise HR pathways. NHEJ usually introduce indel mutations, whereas HR repairs the DSBs more precisely with gene insertion or replacement methods (Shukla et al. 2009).
Unlike ZFNs and TALENs, the construction of CRISPR/Cas system doesn’t require any complex protein engineering steps. CRISPR/Cas system also allows us to edit multiple genes at the same time with simultaneous introduction of DSBs at multiple sites (Mao et al. 2013). These advantages has helped the system in superseding its predecessors and get wide acceptance in scientific community. The first group of studies that demonstrated the applications of CRISPR/Cas system in plants was reported in the model species A. thaliana and N. benthamiana as well as in rice crop (Feng et al. 2013; Nekrasov et al. 2013; Shan et al. 2013a, b; Li et al. 2013). Subsequently, numerous articles were published indicating the potentiality of the CRISPR system in a wide range of crops like rice (Wang et al. 2016), wheat (Wang et al. 2014), maize (Char et al. 2017) and tomato (Pan et al. 2016). Genome editing with CRISPR/Cas9 has been demonstrated in rice for disease resistance (Wang et al. 2016), enhancing the grain weight (Xu et al. 2016), and herbicide resistance (Xu et al. 2014).
Applications of genome editing tools in crop improvement
Genome editing technologies have been efficiently used in targeting genes in a wide range of crops and improve the varieties for higher yield, better quality, enhanced nutritional value and disease resistance (Table 1). The genome editing tools have shown great potentials in enhancing crop resistance to several biotic and abiotic stresses by targeting the traits mainly controlled by negatively regulatory genes (Wang et al. 2016). For example, the DuPont scientists have successfully employed CRISPR–Cas9 method to generate novel variants of ARGOS8, a negative regulator of ethylene responses in maize crop (Shi et al. 2017). They have tried to insert the native maize GOS2 promoter, into the 5′-untranslated region of the native ARGOS8 gene or replace it via homology-directed DNA repair. The study has helped in generating novel variants of ARGOS8 for breeding drought-tolerant crops in maize. Similarly, the targeted mutation of the ERF transcription factor gene OsERF922 in rice enhanced resistance to the rice blast fungal pathogen (Wang et al. 2016). In a recent study, CRISPR/Cas9 technology was used to modify the canker susceptibility gene CsLOB1 in citrus varieties (Jia et al. 2017). This study will provide a promising pathway to generate disease-resistant citrus varieties. The CRISPR/Cas system was successfully used to edit three homeoalleles (TaMLO-A, TaMLO-B and TaMLO-D) of the MLO gene that confers resistance to powdery mildew in bread wheat (Wang et al. 2014). Recently, Zhang et al. (2017), reported enhanced disease resistance to powdery mildew disease by modifying of three homoeologs of TaEDR1 gene simultaneously in wheat by CRISPR/Cas genome editing method. Most recently, a study demonstrated genetic modification of the EBEtal7 binding site in the Os09g29100 gene promoter via TALEN editing could be deployed to reduce Tal7 binding, which could potentially reduce disease severity (Cai et al. 2017). This approach is quite promising in engineering rice cultivars with reduced susceptibility to X. oryzae. Kamoun’s group efficiently targeted SlMlo1 gene, one of the major contributor to powdery mildew susceptibility, to generate transgene-free tomato named Tomelo, resistant to powdery mildew disease using CRISPR/Cas9 technology (Nekrasov et al. 2017). The transgene-free genetically edited crops like Tomelo can be widely adopted to meet the growing demands for food worldwide.
Plant viruses cause physiological changes in plants and are a major threat to global food security. Many researchers have demonstrated the application of CRISPR/Cas9 system to confer resistance against plant viruses (Ali et al. 2015b; Ji et al. 2015; Baltes et al. 2015; Iqbal et al. 2016). CRISPR/Cas9 system can be used to target multiple viral strains by co-infiltration of sgRNA targeting Tomato yellow leaf curl virus (TYLCV), Beet curly top virus (BCTV), and Merremia mosaic virus (MeMV) in N. benthamiana plants (Ali et al. 2015a, b). Similarly, virus interference activities have been demonstrated in N. benthamiana against Bean yellow dwarf virus (BeYDV) and BSCTV, respectively (Baltes et al. 2015; Ji et al. 2015). Cas9/sgRNA delivery through gemini virus replicons (GVRs) can be used to confer broad spectrum gemini virus resistance by targeting Cotton leaf curl Kokhran virus (CLCuKoV) and also showed that targeting the conserved mononucleotide sequence can simultaneously target multiple begomovirus (CLCuKoV, TYLCV, TYLCSV, MeMV, BCTV-Worland, and BCTV-Logan) (Ali et al. 2016). A multiplexed CRISPR/Cas9 system was used to target the whole cotton leaf curl disease (CLCuD)-associated begomovirus complex along with its associated satellite molecules for broad-spectrum resistance against begomovirus in cotton (Iqbal et al. 2016). CRISPR/Cas9 technology was used to develop virus resistance in cucumber (Cucumis sativus L.) by targeting the eIF4E (eukaryotic translation initiation factor 4E) gene (Chandrasekaran et al. 2016). The non-transgenic mutated lines exhibited broad resistance to viruses like Cucumber vein yellowing virus (CVYV), Zucchini yellow mosaic virus (ZYMV) and Papaya ringspot mosaic virus-W (PRSV-W). These studies suggest that CRISPR/Cas9 genome editing is an efficient technique for targeting multiple sites for multiple viruses to confer broad-spectrum resistance in major crops and model plants.
