Abstract
The current rate of genetic gains in crop improvement should rise to match growing need for sustainable food production and environmental safety. Recent years have seen genome editing being emerged as a promising tool to tailor a variety of traits that improve plant performance. In the context, sequence-specific nucleases like zinc finger nuclease (ZFN), transcription activator-like effector nucleases (TALENs) and more recently, clustered regularly interspaced short palindromic repeats (CRISPR/Cas) have enabled rapid and precise modification of the genomes. The CRISPR/Cas system has revolutionized targeted gene modification approaches owing of its capacity to produce allelic series with high precision in both domesticated and crop wild species. Recent examples demonstrating simultaneous mutagenesis of multiple genes lends credence to targeted genome editing for tailoring complex quantitative traits. In parallel, oligogenic traits like disease resistance can be improved by precise base editing by accurate protein remodelling. Notwithstanding encouraging results on plant genome editing, adoption of gene-edited plants remains a moot point. To realize immense potential of genome editing, emphasis should be given on resolving the technical and regulatory apprehensions associated with the adoption of gene-edited plant products. This article presents latest advances in techniques grouped under “genome editing”, with a brief discussion on the current status of genome edited plants. We also highlight current challenges that limit widespread applications of targeted genome modification in crop improvement for sustainable food security.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Practise of plant breeding started nearly 10,000 years ago that brought first grain crops under domestication and selective breeding (Hickey et al. 2019). Subsequent discovery of Mendel’s law, hybrid vigour and experimental designs not only improved the understanding of genetic elements underlying various plant traits, but also rendered plant breeding more systematic and efficient. Conventional plant breeding remains a key technology to facilitate crop improvement; however, it has limitations such as polyploidy, zygosity and longer generation time. Also, trait introgression from wild to cultivated varieties through hybridization and selection is extremely difficult (Zamir 2001; Warschefsky et al. 2014). Similarly, utilization of mutants generated through chemicals and/or irradiation is restricted either due to the mutational load or low mutation frequency in the targeted genomic region controlling trait(s) (Jung et al. 2018). Now molecular breeding approaches that integrate genomics and high-throughput phenomics and multipotent genetic material offer faster delivery of improved varieties (Varshney et al. 2009, 2018; Appels et al. 2013, 2015). Though biotechnological tools that could precisely engineer plant traits are available such as genetic transformation, these face challenges from regulators and policy makers. Furthermore, the cost of regulating GMOs is much higher than non-GMO crops and the entire process consumes considerable time even after developing improved products (Sprink et al. 2016). To address these challenges, precise modification of crop genes and/or regulatory elements has now become possible through genome editing. Recent years have seen genome editing gaining attention of researchers because it offers predictable allelic series to optimize both quantitative and qualitative traits (Kumar et al 2020; Scheben and Edwards 2018; Biswal et al. 2019).
Domestication and modern breeding practices favouring certain genomic regions have eroded genetic variation in current cultivated pools of different crop species. For instance, transition of domesticated rice from prostrate (wild rice) to erect growth (modern rice cultivars) resulted from the selection of an important single mutation prostrate growth 1 (PROG1) gene (Jin et al. 2008; Tan et al. 2008). Therefore, endeavouring precise modification of crop gene(s) by generating beneficial alleles with site-specific nucleases for desire phenotype will make huge impact on trait discovery and accelerate domestication of crop species (Scheben and Edwards 2018; Nogué et al. 2016). And with genome editing tools in place, it is possible to achieve this in much shorter duration (Scheben and Edwards 2018); however, their acceptance is still in obscurity. The onus is thus on the scientific community to provide ample evidences and generate awareness regarding technically different nature of genome editing products that lack foreign DNA, thus rendering this similar to the plants improved using conventional breeding tools. Researchers argue that the edited plants developed through genome editing should not be treated as GMOs (Araki and Ishii 2015). These technologies should be kept free from the hurdle of GMO legislation to allow their speedy adoption in routine genetic improvement programmes not only in developed countries but also in developing countries.
The present review aims to underscore the potential of modern genome editing tools for developing improved crop varieties for sustainable food production. This article evaluates genome editing with respect to environment and consumer risk. Also, the constraints that limit adoption of the crops improved with genome editing are briefly discussed.
10.2 Introducing Mutations Through Advanced Genome Editing Tools
10.2.1 Zinc Finger Nuclease (ZFN)
The term ZFN was initially used by Lusser et al. (2011) and successively by new technique working group (NTWG) in 2012. In this technique, a synthetic restriction endonuclease is customized to cut double-stranded deoxyribonucleic acid (DNA) at specific sequences (Wyman and Kanaar 2006). It comprises a zinc finger domain that allows recognition of a specific DNA sequence, enabling both site-specific mutation and integration of gene(s) into the plant genome (Bibikova et al. 2002; Wyman and Kanaar 2006). ZFN acts as a heterodimer, and therefore, ZFN transcribing genes are transported in a designed expression vector to plant cells (Söllü et al. 2010). The transfer of gene through ZFN technology involves electroporation (Wright et al. 2005), transfection (Szczepek et al. 2007), whiskers (Shukla et al. 2009), microparticle bombardment (Ainley et al. 2013), and Agrobacterium (De Pater et al. 2009). The viral vectors are also used for gene(s) transfer into the plant genome. ZFN causes double strand breaks at unambiguous site in the genome, which activate the repair mechanism of the host plant (Petolino 2015). Afterwards, both homologous recombination (HR) and DNA inclusion take place (Fig. 10.1a, e). This technique involves three artificial restriction enzymes, namely ZFN-1, ZFN-2 and ZFN-3 (Bibikova et al. 2001). (1) ZFN-1: Here ZFN is transported to the plant genome without taking repair template. Once it reaches the plant genome, it creates double-stranded breaks (DSB) to the host DNA that leads to non-homologous end-joining (NHEJ) of DNA (Puchta 2005), which either generates site-specific random mutations or small insertion or deletion. (2) ZFN-2: In contrast to ZFN1, a homology-directed repair (HDR) along with short repair template is delivered to plant genome along with ZFN enzyme (Lusser et al. 2011). The template DNA is homologous to target DNA, which binds to specific sequence causing a double-stranded break. The template starts repairing competing with endogenous repair machinery which led to site-specific point mutations through homologous recombination (HR). (3) ZFN-3: When ZFN transcribing gene is transported to the plant genome along with large repair template (for gene addition or replacement), it is called ZFN3 (Lusser et al. 2011; Araki et al. 2014). It binds to double-stranded DNA and causes site-specific double-stranded cleavage followed by HR. The end sequence flanking the double-stranded cleavage is the homologous results insertion of DNA stretch in a site-specific manner. ZFN-3 also helps in addition or replacement of the gene of interest, and for trait stacking in crops, such as herbicide resistance in plants (Townsend et al. 2009).