While most of the genome editing tools have so far being aimed at creating null alleles through engineering mutations in the coding sequences, recent development also suggested that various elements of CRISPR/Cas9 system could be incorporated at creating novel allelic variations through engineering diverse types of cis-regulatory mutations (Čermák et al. 2017). Scientists at Cold Spring Harbor Laboratory (CSHL) have successfully used CRISPR/Cas9 technology to overcome yield barriers in crops and accelerate crop improvement. Lippman and his group have reported a seminal work on the development of novel variations in fruit size of tomato through targeted editing of a cis regulatory element (CRE) in the CLAVATA-WUSCHEL stem cell circuit (Rodríguez-Leal et al. 2017). Wild tomato species Solanum pimpinellifolium carrying a CRISPR/Cas9 mediated 4-bp deletion in the CArG promoter element of the putative tomato WUS (SlWUS) gene resulted in increased locule number and thus the fruit size. A similar experiment in a domesticated tomato (S. lycoperson) increased the fruit size by upto 70% (Rodríguez-Leal et al. 2017). As the fascinated (fas) and locule number (ls) QTLs that are generated through mutation in the promoter regions of SlWUS are considered as major contributors to fruit size in domesticated tomato (Xu et al. 2015; Somssich et al. 2016), it is tangible to believe that CRISPR/Cas9 technology could be used to engineer QTLs by mutating CREs with distinct functions.
Grain weight is one of the most important quantitative traits in rice production. Improving the grain weight in rice helps in improving rice production. CRISPR/Cas9-mediated multiplex gene editing was used for rapid pyramiding to improve grain weight in LH422, by targeting three major genes (GW2, GW5 and TGW6) that negatively regulate rice grain weight (Xu et al. 2016). Pyramiding null mutations of major genes using CRSIPR/Cas9 has not only improved the grain weight in rice but also facilitates the study of quantitative traits and their applications in crop breeding. Similarly, rice heading date is also an important agronomic trait that determines rice distribution and production. Traditional breeding methods are time consuming and laborious. Thus, Chinese scientists have efficiently used the CRISPR/Cas9 mediated multiplex genome editing to target three major genes (Hd2, Hd4 and Hd5) that negatively regulate the heading date of rice varieties to develop early maturity rice varieties (Li et al. 2017a, b).
The enhancement in nutritional properties of starch in rice grain has been possible due to the generation of high amylose rice plants by targeting starch-associated genes (SBEII b and SBEI) through CRISPR/Cas9-mediated genome editing (Sun et al. 2017). Liang et al. (2014) discussed the presence of anti-nutritional compound Phytic acid (PA), myo-inositol 1, 2, 3, 4, 5, 6-hexakisphosphate in maize. PA is poorly digested in humans and possesses a threat to the environment, thus, PA content of maize seeds was reduced by designing two gRNAs targeting the ZmIPK (inositol phosphate kinase) gene that catalyses a key step in PA biosynthetic pathway. The highly efficient ISU Maize CRISPR system developed by Bing Yang and his group is simpler, easy to construct and has increased frequencies of mutagenesis as compared to other systems. As the mutant lines generated through this system does not contain any foreign DNA sequences, it can be readily used for trait improvement in crops and widely accepted by research community (Char et al. 2017). Multicopy genes were targeted in Hordeum vulgare investigating the use and target specificity requirements of Cas9 editing (Lawrenson et al. 2015). They targeted two copies of HvPM19 gene encoding an ABA-inducible plasma membrane protein, which in wheat acts as a positive regulator of grain dormancy, an important agronomic trait in cereals.
Oils with high oleic acid are considered to have a longer shelf life, enhanced oxidative stability and does not require hydrogenation process. In soybean, the oil quality was improved by targeting FAD2-1A and FAD2-1B genes that convert oleic acid to linoleic acid using TALENs (Haun et al. 2014). The mutated lines showed almost four times more oleic acid than the wild-type parents. Further, the mutated soybean lines were also found to be transgene free. This study indicates the potential application of genome editing technology in improving the oil quality in soybean. Cold storage of potatoes enhances their postharvest shelf life and make them available throughout the year. But long-term storage leads to accumulation of reducing sugars due to cold-induced sweetening (CIS) and further leads to the formation of acrylamide, a potential carcinogen (Clasen et al. 2016). Zhang and group have effectively knocked out the vacuolar invertase gene (VInv) using TALENs to reduce the accumulation of reducing sugars (Clasen et al. 2016). VInv is a gene which encodes a protein that breaks down sucrose into glucose and fructose. The knockout lines resulted in lower levels of reducing sugars and lacked foreign DNA.
Recent advances in genome editing
CRISPR–Cpf1: a new system for genome editing
CRISPR–Cpf1 (CRISPR from Prevotella and Francisella 1) is identified as a new system for targeted genome editing in mammalian systems (Zetsche et al. 2015). Cpf1 is class II type V endonuclease, that recognizes a T-rich PAM (5′-TTTN-3′) present in the 5′ end of the target site and requires a single crRNA of ~ 44-nucleotide (nt) length for cleavage. Cpf1 generates sticky or cohesive ends unlike blunt ends in case of SpCas9, which enhances the efficiency of gene insertion at a precise genome location. Cpf1 enzymes have also been shown to have lower rates of off-target activity as compared to Cas9 nucleases (Kim et al. 2016). These advanced features make it a more desirable editing system in plants as compared to SpCas9 (Zaidi et al. 2017). Several reports have demonstrated this new system to be an effective DNA-free genome-editing tool for plant genome editing (Kim et al. 2017; Xu et al. 2017). Cpf1 system was used for multiplexed gene editing in rice by editing four OsBEL genes (Wang et al. 2017a, b). Most recently, Yang and colleagues have successfully generated stable and heritable mutations in rice by selecting two genome targets in the OsPDS and OsBEL genes (Xu et al. 2017).
Begemann and his colleagues screened the Cpf1 nucleases from Francisella novicida (FnCpf1) and Lachnospiraceae bacterium ND2006 (LbCpf1) for their ability to induce targeted gene insertions via homology-directed repair (Begemann et al. 2017). Chlorophyllide-aoxygenase gene of rice (CAO1) was selected as the target gene. Scientists have successfully demonstrated that both FnCpf1 and LbCpf1, has the capability to generate precise gene insertions as well as indel mutations in rice genome when used together with crRNA and repairing template DNA. This study indicates the wide adoption of Cpf1 genome editing technology can make a huge impact on plant biotechnology. CRISPR–Cpf1 system has wide applications in plant genome editing like functional screening based on gene knockouts, transcriptional repression using catalytically inactivated Cpf1 (dCpf1), or transcriptional activation using dCpf1 fused with a transcription activator domain, epigenome editing with dCpf1 fused to epigenetic modifiers, and the tracking of cell lineages with DNA-barcoding techniques. These advanced applications will enhance the improvement of yield and quality of crops and help in attaining food security and sustainability (Zaidi et al. 2017).
Yiping Qi and his colleagues demonstrated the efficacy of CRISPR/Cpf1 system (AsCpf1 and LbCpf1) as transcriptional repressors and their role in targeted gene repression in vivo (Tang et al. 2017). In the study, they demonstrated that when LbCpf1 is coupled with Pol II-promoter and a double ribozyme system to express and mature the crRNAs, acts as an effective mutagen in rice. CRISPR–Cpf1 technology was used to knock out an early developmental gene EPFL9 (epidermal patterning factor like-9) in rice to study the loss of function of genes (Yin et al. 2017a, b). OsEPFL9, also known as stomagen, is a developmental gene in rice which regulates leaf stomatal density. The mutated plants showed eightfold reduction in stomatal density without having any off-target activity. The study not only helps in understanding the loss of functions of the gene but also helps in understanding the early development of plant.
DNA-free genome editing in plants
The critical objectives to be considered during the escalation of CRISPR/Cas9 method are the prevention of transgene assimilation and minimization of off-target activity of Cas9. Accordingly, it is a goal to design a CRISPR/Cas9 Ribonucleoproteins (RNP)-mediated genome editing technology to generate adequate and definitive genome editing. The assessment of CRISPR/Cas9 RNP demonstrated strong editing activity and strong decrease in off-target activity in-vitro. Reserchers have succesfully edited a wide range of plants like A. thaliana, tobacco, lettuce and rice using CRISPR/Cas9RNPs (Woo et al. 2015). Similar studies have also been reported in maize (Svitashev et al. 2016). Recently, Korean scientists have efficiently delivered CRISPR/Cas9 RNPs to the protoplast system of grape and apple to generate transgene-free plants (Malnoy et al. 2016). Researchers have targeted a susceptible gene named MLO-7, to enhance disease resistance against powdery mildew in grape cultivar and DIPM-1, DIPM-2 and DIPM-4 genes to enhance fire blight disease resistance in apple. Gao and group from China have effectively demonstrated CRISPR/Cas9RNP mediated genome editing in immature embryo cells of bread wheat (Liang et al. 2017).
Base editing in cereal crops
Single-base changes generates elite trait variations in crop plants which helps in accelerating crop improvement (Zhao et al. 2011; Voytas and Gao 2014). Genome editing in plants using CRISPR–Cas9 system via homology directed repair (HDR) is quite challenging due to low frequency and efficiency of delivery of template DNA. Base editing which enables single base change in the genome without the requirement of a DSB or foreign DNA donor template could be an efficient and advanced approach for crop improvement (Komor et al. 2016; Nishida et al. 2016). A catalytically inactive CRISPR–Cas9 domain (Cas9 variant) is fused with a cytosine deaminase domain to form base editors which converts G–C base pairs to A–T base pairs (Nishida et al. 2016). There are few publications which demonstrates the optimization of base-editing in cereal crops (Zong et al. 2017; Shimatani et al. 2017; Lu and Zhu 2017).
Caixia Gao and group from China have successfully demonstrated base editing in cereal crops like rice, wheat and maize plants using a plant base editor named nCas9-PBE, composed of rat cytidine deaminase APOBEC1 and a Cas9 variant [Cas9-D10A nickase (nCas9)] (Zong et al. 2017). Researchers have designed a sgRNA for each of three rice genes (OsCDC48, OsNRT1.1B and OsSPL14), three different sgRNAs (S1, S2 and S3) for wheat TaLOX2 and one sgRNA for maize ZmCENH3. The rice gene OsCDC48 which regulates senescence and cell death was targeted using Agrobacterium-mediated transformation and the mutated plants revealed a mutation efficiency up to 43.48%. In case of wheat, pnCas9-PBE and pTaU6-LOX2-S1-sgRNA constructs were delivered into immature wheat embryos by particle bombardment. Sequencing results revealed C to T substitutions at positions 3, 6 and 9 in T0–3 mutated plant and one C to T substitution at position 3 in T0–7 mutated plants. T0 transgenic maize lines also revealed C–T mutations. The results indicated that nCas9-PBE can be used as a potential tool for producing point mutations useful in the precision breeding of wheat, rice and maize. Targeted point mutations was also reported in rice by targeting two genes OsPDS, which encodes a phytoene desaturase, and OsSBEIIb, which encodes a starch branching enzyme IIb using nCas9 fused with a cytidine deaminase enzyme and the uracil glycosylase inhibitor (UGI) (Li et al. 2017a, b). The results revealed single base mutations with more than 40% mutagenesis efficiency.
Japanese scientists have successfully demonstrated targeted base editing in tomato and rice plants using Target-AID (target-activation induced cytidine deaminase) with a construct comprising nuclease-deficient Cas9 (dCas9) or nickase CRISPR/Cas9 (nCas9) fused to Petromyzon marinus cytidine deaminase (PmCDA1)1 and sgRNAs (Shimatani et al. 2017). Researchers have induced multiple herbicide-resistance point mutations in rice plants by multiplexed editing using herbicide selection. Acetolactate synthase (ALS) enzyme confers herbicide tolerance when mutated. Target-AID was used to mutate C287 in rice using an appropriate sgRNA and then the sgRNA together with dCas9Os-PmCDA1Ator nCas9Os-PmCDA1At was transformed into rice calli by the Agrobacterium method. Results revealed spontaneous resistance mutations in W548C/L regardless of Target-AID treatment at a frequency of 1.56%, but nCas9OsPmCDA1At induced 3.41% IMZ tolerance. In case of tomato, marker-free plants were generated harbouring stable DNA substitutions. Two endogeneous tomato genes, DELLA and ETRI that regulate plant hormone signalling were targeted using CRISPR/Cas9 system Cas9At. D10A mutant nCas9At was fused with either a human codon-optimized PmCDA1 (nCas9At-PmCDA1Hs) or a version codon-optimized for Arabidopsis (nCas9At-PmCDA1At). Indels or C-to-T or C-to-G substitutions were observed in transformed lines indicating gain or loss of function in genomes. The above studies indicate that precise base editing is a useful and efficient approach to generate point mutations and accelerate crop improvement.