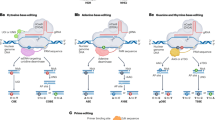
Structural representation of nucleases. (a) Structure of ZFN. ZFP represents zinc figure protein. The ZFN recognizes target site by the left and right ZFPs, and each engineered ZFP can recognize a target nucleotide. The ZFN monomer is contained a NLS (red) domain at N-terminal. The C-terminal comprises the Fok I endonuclease. The target sequence recognized by the left and right ZFPs which undergo for the dimerization of the Fok I endonuclease for activity. (b) TALEN contains an N-terminal domain comprising a nuclear localization signal (NLS); an essential domain typically formed of tandem TALE repeats to recognition a specific target DNA sequence; and a C-terminal domain with functional endonuclease Fok I. Each TALE repeat consists of 34-amino-acid with a variation at 12th and 13th amino acid position: NI (recognizes nucleotide A), NG (recognizes T), HD (recognizes C) or NN (recognizes G) (marked in black box). (c) Mode of action for TALEN. (d) Schematic representation of the CRISPR/Cas9 system structure and the principle for mutation induced through CRISPR. The synthetic guide RNA (sgRNA) complementary to the target DNA binding site and stem loops facilitates the binding of the Cas9 protein. The protospacer adjacent motif (PAM, NGG) is required for DSB which facilitate genome editing through error prone non-homologous end-joining (NHEJ) and homology-directed repair (HDR) repair pathway. (e) Mode of action for nucleases. The DNA double-stranded break DSB is repaired through HDR/NHEJ which causes base change or gene insertion or deletion in the target region (Adapted from Kumar et al. 2021)
10.2.2 Transcription Activator-like Effector Nucleases (TALEN)
Transcription activator-like effector (TALE) proteins were discovered in the bacterial Xanthomonas sp. (Bonas et al. 1989). Bacterial system utilizes this to infect plants through injecting TALE protein in plant cell via the Type III secretion system, triggering effector specific genes in host (Römer et al. 2007). TALEs consist of effector proteins, which facilitate localization, activation and specific DNA binding (Miller et al. 2011). The DNA binding domain consists of TALE effector proteins that are highly conserved, and possesses tandem repeats of 5–30 (average of 17.5) amino acids which specifically recognizes target DNA sequences (Boch and Bonas 2010). The highly conserved domain shows variation at 12th and 13th position called as “repeat variable di-residues” (RVDs); it primarily determines the DNA specificity of TALE (Bogdanove and Voytas 2011). However, these tandem repeats end abruptly, leading to truncated repeated are termed as “half repeats” (Boch and Bonas 2010; Miller et al. 2011). The DNA binding efficacy of RVDs of TALEN to nucleotides (A, C, G and T) depends upon the amino acids Asn-Ile, His-Asp, Asn-Asn, Asn-Lys and Asn-Gly (Moscou and Bogdanove 2009). The deeper understanding of RVDs has allowed molecular biologist to modify naturally occurring TALEs for genome editing (Römer et al. 2007). The fusion of nikase Fok I to the C-terminus of TALEs results in development of specific TALEN for genome editing (Fig. 10.1b, c, e). The Fok I enzymes work in a dimeric state, hence pair of TALENs is required to facilitate DNA binding by Fok I heterodimer (Zu et al. 2013; Shin et al. 2014). Then the Fok I dimer cuts specific DNA region at the spacer site to create DSB. These DSB are repaired through NHEJ, which often yield indels within the target site of the genome. Further, the TALE protein can be fused with activator, repressor, nuclease or methylase to improve TALE based proteins for genome editing (Chen and Gao 2013). The application of TALENs was extended for—(a) introduction of exogenous sequences, e.g. fluorescent tags, etc.; (b) conditional gene expression and specific gene knockout; (c) controllable rearrangements of genomic DNA through deletions, inversions/reversions (Quétier 2016). Though widely used in animals, limited attempts have been reported so far in case of plant system due to the complex nature of TALEN construct (Araki and Ishii 2015).
10.2.3 CRISPR: A Modern Editing Tool to Assist Plant Breeding
Bacterial and archaea genomes encode nucleases that trim invaders (bacteriophages) DNA. These small segments of foreign DNA are incorporated into the host genome as a long term permanent records of infectious genome (Barrangou and Doudna 2016). This yields direct repeats in bacterial genome intervened by short unique sequences (proto-spacers, 32 nucleotides), indeed representing a short sequence of foreign genome (Quétier 2016). The term CRISPR is an abbreviation for “clustered regularly interspaced short palindromic repeats”, whereas Cas represents the nuclease associated with CRISPRs assembly. Recent genome sequencing experiments have revealed occurrence of CRISPRs in almost 40% of bacteria and 90% archaea species (Horvath and Barrangou 2010). The bacterial genome encodes a range of Cas proteins, of which Cas9 represents Type II CRISPR/Cas system (Song et al. 2016). The CRISPR/Cas system was initially discovered in 1987 by Ishino and colleagues (Ishino et al. 1987). The principle that underlies CRISPR/Cas9 system was elucidated later in 2011 (Fig. 10.1d). The Cas9 associates with trans-activator crRNA (tracrRNA) and CRISPR RNA (crRNA, transcript of a protospacer) to create a double strand break in foreign DNA that matches the crRNA (Fig. 10.1d) (Deltcheva et al. 2011). Interestingly, these spacers are transcribed after each invasion and aligned with complementary nucleotides bases present in the foreign DNA, causing CRISPR/Cas mediated degradation of invaded DNA. The Cas9 protein consists of RuvC and HNH domains that create a blunt end DSB at the three base pairs upstream of protospacer at the 3´ end (Garneau et al. 2010). The DSB is repaired by NHEJ or HDR (Fig. 10.1e) mechanism which often results mutation such as indels (Xiong et al. 2015). Furthermore, the specificity of Cas9 also depends on its three-dimensional conformation. The nuclear DNA regulates the differential binding and residence time. For instance, extended binding time with target DNA sequences, whereas a shorter period for off-targets (Knight et al. 2015). To make this technique more robust, researcher fused the tracrRNA and crRNA to a single guide RNA molecule (sgRNA) (Jinek et al. 2012). The Cas9 nuclease specifically cleaves the RNA/DNA complex followed by DNA repair. With this modification CRIPSR/Cas9 genome engineering is achieved with much higher efficiency. A recent modification involves development of Cas9 variant using Fok I (from Streptococcus pyogenes), Cpf1 (Cas12; from Francisella novicida U112) and C2c2 (Cas13; from Alicyclobacillus acidoterrestris) nucleases (Tsai et al. 2015; Shmakov et al. 2015; Zetsche et al. 2015). Genome editing CRISPR technology has been extended beyond site-specific mutagenesis (Barrangou and Doudna 2016). Recent research has shown transcriptional regulation by deactivating the Cas9, and fusing the guide RNA with activator or repressor (Fig. 10.2) (Qi et al. 2013; Gilbert et al. 2014). Likewise, fusion of fluorophores enables Cas9 sequence-specific DNA visualization or chromatin imaging (Chen et al. 2012; Mao et al. 2016). Additionally, RNA manipulation has been reported using CRISPR/Cas13 in eukaryotes, including plants. RNA editing is a post-transcriptional mechanism, which converts adenosine to inosine (A to I) (Matsoukas 2018). Cox et al. (2017) reported that CRISPR/Cas13 in a programmable manner to alter the coding potential in mammalian cells. Further, Abudayyeh et al. (2017) and Aman et al. (2018) used Cas13 system to target mammalian and plant cells to knockdown of either endogenous or reporter transcripts and RNA virus, clearly indicating the potential applications in agricultural biotechnology (Ali et al. 2018). In recent years, CRISPR application has been extended to epigenetic modifications in genome to activate gene through promoters and enhancers by fusing to acetyltransferases to Cas9 (Hilton et al. 2015; Kearns et al. 2014). Unlike ZFNs and TALENs, CRISPR/Cas9 offers RNA guided genome editing in an cost-efficient and user-friendly manner (Nagamangala Kanchiswamy et al. 2015). These advancements have inspired increasing use of CRISPR/Cas9 technology in crop and animal breeding (Quétier 2016; Song et al. 2016).
Schematic illustration of crop improvement. (a) Loss of genetic diversity prior to domestication resulting in domesticated crop with desired trait(s), (b) conventional breeding approach for crop improvement which involves up to 6–15 yrs., (c) genome editing assisted breeding for faster improvement of existing elite cultivars (Adapted from Kumar et al. 2021)
10.3 Showcasing of the Candidate Genes Through Genome Editing of Crop Plants
Site-specific nucleases have allowed the introduction of targeted sequence-specific changes in both plant and animal system. Initially adopted in animal systems for targeted genome modification, ZFN and TALEN protein-guided recognition tools were later extended to create mutations or indels in the target gene of various plant species (Table 10.1) (Gaj et al. 2013; Lor et al. 2014; Sawai et al. 2014). In model plant Arabidopsis ZFN technique was employed to generate several mutants (Qi et al. 2013). For instance, Arabidopsis loss of function mutant for endogenous gene aba-insensitive-4 (ABI4) was generated for ABA and glucose insensitivity (Osakabe et al. 2010), deletion mutants for alcohol dehydrogenase-1(ADH1) and transparent testa-4 (TT4) which have shown heritable behaviour (Zhang et al. 2010). In maize, ZFN technique conferred herbicide tolerance through disruption of target gene IPK1, which alters inositol phosphate profile (Shukla et al. 2009). A similar approach in tobacco demonstrated disruption of an endogenous endochitinase gene CHN50 through a ZFN construct that consisted of a herbicide resistance PAT gene flanked by short stretches of endochitinase (Cai et al. 2009). Similarly, mutations in acetolactate synthase genes—SuRA and SuRB of tobacco improved tolerance against herbicides (Townsend et al. 2009). The heritable nature of the genetic modifications caused by gene editing was confirmed in soybean for target 10 genes: a transgene “GFP transgene” and nine endogenous genes (DCL1a, DCL1b, DCL2a, DCL2b, DCL4a, DCL4b, RDR6a, RDR6b and HEN1a) (Curtin et al. 2011). Recent examples for ZFN mediated modifications in plants include apple and fig (Peer et al. 2015), populus (Lu et al. 2016), tomato (Hilioti et al. 2016) and tobacco (Schneider et al. 2016).