Off-target activity: a major concern
The off-target activity of Cas9 is a major issue in CRISPR–Cas9 system (Fu et al. 2013). Improper concentration in the Cas9: sgRNA ratio, the presence of promiscuous PAM sites and insufficient Cas9 codon optimization are some of the factors responsible for undesired cleavage of the DNA regions (Song et al. 2016). Off-target effects may not be a serious problem in plants as the unwanted mutations can be removed by repeated backcrossing. But backcrossing is time-consuming and affects the progress in crop improvement. In case of plants with higher genome size but without genome sequence information, three different strategies like double nicking strategy, SpCas9-FokI strategy and SpCas9 strategy can be used for minimizing off-target activity (Ran et al. 2015; Tsai et al. 2014). Truncated sgRNA strategy can be used in plants with fully sequenced genomes to reduce off-target effects (Fu et al. 2014). Moreover, several computational algorithms such as Cas-OFFinder, CRISPR-P, CRISPR-Plant and CRISPR Primer Designer have been used to create gene-specific sgRNAs with minimal off-target effects (Bae et al. 2014; Yan et al. 2015b). Lawrenson et al. (2015) reported that a reduction in the expression levels of Cas9/sgRNA reagents could significantly decrease the off-target effects. Furthermore, next-generation sequencing methods like GUIDE-seq, Digenome-seq and ChIP-seq can also identify the off-target sites for Cas9/gRNA (Tsai et al. 2015; Kim et al. 2015). Shortening the gRNA spacer sequence to 17–18 nt increases targeting fidelity (Fu et al. 2014). Recently, high-fidelity Cas9 variants have been engineered by substituting 3–4 amino acids. These Cas9 variants have been quite effective in addressing off-target issue in plants (Kleinstiver et al. 2016).
Future prospects and research opportunities
Recent advances in genome editing techniques have revolutionized the field of basic and applied biology. However, there are certain issues and challenges like the SSN/DNA delivery methods, off-target effects and the balance between HR/NHEJ pathways that has to be addressed for better efficiency and output. Although off-target activity is negligible in plants as compared to animal systems, still there is a need to address the off-target mutations systematically in major crops and other plant species. Moreover, some aspects of the editing system are still unclear, including the catalytic activity of Cas9, target sites identification and the importance of PAM sites. A thorough understanding of these aspects will help solving the problems associated with it and enhance the efficiency of the editing system. Several approaches like Digenome-seq and GUIDE-seq have been developed to detect off-target activities in human cells (Kim et al. 2015; Tsai et al. 2015) and need to be adapted to plants too for better efficiency and specificity of Cas9.
High-precision genome editing in plants, especially in transformation-recalcitrant species has been a major challenge in current times. Transformation methods are usually genotype specific and also raise regulatory concerns due to Agrobacterium-mediated transformation in plants (Yin et al. 2017a, b). Therefore, the DNA delivery methods and plant transformation issues have to be addressed. Recently, Lowe demonstrated that over-expression of Baby boom (Bbm) and Wuschel2 (Wus2) genes from maize (Zea mays) increases transformation frequencies in different crops such as rice, maize and sorghum. (Lowe 2016). This approach is genotype independent and may be useful in improving transformation of recalcitrant crop species. Identification of boom and wuschel like genes can improve the transformation efficiencies in recalcitrant crop species and broaden the genome editing applications for more crops in future.
Till date, the genome editing systems are mainly used to destroy genes in plants by inducing DSBs following NHEJ repair that introduces Indels at the target site. Precision editing by sequence replacement and fragment knock-in via homologous recombination (HR) has more important implications for crop improvement because more desired traits relay on gain of function mutation. However, the precision editing is still difficult because the efficiency of HR is very low in plants. Base editing has emerged as an advanced and precise tool in generating point mutations in crops. However, there is still a lot of scope for improvisation and its application in major cereal crops and plants. Plant cytidine deaminase homologs may also be tested for possible improvements in the efficiency of base-editing in near future.
Conclusion
In the last 5 years, genome editing has flourished as a technology and revolutionized the field of functional genomics and crop improvement in plants. CRISPR/Cas9 has emerged as the most promising approach due to its simplicity, ease of use, versatility and tolerable off-target effects. The genome editing system holds great promise in generating crop varieties with enhanced disease resistance, improved yield and quality and novel agronomic traits which will be beneficial for farmers and consumers. The technology has been successfully used for targeted mutagenesis in various model and other crops. Most recently, CRISPR–Cpf1 has been used as a new and alternative method for plant genome editing which can overcome some limitations of CRISPR/Cas9such as the PAM site requirement, and therefore, widen the scope of genome editing in crops. Nevertheless, increasing case-studies suggest that the CRISPR/Cas9 is an efficient and frequently-used technology that can accelerate basic and applied research towards crop improvement.
References
Abdallah NA, Prakash CS, Mchughen AG (2015) Genome editing for crop improvement: challenges and opportunities. GM Crops Food 6(4):183–205
Ainley WM, Sastry-Dent L, Welter ME, Murray MG, Zeitler B, Amora R, Corbin DR, Miles RR, Arnold NL, Strange TL, Simpson MA, Cao Z, Carroll C, Pawelczak KS, Blue R, West K, Rowland LM, Perkins D, Samuel P, Dewes CM, Shen L, Sriram S, Evans SL, Rebar EJ, Zhang L, Gregory PD, Urnov FD, Webb SR, Petolino JF (2013) Trait stacking via targeted genome editing. Plant Biotechnol J 11:1126–1134
Ali Z, Abulfara A, Idris A, Ali S, Tashkandi M, Mahfouz MM (2015a) CRISPR/Cas9-mediated viral interference in plants. Genome Biol 16:238–249>
Ali Z, Abulfaraj A, Li L, Ghosh N, Piatek M, Mahjoub A, Aouida M, Piatek A, Baltes NJ, Voytas DF, Dinesh-Kumar S, Mahfouz MM (2015b) Efficient virus-mediated genome editing in plants using the CRISPR/Cas9 system. Mol Plant 8(8):1288–1291
Ali Z, Ali S, Tashkandi M, Zaidi SS, Mahfouz MM (2016) CRISPR/Cas9-mediated immunity to gemini viruses: differential interference and evasion. Sci Rep 6:26912
Antunes MS, Smith J, Jantz D, Medford J (2012) Targeted DNA excision in Arabidopsis by a re-engineered homing endonuclease. BMC Biotechnol 12(86):1–12
Bae S, Park J, Kim JS (2014) CAS-OF FINDER- A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30:1473–1475
Baltes NJ, Hummel AW, Konecna E, Cegan R, Bruns AN, Bisaro DM, Voytas DF (2015) Conferring resistance to Gemini viruses with the CRISPR-Cas prokaryotic immune system. Nat Plants 1:15145
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712
Begemann MB, Gray BN, January E, Gordon GC, He Y, Liu H, Wu X, Brutnell TP, Mockler TC, Oufattole M (2017) Precise insertion and guided editing of higher plant genomes using Cpf1 CRISPR nucleases. Sci Rep 7(1):11606
Boch J, Bonas U (2010) Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Plant Biol 48:419–436
Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U (2009) Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326:1509–1512
Cai CQ, Doyon Y, Ainley WM, Miller JC, Dekelver RC, Moehle EA, Rock JM, Lee YL, Garrison R, Schulenberg L, Blue R, Worden A, Baker L, Faraji F, Zhang L, Holmes MC, Rebar EJ, Collingwood TN, Rubin-Wilson B, Gregory PD, Urnov FD, Petolino JF (2009) Targeted transgene integration in plant cells using designed zinc finger nucleases. Plant Mol Biol 69:699–709
Cai L, Cao Y, Xu Z, Ma W, Zakria M, Zou L, Cheng Z, Chen G (2017) A Transcription activator-like effector Tal7 of Xanthomonas oryzae pv. oryzicola activates rice gene Os09g29100 to suppress rice immunity. Sci Rep 7(5089):1–13
Carroll D (2011) Genome engineering with zinc finger nucleases. Genetics 188:773–782
Carroll D (2014) Genome engineering with targetable nucleases. Annu Rev Biochem 83:409–439
Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39(12):e82
Čermák T, Curtin SJ, Gil-Humanes J, Čegan R, Kono TJY, Konečná E, Belanto JJ, Starker CG, Mathre JW, Greenstein RL, Voytas DF (2017) A Multipurpose Toolkit to Enable Advanced Genome Engineering in Plants. Plant Cell 6:1196–1217
Chandrasekaran J, Brumin M, Wolf D, Leibman D, Klap C, Pearlsman M, Sherman A, Arazi T, Galon A (2016) Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol Plant Pathol 17:1140–1153
Char SN, Unger-Wallace E, Frame B, Briggs SA, Main M, Spalding MH, Vollbrecht E, Wang K, Yang B (2015) Heritable site-specific mutagenesis using TALENs in maize. Plant Biotechnol J 13(7):1002–1010
Char SN, Neelakandan AK, Nahampun H, Frame B, Main M, Spalding MH, Becraft PW, Meyers BC, Walbot V, Wang K, Yang B (2017) An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotechnol J 15:257–268
Chen K, Gao C (2014) Targeted genome modification technologies and their applications in crop improvements. Plant Cell Rep 33:575–583
Christian ML, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF (2010) Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186:757–761
Clasen BM, Stoddard TJ, Luo S, Demorest ZL, Li J, Cedrone F, Tibebu R, Davison S, Ray EE, Daulhac A, Coffman A, Yabandith A, Retterath A, Haun W, Baltes NJ, Mathis L, Voytas DF, Zhang F (2016) Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol J 14:169–176
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Curtin SJ, Zhang F, Sander JD, Haun WJ, Starker C, Baltes NJ, Reyon D, Dahlborg EJ, Goodwin MJ, Coffman AP, Dobbs D, Joung JK, Voytas DF, Stupar RM (2011) Targeted mutagenesis of duplicated genes in soybean with zinc-finger nucleases. Plant Physiol 156:466–473
Curtin SJ, Voytas DF, Stupar RM (2012) Genome engineering of crops with designer nucleases. Plant Genome 5:42–50
Datsenko KA, Pougach K, Tikhonov A, Wanner BL, Severinov K, Semenova E (2012) Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system. Nat Commun 3:945
Djukanovic V, Smith J, Lowe K, Yang M, Gao H, Jones S, Nicholson MG, West A, Lape J, Bidney D, Carl Falco S, Jantz D, Alexander L (2013) Male-sterile maize plants produced by targeted mutagenesis of the cytochrome P450-like gene (MS26) using a re-designed I-CreI homing endonuclease. Plant J 76:888–899
Durai S, Mani M, Kandavelou K, Wu J, Porteus MH, Chandrasegaran S (2005) Zinc finger nucleases: custom-designed molecular scissors for genome engineering of plant and mammalian cells. Nucleic Acids Res 33:5978–5990
Feng Z, Zhang B, Ding W, Liu X, Yang D, Wei P, Cao F, Zhu S, Feng Z, Mao Y, Zhu J (2013) Efficient genome editing in plants using a CRISPR/Cas system. Cell Res 23:1229–1232