Like ZFNs, TALENs have also been implemented for the improvement of crop species (Gaj et al. 2013). In monocot species, nearly 12 genes were targeted to generate desirable knockout mutants through TALENs technique (Zhang et al. 2013). In rice, Os11N3 (OsSWEET14, member of SWEET sucrose-efflux transporter family) gene is responsible for bacterial blight susceptibility (Antony et al. 2010; Chen et al. 2012). This gene in rice was mutated through TALEN and thus transgenic plants gained desired resistance to bacterial blight disease (Li et al. 2012). In barley, the promoter of HvPAPhy_a (from phytase gene family) was targeted as it accounts for the maximum of the phytase activity during seed development (Wendt et al. 2013). The mildew-resistance locus (MLO) gene was targeted which encodes for a protein that suppresses defence against powdery mildew disease (Wang et al. 2014). With TALEN technology three homoeoalleles of MLO were disrupted in bread wheat to confer heritable resistance against powdery mildew (Wang et al. 2014). In tomato DELLA protein is encoded by procera (PRO) gene (Carrera et al. 2012), and it negatively regulates the GA signalling pathway (Zentella et al. 2007). Tomato pro mutant possesses enhanced levels of GA, but it partially retained some GA response, suggesting a leaky phenotype of the mutant protein (Van Tuinen et al. 1999). In order to completely block the DELLA protein function, PRO gene mutants of tomato were raised through TALEN, which displayed a similar phenotype as pro mutant (Lor et al. 2014). TALENs have been implicated for improving postharvest quality of potato. The cold storage of potato induces formation of reducing sugars, which react with free amino acids at high temperature to form acrylamide (Kim et al. 2015). Recently, Clasen et al. (2016) obtained TALEN based knockout of the vascular invertase gene, whose tuber produces negligible level of reducing sugars and its processed chips consisted undetectable amount of acrylamide.
A growing body of literature indicate successful application of the CRISPR/Cas9 method in model and crop plants (Shan et al. 2014; Belhaj et al. 2015; Liu et al. 2017; Collonnier et al. 2017). This technique was effectively used to generate mutants in both monocots (rice and sorghum), and dicots (Arabidopsis and tobacco) (Jiang et al. 2013). In wheat, CRISPR/Cas9 system is successfully applied for mutating inositol oxygenase and phytoene desaturase (Upadhyay et al. 2013), and MLO gene (Shan et al. 2013; Wang et al. 2014). Recently, IPK gene function was neutralized in maize by using two sgRNA in the CRISPR/Cas9 (Liang et al. 2014). Mutated chlorophyll a oxygenase 1 (CAO1) and LAZY1 gene in rice caused loss of Chlorophyll b in the mutant leaf and noticeable tiller-spreading during the tillering stage, respectively (Miao et al. 2013). Similar alterations in the promoter regions of OsSWEET14 and OsSWEET11 genes in rice yielded resistance against bacterial blight (Jiang et al. 2013).
The ARGONAUTE7 (AGO7) gene in tomato regulates biogenesis of a group of sgRNAs which control the expression of auxin response factor gene (Husbands et al. 2009). Induction of mutations in tomato AGO7 through CRISPR/Cas9 system resulted in leaf deformities and affected pollen viability (Brooks et al. 2014). Recent work exploring CRISPR/Cas9 system in tomato involved mutagenesis genes such as PDS and phytochrome interacting factor PIF4 (Pan et al. 2016), downy mildew resistance 6 (de Thomazella et al. 2016) and ripening inhibitor (Ito et al. 2015). This technique generates desired mutations at the specific site of interest that are inheritable. Mutagenesis of multiple genes by CRISPR/Cas9 through expressing more than one sgRNAs suggests its immense implications for improving quantitative traits. For example, 30% yield advantage was achieved in rice following CRISPR/Cas9-driven manipulation of 13 genes associated with abscisic acid biosynthesis (Miao et al. 2018). CRISPR/Cas9 approach has been applied in the model plant Arabidopsis (Upadhyay et al. 2013) and tomato (Brooks et al. 2014), and monocot plants like rice (Zhang et al. 2014). Interestingly, a deletion of 10–1000 nucleotides can be created through multiplexing the sgRNA (Belhaj et al. 2013), thus can also lead to deletion of gene clusters due to chromosomal deletion (Zhou et al. 2014). Other examples of CRISPR/CAS9 based modification in plants include targeting multiple loci in Arabidopsis to enhance yield and resistance (Mao et al. 2016; Osakabe et al. 2016; Peterson et al. 2016), gemini virus resistance in tobacco (Zaidi et al. 2016), disease resistance in tomato (de Thomazella et al. 2016), starch modification and herbicide resistance in rice (Baysal et al. 2016; Sun et al. 2016, Wang et al. 2017), improvised fatty acid accumulation in Camelina (Jiang et al. 2016), resistance against Phytophthora sojae in soybean (Fang and Tyler 2016), canker resistance in citrus (Peng et al. 2017), starch modification in wheat (Liang et al. 2017), gibberellins metabolism in rice (Lu et al. 2016), etc.
Change in the expression level and/or organization of the genes resulting from mutations in cis-regulatory regions is known to create quantitative and qualitative variation of the traits (Wittkopp and Kalay 2012). Gene expression is fine-tuned by cis-regulatory elements (CREs) present in the promoter region. Recently, Rodriguez-Leal et al. (2017) used the CRISPR/Cas9 to modify the CREs in the promoters of tomato WUS (SlWUS) and CLV3 (SlCLV3) genes, that control fruit size, and inflorescence architecture. The induced novel cis-regulatory mutant alleles increased the tomato fruit size and locule number, similar to the natural QTL variants. Base-editors (BEs) are another CRISPR/CAS9 technique, which enables direct, irreversible conversion of one base to another at a target locus. Given the majority of the agronomic traits are controlled by point mutations (Huang et al. 2010), recent findings indicated that by fusing a nuclease-deactivated Cas9 (dCAS9) to a cytidine deaminase or adenosine deaminase induces C.G and A.T base pairs (bps) to T.A and G.C (Brooks and Gaj 2018). Though BEs approach was initially applied in mammalian systems, and the same was successfully employed in rice, wheat, maize, and tomato (Zong et al. 2017; Shimatani et al. 2017; Lu et al. 2016).
10.4 Challenges for Genome Editing
One of the major challenges in successfully achieving genome editing is plant genetic transformation and regeneration, which are the bottlenecks in agriculture biotechnology. Various technologies are available to improve plant transformation. For example, floral dip transformation is an attractive solution, it eliminates the plant tissue culture step, but the limitation is that not compatible to several crop plants, except in Arabidopsis and Camelina sativa (Lu and Kang 2008) and its transformation efficiency is very low. Further, to improve plant transformation and regeneration methods, high throughput, efficiency and novel transformation technologies are required. Development of novel methodologies could dramatically enhance plant genomics knowledge to feed the world (Altpeter et al. 2016).
Designing of ZFNs and TALENs is one of the most pain stacking jobs. The commercially available ZFNs are more efficient, but very expensive, than the publicly designed (Ramirez et al. 2008). In contrast, TALENs designing has become easy and efficient by using Golden Gate cloning—a DNA assembly technique (Engler et al. 2008). On the basis of available literature, some of the most important problems associated with genome editing are their low efficacy, regulatory vagueness and social acceptance (Shukla et al. 2009). Yet, the accurate estimation of efficiency is very difficult because efficiency depends on various factors such as the crop plant selection, methodology used, target gene and marker genes. For example, the efficiency of ZFN induced mutation in Arabidopsis is reported to be around 2% (de Pater et al. 2009) whereas, in the case of tobacco, it is 40% (Townsend et al. 2009). Over and above, one of the problems associated with ZFN and TALENs technologies is its non-specific binding which leads to create non-specific mutations (Pattanayak et al. 2011). These off target effects are also associated with CRISPR/Cas9 technology (Song et al. 2016). An improper concentration between Cas9 and sgRNA, or promiscuous PAM sites, or poor codon optimization of Cas9 during translation results off target/undesired cleavage of DNA sequence. It has been reported that high off-targets were found in humans, but low in mice, zebrafish and plants (Fu et al. 2013; Pan et al. 2016). Depending upon the species/cultivar the efficiency of the technology shows discrepancy. At the same time, T-DNA (foreign DNA) will be removed before proceeding for commercialization (Schaart et al. 2010). However, off target effects are expected with any genome editing tools as these are driven by several factors including sequence similarities. Nevertheless, researcher always selected the best phenotypic variants from genetically engineered lines, ruling out off target effect.
The regulatory cost of new plant breeding techniques is very high and the regulatory process alone takes 5–7 years, hence the acceptance of these techniques is low. When products become GMOs, it costs even higher and more time consuming compared to non-regulated classical breeding techniques (Kalaitzandonakes et al. 2007). Therefore, usage of these new techniques is limited. In particular, small companies are using these techniques only for limited traits of high value crops (Miller et al. 2011). Hence, it will be hard for plant breeders to invest in ventures where regulatory cost has a direct impact on the economic potential of the crops such as orphan crop and GM approaches-based product.