Fu Y, Foden JA, Khayter C, Maeder ML, Reyon JK, Joung JD, Sander (2013) High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31:822–826
Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK (2014) Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32:279–284
Gao H, Smith J, Yang H, Jones S, Djukanovic V, Nicholson MG, West A, Bidney D, Falco SC, Jantz D, Lyznik LA (2010) Heritable targeted mutagenesis in maize using a designed endonuclease. Plant J 61:176–187
Gasiunas G, Barrangou R, Horvath P, Siksnys V (2012) Cas9-crRNA ribonucleoproteins complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci USA 109:E2579–E2586
Georges F, Ray H (2017) Genome editing of crops: a renewed opportunity for food security. GM Crops Food 8:1–12
Govindaraj M, Vetriventhan M, Srinivasan M (2015) Importance of genetic diversity assessment in crop plants and its recent advances: an overview of its analytical perspectives. Genet Res Int 2015:1–14
Haun W, Coffman A, Clasen BM, Demorest ZL, Lowy A, Ray E, Retterath A, Stoddard T, Juillerat A, Cedrone F, Mathis L, Voytas DF, Zhang F (2014) Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol J 12(7):934–940
Iqbal Z, Sattar MN, Shafiq M (2016) CRISPR/Cas9: a tool to circumscribe cotton leaf curl disease. Front in Plant Sci 7:475
Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A (1987) Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol 169:5429–5433
Ji X, Zhang H, Zhang Y, Wang Y, Gao C (2015) Establishing a CRISPR-Cas-like immune system conferring DNA virus resistance in plants. Nat Plants 1:15144
Jia H, Zhang Y, Orbović V, Xu J, White FF, Jones JB, Wang N (2017) Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol J 15:817–823
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Joung JK, Ramm EI, Pabo CO (2000) A bacterial two-hybrid selection system for studying protein–DNA and protein–protein interactions. Proc Nat Acad Sci USA 97:7382–7387
Khandagale K, Nadaf A (2016) Genome editing for targeted improvement of plants. Plant Biotechnol Rep 10:327–343
Kim D, Bae S, Park J, Kim E, Kim S, Yu HR, Hwang J, Kim JI, Kim JS (2015) Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat Methods 12(3):237–243
Kim D, Kim J, Hur JK, Been KW, Yoon SH, Kim JS (2016) Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat Biotechnol 34:863–868
Kim H, Kim ST, Ryu J, Kang BC, Kim JS, Kim SG (2017) CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat Commun 8:1–7
Klap C, Yeshayahou E, Bolger AM, Arazi T, Gupta SK, Shabtai S, Usadel B, Salts Y, Barg R (2017) Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant Biotechnol J 15(5):634–647
Kleinstiver BP, Tsai SQ, Prew MS, Nguyen NT, Welch MM, Lopez JM, Mccaw ZR (2016) Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat Biotechnol 34:869–874
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR (2016) Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533:420–424
Kumar S, Alabed D, Worden A, Novak S, Wu H, Ausmus C, Beck B, Robinson H, Minicks T, Hemingway D (2015) A modular gene targeting system for sequential transgene stacking in plants. J Biotechnol 207:12–20
Lawrenson T, Shorinola O, Stacey N, Li C, ØstergaardL, Patron N, Uauy C, Harwood W (2015) Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol 1–13
Li T, Huang S, Jiang WZ, Wright D, Spalding MH, Weeks DP, Yang B (2011) TAL nucleases (TALNs): hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain. Nucleic Acids Res 39:359–372
Li T, Liu B, Spalding MH, Weeks DP, Yang B (2012) High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat Biotechnol 30:390–392
Li J, Norville JE, Aach J, Mccormack M, Zhang D, Bush J, Church GM, Sheen J (2013) Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol 31:688–691
Li Q, Zhang D, Chen M, Liang W, Wei J, Qi Y, Yuan Z (2016) Development of japonica photo-sensitive genic male sterile rice lines by editing carbon starved anther using CRISPR/Cas9. J Genet Genomics 43(6):415–419
Li X, Zhou W, Ren Y, Tian X, Lv T, Wang Z, Fang J, Chu C, Yang J, Bu Q (2017a) High-efficiency breeding of early-maturing rice cultivars via CRISPR/Cas9-mediated genome editing. J Genet Genomics 44(3):175–178
Li J, Sun Y, Du J, Zhao Y, Xia L (2017b) Generation of targeted point mutations in rice by a modified CRISPR/Cas9 system. Mol Plant 10(3):526–529
Liang Z, Zhang K, Chen K, Gao C (2014) Targeted mutagenesis in Zea mays using 939 TALENs and the CRISPR/Cas system. J Genet Genomics 41:63–68
Liang Z, Chen K, Li T, Zhang Y, Wang Y, Zhao Q, Liu J, Zhang H, Liu C, Ran Y, Gao C (2017) Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoproteins complexes. Nat Commun 8:14261
Lloyd A, Plaisier CL, Carroll D, Drews GN (2005) Targeted mutagenesis using zinc-finger nucleases in Arabidopsis. Proc Natl Acad Sci USA 102:2232–2237
Lor VS, Starker CG, Voytas DF, Weiss D, Olszewski NE (2014) Targeted mutagenesis of the tomato PROCERA gene using TALENs. Plant Physiol 166:1288–1291
Lowe K (2016) Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 28:1998–2015
Lu Y, Zhu JK (2017) Precise editing of a target base in the rice genome using a modified CRISPR/Cas9 system. Mol Plant 10:523–525
Lusser M, Parisi C, Plan D, Rodriguez-Cerezo E (2012) Deployment of new biotechnologies in plant breeding. Nat Biotechnol 30:231–239
Malnoy M, Viola R, Jung MH, Koo OJ, Kim S, Kim JS, Velasco R, Kanchiswamy CN (2016) DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front Plant Sci 7:1904