Once the plant is classified as GMOs, it has to be a method for identification and actual quantification on the newly introduced gene/s and has to be mentioned before going to the market (Kuzma and Kokotovich 2011). All contemporary available standard methods for GMO detection basically depends on the quality of DNA and efficiency of the techniques (PCR, qPCR, ELISA, etc.,). In order to evaluate the changes brought about by these genomes editing, those are mostly monitored by an expert committee, which is considered to be an important element of risk management (Glandorf et al. 2011). The prior knowledge of DNA sequence has an imperative role in the detection and identification of GMOs. The plant produced through ZFN1, ZFN2 and ZFN3 techniques can be detected by DNA based approaches only when there is prior information of flanking sequences of introduced modifications.
Genome editing has been mostly implemented in plant breeding to generate disease resistance and yield advantage for crops. Its application has to widen such as abiotic stress tolerance, nutritional quality enhancement and allergenicity elimination from various crops. There has been some report on RNAi based reduction of allergens from apple (Gilissen et al. 2005). Similarly, peanut allergens and gluten gene from various crops such as wheat, rye and barley can be reduced or abolished using these techniques (Gilissen et al. 2014; Smulders et al. 2015). Now breeders are opting for developing superior varieties through grafting. In case of grafting, plants are produced by joining of scions and rootstocks. When a non-GM scion is attached to the GM rootstocks, detection of scion derived products becomes impossible, but rootstock can be identified using usual genomics tools used for GM crops.
10.5 Genome Edited Crop: Social Acceptance and Regulatory Framework
Since the domestication of first agricultural plants nearly 10,000 years ago, plant breeding techniques have tremendously improved crop yields that can feed more than 70 million peoples (Palmgren et al. 2015). However, new breeding techniques and agronomic practices are required for a sustainable food future of 10 billion people by 2050. The unfavourable conditions like biotic and abiotic stress are conspicuous factors which have increased the losses of crop productivity over the years. Thus, the pressing demand for resilient crop species has invigorated researchers to discover the possibility through reverse genetic or genome editing. Although recent genetic engineering approaches in crop species have achieved considerable progress in crop improvement, its social acceptance is negligible due to lack of strong global policies (Araki and Ishii 2015). Argument for the accepting GMOs or genome edited plants occur not with the public; but surprisingly, it had also created a debate between researchers (Tanaka 2012; Freedman 2013; Lucht 2015). It is projected that acceptance of these genome edited plants will adversely affect the native crop germplasm resources and also human health. In order to broaden the public acceptance, constant discussion with the society is a prerequisite (Palmgren et al. 2015), excellent reviews have been published on regulatory vagueness and social acceptance (Jones 2015; Araki and Ishii 2015). In 2007, the European Union commission and member states decided to set up an expert committee on NPBTs to evaluate these new techniques with respect to GMO legislation (Schaart et al. 2016), and the commission highlighted an array of legal and social issues associated with GMOs (Lusser et al. 2012). According to committee view, these techniques may or may not involve genome alteration of the target plant species given their heterogeneous nature. The EU declaration defines GMO as “any organism having altered genetic material which does not occur by natural mating or by natural recombination” (Directive 2001/18/EC 2001).
Anthropogenic activity has dramatically changed agricultural strategies such as large-scale cultivation of new varieties in combination with affecting natural habitats of ancestral wild species of crop plants (von Wettberg et al. 2018). Notably, the important alleles and genetic variations present in the plant wild species allow sustainable growth in extreme environmental conditions and distant geographical regions (Hajjar and Hodgkin 2007; Lu 2013; Brozynska et al. 2016). Recent success in the field of genetic engineering has also overlooked the huge potential of wild species and their use in prebreeding for certain extent, as these modern techniques have potential to improve elite crops or domestication of crop wild relatives in shorter duration (Palmgren et al. 2015; Li et al. 2018). Numerous researchers believe that worldwide acceptance of these genome edited plants can severely affect the diversity of plant wild species, and even it could lead to extinction of some rare species (Stewart et al. 2003; Castañeda-Álvarez et al. 2016). Questions remain on the legal acceptance for the applied strategies, and also on the social, economic and ethical acceptance of them (Tanaka 2012). Hence, the major concern with the release of living modified organisms (LMOs) is their impact on the environment, biodiversity conservation and a human health risk due to consistent consumption of GMOs (Lucht 2015).
According to the Cartagena Protocol, plants raised using genome editing can be out boxed from GMO regulation as they do not possess any transgene (http://bch.cbd.int/protocol/text/). It is quite interesting that, the stringency of the regulation of GMO or LMOs considerably varies within countries. For example, in New Zealand and Europe, food obtained from the plants derived from the precision mutagenesis techniques should be compared similar to the food derived from the traditional mutagenic techniques (Lusser et al. 2011; Palmgren et al. 2015). However, the foremost challenge for GMOs is acceptance of them in public domain, which greatly relies on the mindset of citizen, farmers and decision makers (Araki and Ishii 2015). The controversies related to transgenic have led to their widespread public rejections, and limited commercialization. For instance, the expert committee of new plant biotechnology declares that ZFN-1 and 2 both create GMO and therefore, both fall under the directive of 2001/18/EC or Directive 2009 41/EC 2009 (Schiemann et al. 2009; Sprink et al. 2016). Also, the plant produced using ZFN-3 technology is transgenic and therefore, comes under the directive of 2001/18/EC (Schiemann et al. 2009; Araki et al. 2014). Similarly, several CRISPR/Cas9 mediated products including rice, maize, soybean, etc., are already developed and waiting for the approval of government regulatory bodies. Very recently, the Court of Justice of the European Union (ECJ) subjected CRISPR edited plants under tough GM laws by subjecting these plants to a 2001 directive, previously developed to control GM crops for food (Callaway 2018). However, researchers and plant breeders argue that CRISPR/Cas9 edited plants should be treated same as irradiation mutagenesis because it causes changes in DNA and does not involve the insertion of foreign genes, thus they can be exempt from the directive. Currently, the adoption of CRISPR system in agronomy has been remarkably increasing (Ricroch et al. 2017). As a result, several countries like USA, Canada, China, etc., have showed positive response towards CRISPR/Cas edited crop products (Lassoued et al. 2019); however, the developed edible food products from edited crops are of major concern. In fact, globally, the impact of genetically modified crops has been realized, especially due to the recent economic analysis obtained for the modified global crops such as maize and cotton (Brookes and Gaj 2018). For instance, in Spain and Portugal, over the 21-year period (1998 and 2018) the insect-resistant (IR) maize (aka corn) has increased farmers income by €285.4 million (US$322.9 million) by saving money on insecticides and producing more crop yields (Brookes and Gaj 2018). Additionally, use of IR maize maintained the required production by using lesser arable land because for the same production with conventional breeding material the farmers would have required an additional 15,240 hectares in the two countries. In 2014, the genetically modified soybean, cotton and canola saved 19, 9 and 1.5 million hectares of land globally (https://www.pgeconomics.co.uk/pdf/2017globalimpactstudy.pdf).
Recent reports have suggested that the genome editing tools have faded the boundaries between edited crops and regulatory bodies for social acceptance (Ishii and Araki 2016). Many products delivered through TALEN approach has been accepted (see Table 10.2). For instance, a TALEN mediated SU (sulfonylurea) Canola launched by Cibus (https://www.cibus.com/products.php) was commercially approved by the Canadian and United States governments in 2015 (Table 10.3). In the next 5 years other products from TALEN mediated genome edited products from Cibus such as glyphosate tolerant flax, soybean and maize breeds are under evaluation in the United States (Li et al. 2016) (Table 10.3). No wonder, more crop TALEN mediated genome edited products would be pushed to the market, as TALEN has proved its potential and critical role in genome editing breeding. It is obvious that the growing demand of food supply coupled with agronomic losses due to increased prevalence of diseases and abiotic stress needs supports of genome edited crops, which can provide elite varieties in very short duration. We speculate, in next two decades CRISPR/Cas9 mediated crop will have more products directly developed from the domestication of crop wild relatives (Li et al. 2018), which known to have several important features including higher nutritional quality and disease resistance, these products should be globally accepted to fulfil hunger need.
10.6 Intellectual Property Rights (IPRs) Protection and Freedom to Operate (FTO)
Biotechnology research has increased the availability of crop plants that are high-yielding, nutritious, stress tolerant, etc. (Díaz de la Garza et al. 2004; Vinocur and Altman 2005; Storozhenko et al. 2007; Nunes et al. 2009; Tamás et al. 2009; Varshney et al. 2011). However, the success of these genetically engineered plants is greatly dependent on the inventor incentives because of IPRs. IPRs include a set of laws to provide a legal protection to inventor or innovators for a fixed period of time against direct exploitation of their product or method (Malik and Zafar 2005). IPRs protect the biotechnology material through two major systems: patents and rights in plant varieties, but for a limited period of time. Patents provide a wide range of legal rights to retain, use, transfer it by sale or as a gift, and restrict others from similar rights for a duration of 17–20 yrs. (Gold et al. 2002; Graff et al. 2003). According to International Union for the Protection of New Varieties of plants (UPOV), a plant variety can be protected only if it is unique, stable, uniform and fulfil the novelty requirements (Jördens 2005). This grant provides an exclusive right to the owner to sell the plant materials such as reproductive organ or whole plant, which can be up to a period of 20–30 years. In the field of agro-biotechnology there is an exponential increase of the counts for filed application patents in USA, Germany and Japan (Graff et al. 2003). This surge was motivated by the royalties which can be obtained from invention (Barrows et al. 2014). The seed companies protect their genetically engineered seeds by IPRs (Oczek 2000; Frison et al. 2010), and sell their seed to farmers at monopolistic prices. It has also affected the conventional plant breeding, which is slow due to their need on the availability of the desired traits from the ancestral or closely related species. However, the rapid increase of the advancement in agricultural plant biotechnologies has increased the monitoring responsibility for the crop biosecurity which protects from the bioterrorism, biopiracy, genetic erosion, etc., (Evenson 1999; Chen and Puttitanun 2005). Furthermore, the surge of proprietary protection in crop species has intricate the exchange of germplasm which are required to develop new cultivars against destructive disease and environmental stress (Graff et al. 2003; Luby and Goldman 2016). This has led to an inability of researchers and breeders to obtain seed without acquiring permission through entering into an agreement such as material transfer certificate, license, etc. (Chi-Ham et al. 2012; Luby et al. 2015), hence, deprive researchers and breeders from genetic gain. These circumstances restrict the reach of quantity breed from a breeder and the researcher, and limit FTO for the purpose of crop breeding (Binenbaum et al. 2003; Le Buanec 2005). FTO allows researcher/people to determine whether a commercialization or testing can be done without violating any valid IPRs of others (Luby et al. 2015; Bjørnstad 2016; Zanga et al. 2016). To increase the accessibility of germplasm to breeders and researcher several open resource centre available such as an open source seed initiative (OSSI), Chinese crop germplasm information system (CCGIS), national small grains collection (NSGC), USDA soybean germplasm collection, tomato genetics resource centre (tgrc.ucdavis.edu/), etc., (Sachs 2009). Recently, efforts have been made to enhance the FTO in corn and carrot breeding through development of open source populations (Luby and Goldman 2016; Zanga et al. 2016). Similar attempts are required for improving the crop breeding because today crop genetics resources are intensely secure with IPRs.
10.7 Conclusion
The expectations of genome editing have risen that the technology would expedite progress towards sustainable crop productivity. Some products are at the final stage of development. The commercial use of the technology is relatively new in crop improvement. Genome editing allows generation of superior plants rapidly with higher efficiency and in an environmentally safe manner. Majority of the commercial crop varieties developed over the last 20 years by transgenesis, conventional and molecular breeding are now being explored through genome editing. Considering wider applications, CRISPR/Cas9 has gained more attention from researchers and breeders as compared to ZFNs, TALENs, grafting, reverse breeding, etc. As a result, breeders are now encouraging CRISPR edited plants crops to combat climate change and associated yield loss. The genome editing is accepted by the commercial sector because of its impending financial gain over alternative traditional techniques. However, a wider adoption of products derived from these techniques depends on several factors, including regulatory jurisdiction, the efficiency of the techniques and political expediency. Genome edited crop products are now available in a few countries such as the U.S. and Canadian government has approved genome edited canola and mushroom. We anticipate that CRISPR/Cas9 technology is likely to bridge the gap between GMO and society. The genome editing will be instrumental in meeting the challenge of feeding 12 billion people by the end of the twenty-first century.
Abbreviations
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- EU:
-
European union
- FAO:
-
Food and agriculture organization
- FTO:
-
Freedom to operate
- GMO:
-
Genetically modified organism
- IPR:
-
Intellectual property rights
- NCA:
-
National competent authority
- NPBTs:
-
New plant breeding techniques
- NTWG:
-
New technique working group
- SDN:
-
Site-directed nuclease
- SG:
-
Synthetic genomics
- TALEN:
-
Transcription activator-like effector nucleases
- ZFN:
-
Zinc finger nuclease
References
Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J et al (2017) RNA targeting with CRISPR-Cas13. Nature 550:280–284
Ainley WM, Sastry-Dent L, Welter ME, Murray MG, Zeitler B, Amora R, Corbin DR, Miles RR et al (2013) Trait stacking via targeted genome editing. Plant Biotechnol J 11:1126–1134
Ali Z, Mahas A, Mahfouz M (2018) CRISPR/Cas13 as a tool for RNA interference. Trends Plant Sci 23:374–378
Altpeter F, Springer NM, Bartley LE, Blechl AE, Brutnell TP, Citovsky V, Conrad LJ, Gelvin SB, Jackson DP, Kausch AP et al (2016) Advancing crop transformation in the era of genome editing. Plant Cell 28:1510–1520
Aman R, Ali Z, Butt H, Mahas M, Aljedaani F, Khan MZ, Ding S, Mahfouz M (2018) RNA virus interference via CRISPR/ Cas13a system in plants. Genome Biol 19:1
Antony G, Zhou J, Huang S, Li T, Liu B, White F, Yang B (2010) Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell 22:3864–3876
Appels R, Barrero R, Bellgard M (2013) Advances in biotechnology and informatics to link variation in the genome to phenotypes in plants and animals. Funct Integr Genomics 13(1):1–9
Appels R, Nystrom J, Webster H, Keeble-gagnere G (2015) Discoveries and advances in plant and animal genomics. Funct Integr Genomics 15:121–129
Araki M, Ishii T (2015) Towards social acceptance of plant breeding by genome editing. Trends Plant Sci 20:145–149
Araki M, Nojima K, Ishii T (2014) Caution required for handling genome editing technology. Trends Biotechnol 32:234–237
Barrangou R, Doudna JA (2016) Applications of CRISPR technologies in research and beyond. Nat Biotechnol 34:933–941
Barrows G, Sexton S, Zilberman D (2014) Agricultural biotechnology: the promise and prospects of genetically modified crops. J Econ Perspect 28:99–120
Baysal C, Bortesi L, Zhu C et al (2016) CRISPR/Cas9 activity in the rice OsBEIIb gene does not induce off-target effects in the closely related paralog OsBEIIa. Mol Breed 36:1–11
Belhaj K, Chaparro-Garcia A, Kamoun S et al (2013) Plant genome editing made easy: targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods 9:39
Belhaj K, Chaparro-garcia A, Kamoun S et al (2015) ScienceDirect editing plant genomes with CRISPR/Cas9. Curr Opin Biotechnol 32:76–84
Bibikova M, Carroll D, Segal DJ, Trautman JK, Smith J, Kim YG, Chandrasegaran S (2001) Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol Cell Biol 21(1):289–297
Bibikova M, Golic M, Golic KG, Carroll D (2002) Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 161(3):1169–1175
Binenbaum E, Nottenburg C, Pardey PG et al (2003) South-north trade, intellectual property jurisdictions, and freedom to operate in agricultural research on staple crops. Econ Dev Cult Change 51:309–335
Biswal AK, Mangrauthia SK, Reddy MR et al (2019) CRISPR mediated genome engineering to develop climate smart rice: challenges and opportunities. Semin Cell Dev Biol 96:100–106
Bjørnstad Å (2016) “Do not privatize the giant” shoulders’: rethinking patents in plant breeding. Trends Biotechnol 34:609–617
Boch J, Bonas U (2010) Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol 48:419–436
Bogdanove AJ, Voytas DF (2011) TAL effectors: customizable proteins for DNA targeting. Science 333(6051):1843–1846. https://doi.org/10.1126/science
Bonas U, Stall RE, Staskawicz B (1989) Genetic and structural characterization of the avirulence gene avrBs3 from Xanthomonas campestris pv. Vesicatoria. Mol Gen Genet 218:127–136
Brooks AK, Gaj T (2018) Innovations in CRISPR technology. Curr Opin Biotechnol 52:95–101
Brooks C, Nekrasov V, Lippman ZB, Van Eck J (2014) Efficient gene editing in tomato in the first generation using the clustered regularly interspaced short palindromic repeats/CRISPR-Associated9 System1. Plant Physiol 166:1292–1297
Brozynska M, Furtado A, Henry RJ (2016) Genomics of crop wild relatives: expanding the gene pool for crop improvement. Plant Biotechnol J 14:1070–1085
Cai CQ, Doyon Y, Ainley WM et al (2009) Targeted transgene integration in plant cells using designed zinc finger nucleases. Plant Mol Biol 69:699–709
Callaway E (2018) CRISPR plants now subject to tough GM laws in European Union. Nature 560:16
Carrera E, Ruiz-Rivero O, Peres LEP et al (2012) Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol 160:1581–1596
Castañeda-Álvarez NP, Khoury CK, Achicanoy HA, Bernau V, Dempewolf H, Eastwood RJ et al (2016) Global conservation priorities for crop wild relatives. Nat Plants 2(4):16022
Chen K, Gao C (2013) TALENs: customizable molecular DNA scissors for genome engineering of plants. J Genet Genomics 40:271–279
Chen Y, Puttitanun T (2005) Intellectual property rights and innovation in developing countries. J Dev Econ 78:474–493
Chen L-Q, Qu X-Q, Hou B-H et al (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335:207–211
Chi-Ham CL, Boettiger S, Figueroa-Balderas R et al (2012) An intellectual property sharing initiative in agricultural biotechnology: development of broadly accessible technologies for plant transformation. Plant Biotechnol J 10:501–510
Clasen BM, Stoddard TJ, Luo S et al (2016) Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol J 14:169–176
Collonnier C, Epert A, Mara K, Maclot F, Guyon-Debast A, Charlot F, White C, Schaefer D, Nogué F (2017) CRISPR-Cas9-mediated efficient directed mutagenesis and RAD51-dependent and RAD51-independent gene targeting in the moss Physcomitrella patens. Plant Biotechnol J 15:122–131
Cox DB, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, Zhang F (2017) RNA editing with CRISPR-Cas13. Science 358:1019–1027
Curtin SJ, Zhang F, Sander JD et al (2011) Targeted mutagenesis of duplicated genes in soybean with zinc-finger nucleases. Plant Physiol 156:466–473
De Pater S, Neuteboom LW, Pinas JE et al (2009) ZFN-induced mutagenesis and gene- targeting in Arabidopsis through agrobacterium-mediated floral dip transformation. Plant Biotechnol J 7:821–835
de Thomazella DPT, Brail Q, Dahlbeck, D, Staskawicz, BJ (2016) CRISPR-Cas9 mediated mutagenesis of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. bioRxiv 064824.
Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E (2011) CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471:602–607
Díaz de la Garza R, Quinlivan EP, Klaus SMJ et al (2004) Folate biofortification in tomatoes by engineering the pteridine branch of folate synthesis. Proc Natl Acad Sci U S A 101:13720–137205
Directive 2001/18/EC (2001) Directive 2001/18/EC of the European Parliament and of the Council on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC. Official Journal of the European Union, L 106, 17.4.2001
Directive 2009/41/EC (2009) Directive 2009/41/EC of the European Parliament and of the Council of 6 May 2009 on the contained use of genetically modified micro-organisms (Recast) (OJ L 125:75–97
Engler C, Kandzia R, Marillonnet S (2008) A one pot, one step, precision cloning method with high throughput capability. PLoS One 3:e3647
Evenson RE (1999) Intellectual property rights, access to plant germplasm, and crop production scenarios in 2020. Crop Sci 30(6):1630–1635
Fang Y, Tyler BM (2016) Efficient disruption and replacement of an effector gene in the oomycete Phytophthora sojae using CRISPR/Cas9. Mol Plant Pathol 17:127–139
Freedman DH (2013) The truth about genetically modified food. Sci Am 309:107–112
Frison C, Dedeurwaerdere T, Halewood M (2010) Intellectual property and facilitated access to genetic resources under the international treaty on plant genetic resources for food and agriculture. Eur Intellect Prop Rev 32:1–8
Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD (2013) High-frequency off-target mutagenesis induced by CRISPR–Cas nucleases in human cells. Nat Biotechnol 31:822–826
Gaj T, Gersbach CA, Barbas CF (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31:397–405
Garneau JE, Dupuis ME, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadán AH, Moineau S (2010) The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468:67–71
Gilbert LA, Horlbeck MA, Adamson B et al (2014) Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159:647–661
Gilissen LJ, Bolhaar ST, Matos CI, Rouwendal GJ, Boone MJ, Krens FA et al (2005) Silencing the major apple allergen mal d 1 by using the RNA interference approach. J Allergy Clin Immunol 115:364–369
Gilissen LJWJ, van der Meer IM, Smulders MJM (2014) Reducing the incidence of allergy and intolerance to cereals. J Cereal Sci 59:337–353
Glandorf B, de Loose M, Davies H (2011) Evaluation of changes in the genome of plants through application of new plant breeding techniques. Annex 15. In New plant breeding techniques. State-of-the-art and prospects for commercial development. JRC technical report EUR 24760 EN. (eds. Lusser M, Parisi C, Plan D, Rodríguez-Cerezo E) pp 141–155
Gold ER, Castle D, Cloutier LM et al (2002) Needed: models of biotechnology intellectual property. Trends Biotechnol 20:327–329
Graff GD, Cullen SE, Bradford KJ et al (2003) The public – private structure of intellectual property ownership in agricultural biotechnology. Nat Biotechnol 21:989–995
Hajjar R, Hodgkin T (2007) The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica 156:1–13
Halterman D, Guenthner J, Collinge S, Butler N, Douches D (2016) Biotech potatoes in the 21st century: 20 years since the first biotech potato. Am J Potato Res 93:1–20
Hickey LT, Hafeez AN, Robinson H, Jackson SA et al (2019) Breeding crops to feed 10 billion. Nat Biotechnol 37(7):744–754. https://doi.org/10.1038/s41587-019-0152-9
Hilioti Z, Ganopoulos I, Ajith S et al (2016) A novel arrangement of zinc finger nuclease system for in vivo targeted genome engineering: the tomato LEC1-LIKE4 gene case. Plant Cell Rep 35:1–15
Hilton IB, D’Ippolito AM, Vockley CM et al (2015) Epigenome editing by a CRISPR/Cas9- based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 33:510–517
Holme IB, Wendt T, Gil-Humanes J, Deleuran LC, Starker CG, Voytas DF, Brinch-Pedersen H (2017) Evaluation of the mature grain phytase candidate HvPAPhy_a gene in barley (Hordeum vulgare L.) using CRISPR/Cas9 and TALENs. Plant Mol Biol 95:111–121
Horvath P, Barrangou R (2010) CRISPR/Cas, the immune system of bacteria and archaea. Science 327:167–170
Huang X, Sang T, Zhao Q, Feng Q, Zhao Y et al (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet 42:961–967
Husbands AY, Chitwood DH, Plavskin Y, Timmermans MC (2009) Signals and prepatterns: new insights into organ polarity in plants. Genes Dev 23:1986–1997
Ishii T, Araki M (2016) Consumer acceptance of food crops developed by genome editing. Plant Cell Rep 35:1507–1518
Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A (1987) Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol 169:5429–5433
Ito Y, Nishizawa-Yokoi A, Endo M et al (2015) CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem Biophys Res Commun 467:76–82
Jiang W, Zhou H, Bi H et al (2013) Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucl Acids Res 41(20):e188
Jiang WZ, Henry IM, Lynagh PG, Comai L, Cahoon EB, Weeks DP (2016) Significant enhancement of fatty acid composition in seeds of the allohexaploid, Camelina sativa, using CRISPR/Cas9 gene editing. Plant Biotechnol J 15:648–657
Jin J, Huang W, Gao J, Yang J, Shi M, Zhu M, Luo D, Lin H (2008) Genetic control of rice plant architecture under domestication. Nat Genet 40:1365–1369
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Jones HD (2015) Regulatory uncertainty over genome editing. Nat Plants 1:1–3
Jördens R (2005) Progress of plant variety protection based on the international convention for the protection of new varieties of plants (UPOV convention). World Pat Inf 27:232–243
Jung C, Capistrano-Gossmann G, Braatz J, Sashidhar N, Melzer S (2018) Recent developments in genome editing and applications in plant breeding. Plant Breed 137:1–9
Kalaitzandonakes N, Alston JM, Bradford KJ (2007) Compliance costs for regulatory approval of new biotech crops. Nat Biotechnol 25:509–551
Kearns NA, Genga RM, Enuameh MS, Garber M, Wolfe SA, Maehr R (2014) Cas9 effector-mediated regulation of transcription and differentiation in human pluripotent stem cells. Development 141:219–223
Kim H, Kim ST, Kim SG, Kim JS (2015) Targeted genome editing for crop improvement. Plant Breed Biotechnol 3:283–290
Knight SC, Xie L, Deng W, Guglielmi B, Witkowsky LB, Bosanac L, Zhang ET et al (2015) Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science 350:823–826
Kumar A, Kumar R, Singh N, Mansoori A (2020). Regulatory framework and policy decisions for genome-edited crops. In: Concepts and strategies in plant sciences. Springer, Berlin, pp 193–201
Kumar R, Sharma V, Suryavanshi SS, Ramrao DP, Veershetty V et al (2021) Understanding omics driven plant improvement and de-novo crop domestication: some examples. Front Genet https://doi.org/10.3389/fgene.2021.637141
Kuzma J, Kokotovich A (2011) Renegotiating GM crop regulation. EMBO Rep 12:883–888
Lassoued R, Macall DM, Hesseln H, Phillips PW, Smyth SJ (2019) Benefits of genome-edited crops: expert opinion. Transgenic Res 28:247–256
Le Buanec B (2005) Plant genetic resources and freedom to operate. Euphytica 146:1–8
Li T, Liu B, Spalding MH et al (2012) High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat Biotechnol 30:390–392
Li X, Yue W, Zhenqi L, Yan S (2016) Genome editing and breeding technology and domestic and international development trend analysis. Biotechnol Business 4:37–42
Li T, Yang X, Yu Y, Si X, Zhai X et al (2018) Domestication of wild tomato is accelerated by genome editing. Nat Biotechnol 36:1160–1163. https://doi.org/10.1038/nbt.4273