Mao Y, Zhang H, Xu N, Zhang B, Gou F, Zhu JK (2013) Application of the CRISPR-Cas system for efficient genome engineering in plants. Mol Plant 6:2008–2011
Miglani GS (2017) Genome editing in crop improvement: Present scenario and future prospects. J Crop Improv 31(4):453–559
Moscou MJ, Bogdanove AJ (2009) A simple cipher governs DNA recognition by TAL effectors. Science 326:1501
Nekrasov V, Staskawicz B, Weigel D, Jones JD, Kamoun S (2013) Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol 31:691–693
Nekrasov V, Wang C, Win J, Lanz C, Weigel D, Kamoun S (2017) Rapid generation of a transgene free powdery mildew resistant tomato by genome deletion. Sci Rep 7(482):1–6
Nicolia A, Proux-Wéra E, Åhman I, Onkokesung N, Andersson M, Andreasson E, Zhu LH (2015) Targeted gene mutation in tetraploid potato through transient TALEN expression in protoplasts. J Biotechnol 204:17–24
Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, Tabata M, Mochizuki M, Miyabe A, Araki M, Hara KY, Shimatani Z, Kondo A (2016) Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Sci 353(6305):aaf8729
Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, Ishitani R, Zhang F, Nureki O (2014) Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156:935–949
Osakabe K, Osakabe Y, Toki S (2010) Site-directed mutagenesis in Arabidopsis using custom-designed zinc finger nucleases. Proc Natl Acad Sci USA 107(26):12034–12039
Pan CC, Ye L, Qin LI, Liu X, He Y, Wang X, Chen LI, Lu G (2016) CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Sci Rep 6:247–255
Paques F, Duchateau P (2007) Mega nucleases and DNA double-strand break-induced recombination: perspectives for gene therapy. Curr Gene Ther 7:49–66
Petolino JF, Srivastava V, Daniell H (2016) Editing plant genomes: a new era of crop improvement. Plant Biotechnol J 14:435–436
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, Koonin EV, Sharp PA, Zhang F (2015) In vivo genome editing using Staphylococcus aureus Cas9. Nature 520:186–191
Rodríguez-Leal D, Lemmon ZH, Man J, Bartlett ME, Lippman ZB (2017) Engineering quantitative trait variation for crop improvement by genome editing. Cell 171(2):470–480
Sander JD, Joung JK (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32(4):347–355
Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, Yeh JR (2011) Targeted gene disruption in somatic zebra fish cells using engineered TALENs. Nat Biotechnol 29:697–698
Schiml S, Puchta H (2016) Revolutionizing plant biology: multiple ways of genome engineering by CRISPR/Cas. Plant Methods 12:8
Scott CT (2005) The zinc finger nuclease monopoly. Nat Biotechnol 23:915–918
Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, Zhang K, Liu J, Xi JJ, Qiu JL, Gao C (2013a) Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol 31:686–688
Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, Zhang K, Liu J, Xi J, Qiu J, Gao JL C (2013b) Targeted genome modification of crop plants using a CRISPR/Cas system. Nat Biotechnol 31:686–688
Shan Q, Zhang Y, Chen K, Zhang K, Gao C (2015) Creation of fragrant rice by targeted knockout of the OsBADH2 gene using TALEN technology. Plant Biotechnol J 13:791–800
Sharma S, Kaur R, Singh A (2017) Recent advances in CRISPR/Cas mediated genome editing for crop improvement. Plant Biotechnol Rep 11(4):193–207
Shi J, Gao H, Wang H, Lafitte HR, Archibald RL, Yang M, Hakimi SM, Mo H, Habben JE (2017) ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J 15(2):207–216
Shimatani Z, Kashojiya S, Takayama M, Terada R, Arazoe T, Ishii H, Teramura H, Yamamoto T, Komatsu H, Miura K, Ezura H, Nishida K, Ariizumi T, Kondo A (2017) Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat Biotechnol 35(5):441–443
Shukla VK, Doyon Y, Miller JC, Dekelver RC, Moehle EA, Worden SE, Mitchell JC, Arnold NL, Gopalan S, Meng X, Choi VM, Rock JM, Wu YY, Katibah GE, Zhifang G, Mccaskill D, Simpson MA, Blakeslee B, Greenwalt SA, Butler HJ, Hinkley SJ, Zhang L Rebar EJ, Gregory PD, Urnov FD (2009) Precise genome modification in the crop species Zea mays using zinc finger nucleases. Nature 459:437–441
Somssich M, Je BI, Simon R, Jackson D (2016) CLAVATA-WUSCHEL signalling in the shoot meristem. Development 143:3238–3248
Song G, Jia M, Chen K, Kong X, Khattak B, Xie C, Li A, Mao L (2016) CRISPR/Cas9: a powerful tool for crop genome editing. Crop J 4:75–82
Soyk S, Müller NA, Park SJ, Schmalenbach I, Jiang K, Hayama R, Zhang L, Van Eck J, Jiménez-Gómez JM, Lippman ZB (2016) Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat Genet 49:162–168
Steinert J, Schiml S, Puchta H (2016) Homology-based double-strand break-induced genome engineering in plants. Plant Cell Rep 7:1429–1438
Sun Y, Zhang X, Wu C, He Y, Ma Y, Hou H, Guo X, Du W, Zhao Y, Xia L (2016) Engineering herbicide-resistant rice plants through crispr/cas9-mediated homologous recombination of Acetolactate synthase. Mol Plant 9(4):628–631
Sun Y, Jiao G, Liu Z, Zhang X, Li J, Guo X, Du W, Du J, Francis F, Zhao Y, Xia L (2017) Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Front Plant Sci 8:1–15
Sundström JF, Albihn A, Boqvist S, Ljungvall K, Marstorp H, Martiin C, Magnusson U (2014) Future threats to agricultural food production posed by environmental degradation, climate change, and animal and plant diseases—a risk analysis in three economic and climate settings. Food Secur 6(2):201–215
Svitashev S, Schwartz C, Lenderts B, Young JK, Mark Cigan A (2016) Genome editing in maize directed by CRISPR-Cas9 ribonucleoproteins complexes. Nat Commun 7:13274