Liang Z, Zhang K, Chen K, Gao C (2014) Targeted mutagenesis in Zea mays using TALENs and the CRISPR/Cas system. J Genet Genomics 41:63–68
Liang Z, Chen K, Li T, Zhang Y, Wang Y, Zhao Q, Liu J, Zhang H, Liu C, Ran Y, Gao C et al (2017) Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat Commun 8:14261
Liu H, Ding Y, Zhou Y, Jin W, Xie K, Chen LL (2017) CRISPR-P 2.0: an improved CRISPR-Cas9 tool for genome editing in plants. Mol Plant 10:530–532
Lor VS, Starker CG, Voytas DF et al (2014) Targeted mutagenesis of the tomato PROCERA gene using transcription activator-like effector nucleases. Plant Physiol 166:1288–1291
Lu BR (2013) Introgression of transgenic crop alleles: its evolutionary impacts on conserving genetic diversity of crop wild relatives. J Syst Evol 51:245–262
Lu C, Kang J (2008) Generation of transgenic plants of a potential oilseed crop Camelina sativa by agrobacterium-mediated transformation. Plant Cell Rep 27:273–278
Lu H, Klocko AL, Dow M et al (2016) Low frequency of zinc-finger nuclease-induced mutagenesis in Populus. Mol Breed 36:121
Luby CH, Goldman IL (2016) Improving freedom to operate in carrot breeding through the development of eight open source composite populations of carrot (Daucus carota L. var. sativus). Sustainability 8:479
Luby CH, Kloppenburg J, Michaels TE, Goldman IL (2015) Enhancing freedom to operate for plant breeders and farmers through open source plant breeding. Crop Sci 55:2481–2488
Lucht JM (2015) Public acceptance of plant biotechnology and GM crops. Viruses 7:4254–4281
Lusser M, Parisi C, Plan D, Rodriguez-Cerezo E (2011) New plant breeding techniques. In: state-of-the-art and prospects for commercial development. Luxembourg: Joint Research Centre-Institute for prospective technological studies, publications Office of the European Union; 2011. EUR 24760 EN, 1–220
Lusser M, Parisi C, Plan D, Rodriguez-Cerezo E (2012) Deployment of new biotechnologies in plant breeding. Nat Biotechnol 30:231–239
Malik KA, Zafar Y (2005) The international union for the protection of new varieties of plants (UPOV) recommendations on variety denominations. Asian Biotechnol Dev Rev 8:7–43
Mao Y, Zhang Z, Feng Z et al (2016) Development of germ-line-specific CRISPR-Cas9 systems to improve the production of heritable gene modifications in Arabidopsis. Plant Biotechnol J 14:519–532
Matsoukas IG (2018) Commentary: RNA editing with CRISPR-Cas13. Front Genet 9:134
Miao J, Guo D, Zhang J et al (2013) Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res 23:1233–1236
Miao C, Xiao L, Hua K, Zou C, Zhao Y, Bressan RA, Zhu JK (2018) Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc Natl Acad Sci U S A 115:6058–6063
Miller JC, Tan S, Qiao G et al (2011) A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 29:143–148
Moscou MJ, Bogdanove AJ (2009) A simple cipher governs DNA recognition by TAL effectors. Science 326:1501
Nagamangala Kanchiswamy C, Sargent DJ, Velasco R et al (2015) Looking forward to genetically edited fruit crops. Trends Biotechnol 33:62–64
Nogué F, Mara K, Collonnier C, Casacuberta JM (2016) Genome engineering and plant breeding: impact on trait discovery and development. Plant Cell Rep 35:1475–1486
Nunes ACS, Kalkmann DC, Aragão FJ (2009) Folate biofortification of lettuce by expression of a codon optimized chicken GTP cyclohydrolase I gene. Transgenic Res 18:661–667
Oczek JP (2000) In the aftermath of the “terminator” technology controversy: intellectual property protections for genetically engineered seeds and the right to save and replant seed. Bost Coll Law Rev 41:627
Osakabe K, Osakabe Y, Toki S (2010) Site-directed mutagenesis in Arabidopsis using custom-designed zinc finger nucleases. Proc Natl Acad Sci U S A 107:12034–12039
Osakabe Y, Watanabe T, Sugano SS et al (2016) Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci Rep 6:26685
Palmgren MG, Edenbrandt AK, Vedel SE et al (2015) Are we ready for back-to-nature crop breeding? Trends Plant Sci 20:155–164
Pan C, Ye L, Qin L et al (2016) CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Sci Rep 6:24765
Parrott W (2018) Outlaws, old laws and no laws: the prospects of gene editing for agriculture in United States. Physiol Plant 164:406–411
Pattanayak V, Ramirez CL, Joung JK, Liu DR (2011) Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat Methods 8:765–770
Peer R, Rivlin G, Golobovitch S et al (2015) Targeted mutagenesis using zinc-finger nucleases in perennial fruit trees. Planta 241:941–951
Peng A, Chen S, Lei T, Xu L, He Y, Wu L, Yao L, Zou X (2017) Engineering canker- resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol J 10:1011–1013
Peterson BA, Haak DC, Nishimura MT et al (2016) Genome-wide assessment of efficiency and specificity in CRISPR/Cas9 mediated multiple site targeting in Arabidopsis. PLoS One 11:e0162169
Petolino JF (2015) Genome editing in plants via designed zinc finger nucleases. Vitr Cell Dev Biol - Plant 51:1–8
Puchta H (2005) The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J Exp Bot 56:1–14
Qi Y, Li X, Zhang Y et al (2013) Targeted deletion and inversion of tandemly arrayed genes in Arabidopsis thaliana using zinc finger nucleases. G3: Genes Genomes Genetics 3:1707–1715
Quétier F (2016) The CRISPR-Cas9 technology: closer to the ultimate toolkit for targeted genome editing. Plant Sci 242:65–76
Ramirez CL, Foley JE, Wright DA, Müller-Lerch F, Rahman SH, Cornu TI et al (2008) Unexpected failure rates for modular assembly of engineered zinc fingers. Nat Methods 5:374–375
Ricroch A, Clairand P, Harwood W (2017) Use of CRISPR systems in plant genome editing: toward new opportunities in agriculture. Emerg Top Life Sci 1(2):169–182
Rodriguez-Leal D, Lemmon ZH, Man J, Bartlett ME, Lippman ZB (2017) Engineering quantitative trait variation for crop improvement by genome editing. Cell 171:470–480
Römer P, Hahn S, Jordan T et al (2007) Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science 318:645–648
Sachs MM (2009) Cereal germplasm resources. Plant Physiol 149:148–151
Sawai S, Ohyama K, Yasumoto S et al (2014) Sterol side chain reductase 2 is a key enzyme in the biosynthesis of cholesterol, the common precursor of toxic steroidal glycoalkaloids in potato. Plant Cell 26:3763–3774
Schaart JG, Krens FA, Wolters AMA, Visser RGF (2010) Transformation methods for obtaining marker-free genetically modified plants. In: Stewart CN, Touraev A, Citovsky V, Tzfira T (eds) Plant transformation technologies. Wiley, Oxford, UK
Schaart JG, van de Wiel CCM, Lotz LAP, Smulders MJM (2016) Opportunities for products of new plant breeding techniques. Trends Plant Sci 21:438–449
Scheben A, Edwards D (2018) Towards a more predictable plant breeding pipeline with CRISPR/Cas-induced allelic series to optimize quantitative and qualitative traits. Curr Opin Plant Biol 45:218–225
Schiemann J, Hartung F, Kühn-institut J (2009) EU perspectives on new plant-breeding techniques. In: New DNA-editing approaches methods, applications and policy for agriculture. National Agricultural Biotechnology Council, New Delhi, India, pp 201–210
Schneider K, Schiermeyer A, Dolls A et al (2016) Targeted gene exchange in plant cells mediated by a zinc finger nuclease double cut. Plant Biotechnol J 14:1151–1160
Shan Q, Wang Y, Li J et al (2013) Targeted geome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol 31:684–686
Shan Q, Wang Y, Li J, Gao C (2014) Genome editing in rice and wheat using the CRISPR/Cassystem. Nat Protoc 9:2395–2410