Tang X, Lowder LG, Zhang T, Malzahn AA, Zheng X, Voytas DF, Zhong Z, Chen Y, Ren Q, Li Q, Kirkland ER, Zhang Y, Qi Y (2017) A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat Plants 3:17018
Townsend JA, Wright DA, Winfrey RJ, Fu F, Maeder ML, Joung JK, Voytas DF (2009) High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature 459:442–445
Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D (2014) Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol 32:569–576
Tsai SQ, Zheng Z, Nguyen NT, Liebers M, Topkar V, Thapar V (2015) GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol 33:187–197
Van de Wiel CCM, Schaart JG, Lotz LAP, Smulders MJM (2017) New traits in crops produced by genome editing techniques based on deletions. Plant Biotechnol Rep 11(1):1–8
Voytas DF (2013) Plant genome engineering with sequence-specific nucleases. Annu Rev Plant Biol 64:327–350
Voytas DF, Gao C (2014) Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol 12(6):e1001877
Wang Y, Cheng X, Shan Q, Zhao Y, Liu J, Gao C, Qiu JL (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 32:947–951
Wang F, Wang C, Liu P, Lei P, Hao W, Gao Y, Guang Y, Zhao K (2016) Enhanced rice blast resistance by CRISPR/ Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS One 11(4):e0154027
Wang F, Wang C, Zheng C, Qin T, Gao Y, Liu P, Zhao K (2017a) Creation of gene-specific rice mutants by AvrXa23-based TALENs. J Integr Agric 16(2):424–434
Wang M, Mao Y, Lu Y, Tao X, Zhu JK (2017b) Multiplex Gene Editing in Rice Using the CRISPR-Cpf1 System. Mol Plant 10(7):1011–1013
Weeks DP, Spalding MH, Yang B (2016) Use of designer nucleases for targeted gene and genome editing in plants. Plant Biotechnol J 14:483–495
Wendt T, Holm PB, Starker CG, Christian M, Voytas DF, Brinch-Pedersen H, Holme IB (2013) TAL effector nucleases induce mutations at a pre-selected location in the genome of primary barley transformants. Plant Mol Biol 83:279–285
Woo JW, Kim J, Kwon SI, Corvalán C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS (2015) DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol 33(11):1162–1164
Wright DA, Townsend JA, Winfrey RJ, Irwin PA, Rajagopal J, Lonosky PM, Hall BD, Jondle MD, Voytas DF (2005) High-frequency homologous recombination in plants mediated by zinc-finger nucleases. Plant J 44:693–705
Xu R, Li H, Qin R, Wang L, Li L, Wei P, Yang J (2014) Gene targeting using the Agrobacterium tumefaciensmediated CRISPR-Cas system in rice. Rice (NY) 7(1):5
Xu C, Liberatore KL, MacAlister CA, Huang Z, Chu YH, Jiang K, Brooks C, Ogawa-Ohnishi M, Xiong G, Pauly M, Van Eck J, Matsubayashi Y, van der Knaap E, Lippman ZB (2015) A cascade of arabinosyl transferases controls shoot meristem size in tomato. Nat Genet 47(7):784–792
Xu R, Yang Y, Qin R, Li H, Qiu C, Li L, Wei P, Yang J (2016) Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J Genet Genom 43:529–532
Xu R, Qin R, Li H, Li D, Li L, Wei P, Yang J (2017) Generation of targeted mutant rice using a CRISPR-Cpf1 system. Plant Biotechnol J 15(6):713–717
Yan M, Zhou SR, Xue HW (2015) CRISPR primer designer: design primers for knockout and chromosome imaging CRISPR-Cas system. J Integr Plant Biol 57(7):613–617
Yin X, Biswal AK, Dionora J, Perdigon KM, Balahadia CP, Mazumdar S, Chater C, Lin HC, Coe RA, Kretzschmar T, Gray JE, Quick PW, Bandyopadhyay A (2017a) CRISPR-Cas9 and CRISPR-Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant Cell Rep 36(5):745–757
Yin K, Gao C, Qiu J (2017b) Progress and prospects in plant genome editing. Nat Plants 3:17107
Zaidi SS, Mahfouz MM, Mansoor S (2017) CRISPR-Cpf1: a new tool for plant genome editing. Trends Plant Sci 22(7):550–553
Zetsche B, Volz SE, Zhang F (2015) A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat Biotechnol 33:139–142
Zhang F, Maeder ML, Unger-Wallace E, Hoshaw JP, Reyon D, Christian M, Li X, Pierick CJ, Dobbs D, Peterson T, Joung JK, Voytas DF (2010) High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases. Proc Natl Acad Sci USA 107:12028–12033
Zhang Y, Zhang F, Li X, Baller JA, Starker CG (2013) Transcription activator-like effector nucleases enable efficient plant genome engineering. Plant Physiol 161:20–27
Zhang Y, Bai Y, Wu G, Zou S, Chen Y, Gao C, Tang D (2017) Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J 91:714–724
Zhao K, Tung CW, Eizenga GC, Wright MH, Ali ML, Price AH, Norton GJ, Islam MR, Reynolds A, Mezey J, McClung AM, Bustamante CD, Mc Couch SR (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Commun 13(2):467
Zhou J, Peng Z, Long J, Sosso D, Liu B, Eom JS, Huang S, Liu S, Cruz CV, Frommer WB, White FF, Yang B (2015) Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J 82:632–643
Zong Y, Wang Y, Li C, Zhang R, Chen K, Ran Y, Qiu JL, Wang D, Gao C (2017) Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat Biotechnol 35:438–440
Acknowledgements
This research was supported by grants from the China Priority Program-Breeding of Seven Major Crops (2017YFD01100100), the Innovation Program of Chinese Academy of Agricultural Sciences (to K. Zhao) and the Talented Young Scientist Program of China (to R. Mishra).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mishra, R., Zhao, K. Genome editing technologies and their applications in crop improvement. Plant Biotechnol Rep 12, 57–68 (2018). https://doi.org/10.1007/s11816-018-0472-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-018-0472-0