Shi J, Gao H, Wang H, Lafitte HR, Archibald RL, Yang M, Hakimi SM, Mo H, Habben JE (2017) ARGOS 8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J 15:207–216
Shimatani Z, Kashojiya S, Takayama M, Terada R, Arazoe T, Ishii H, Teramura H, Yamamoto T, Komatsu H, Miura K et al (2017) Targeted base editing in rice and tomato using a CRISPRCas9 cytidine deaminase fusion. Nat Biotechnol 35:441–443
Shin J, Chen J, Solnica-Krezel L (2014) Efficient homologous recombination-mediated genome engineering in zebrafish using TALE nucleases. Development 141:3807–3818
Shmakov S, Abudayyeh OO, Makarova KS et al (2015) Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol Cell 60:385–397
Shukla VK, Doyon Y, Miller JC et al (2009) Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 459:437–441
Smulders MJM, Jouanin AA, Schaart JG, Visser RGF, Cockram J, Leigh F et al (2015) Development of wheat varieties with reduced contents of celiac-immunogenic epitopes through conventional and GM strategies. In: Koehler P (ed) Proceedings of the 28th meeting of the working group on prolamin analysis and toxicity. Verlag Deutsche Forschungsanstalt für Leben-smittelchemie (DFA), Düsseldorf, pp 47–56
Song G, Jia M, Chen K et al (2016) CRISPR/Cas9: a powerful tool for crop genome editing. Crop J 4:75–82
Söllü C, Pars K, Cornu TI, Thibodeau-Beganny S et al (2010) Autonomous zinc-finger nuclease pairs for targeted chromosomal deletion. Nucleic Acids Res 38(22):8269–8276. https://doi.org/10.1093/nar/gkq720
Sprink T, Eriksson D, Schiemann J, Hartung F (2016) Regulatory hurdles for genome editing: process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep 35:1493–1506
Stewart CN, Halfhill MD, Warwick SI (2003) Transgene introgression from genetically modified crops to their wild relatives. Nature 4:806–817
Storozhenko S, De Brouwer V, Volckaert M et al (2007) Folate fortification of rice by metabolic engineering. Nat Biotechnol 25:1277–1279
Sun Y, Zhang X, Wu C et al (2016) Engineering herbicide resistant rice plants through CRISPR/Cas9-mediated homologous recombination of the acetolactate synthase. Plant Physiol 170:1689–1699
Szczepek M, Brondani V, Büchel J et al (2007) Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nat Biotechnol 25:786–793
Tamás C, Kisgyörgy BN, Rakszegi M et al (2009) Transgenic approach to improve wheat (Triticum aestivum L.) nutritional quality. Plant Cell Rep 28:1085–1094
Tan L, Li X, Liu F, Sun X, Li C et al (2008) Control of a key transition from prostate to erect growth in rice domestication. Nat Genet 40:1360–1364
Tanaka Y (2012) Attitude gaps between conventional plant breeding crops and genetically modified crops, and psychological models determining the acceptance of the two crops. J Risk Res 16:69–80
Tomlinson L, Yang Y, Emenecker R, Smoker M, Taylor J, Perkins S, Smith J, MacLean D, Olszewski NE, Jones JDG (2019) Using CRISPR/Cas9 genome editing in tomato to create a gibberellin-responsive dominant dwarf DELLA allele. Plant Biotechnol J 17:132–140
Townsend J, Wright D, Winfrey RJ et al (2009) High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature 459:442–445
Tsai SQ, Zheng Z, Nguyen NT et al (2015) GUIDE-Seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol 33:187–197
Upadhyay SK, Kumar J, Alok A, Tuli R (2013) RNA guided genome editing for target gene mutations in wheat. G3 3:2233–2238
Van Tuinen A, Peters AHLJ, Kendrick RE et al (1999) Characterisation of the procera mutant of tomato and the interaction of gibberellins with end-of-day far-red light treatments. Physiol Plant 106:121–128
Varshney RK, Nayak SN, May GD, Jackson SA (2009) Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol 27:522–530
Varshney RK, Bansal KC, Aggarwal PK et al (2011) Agricultural biotechnology for crop improvement in a variable climate: Hope or hype? Trends Plant Sci 16:363–371
Varshney RK, Singh VK, Kumar A, Powell W, Sorrells ME (2018) Can genomics deliver climate-change ready crops? Curr Opin Plant Biol 45:205–211
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16:123–132
von Wettberg EJ, Chang PL, Başdemir F, Carrasquila-Garcia N, Korbu LB et al (2018) Ecology and genomics of an important crop wild relative as a prelude to agricultural innovation. Nat Commun 9:649
Waltz E (2018) With a free pass, CRISPR-edited plants reach market in record time. Nat Biotechnol 36:6–7
Wang Y, Cheng X, Shan Q et al (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 32:947–951
Wang M, Lu Y, Botella J, Mao Y, Hua K, Zhu JK (2017) Gene targeting by homology- directed repair in Rice using a Geminivirus-based CRISPR/Cas9 system. Mol Plant 10:1007–1010
Warschefsky E, Varma Penmetsa R, Cook DR, Von Wettberg EJB (2014) Back to the wilds: tapping evolutionary adaptations for resilient crops through systematic hybridization with crop wild relatives. Am J Bot 101:1791–1800
Wendt T, Holm PB, Starker CG et al (2013) TAL effector nucleases induce mutations at a pre-selected location in the genome of primary barley transformants. Plant Mol Biol 83:279–285
Wittkopp PJ, Kalay G (2012) Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nat Rev Genet 13:59–69
Wolt JD, Wang K, Sashital D, Lawrence-Dill CJ (2016) Achieving plant CRISPR targeting that limits off-target effects. Plant Genome 9(3):1–8. https://doi.org/10.3835/plantgenome2016.05.0047
Wright DA, Townsend JA, Winfrey RJ Jr, Irwin PA, Rajagopal J et al (2005) High-frequency homologous recombination in plants mediated by zinc-finger nucleases. The Plant J 44:693–705
Wyman C, Kanaar R (2006) DNA double-strand break repair: all's well that ends well. Annu Rev Genet 40:363–383
Xiong J, Ding J, Li Y (2015) Genome-editing technologies and their potential application in horticultural crop breeding. Horticu Res 2:15019
Zaidi SS, Mansoor S, Ali Z, Tashkandi M, Mahfouz MM (2016) Engineering plants for geminivirus resistance with CRISPR/Cas9 system. Trends Plant Sci 21:279–281
Zamir D (2001) Improving plant breeding with exotic genetic libraries. Nat Rev Genet 2:983–989
Zanga D, Capell T, Zhu C et al (2016) Freedom-to-operate analysis of a transgenic multivitamin corn variety. Plant Biotechnol J 14:1225–1240
Zentella R, Zhang Z-L, Park M et al (2007) Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19:3037–3057
Zetsche B, Gootenberg JS, Abudayyeh OO et al (2015) Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163:759–771
Zhang F, Maeder ML, Unger-Wallace E et al (2010) High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases. Proc Natl Acad Sci U S A 107:12028–12033
Zhang Y, Shan Q, Wang Y et al (2013) Rapid and efficient gene modification in rice and brachypodium using TALENs. Mol Plant 6:1365–1368
Zhang H, Zhang J, Wei P et al (2014) The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol J 12:797–807
Zhou H, Liu B, Weeks DP, Spalding MH, Yang B (2014). Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acid Res 42:10903–10914. https://doi.org/10.1093/nar/gku806
Zong Y, Wang YP, Li C, Zhang R, Chen KL, Ran YD, Qiu JL, Wang DW, Gao CX (2017) Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat Biotechnol 35:438–440
Zu Y, Tong X, Wang Z et al (2013) TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat Methods 10:329–331
Acknowledgement
RK acknowledges Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India for the financial support. A.K sincerely thank University Grants Commission (UGC) start-up grant (No.F.30-392/2017 (BSR) and Madhya Pradesh Council of Science and Technology (Endt. No. 3879/CST/R&D/BioSci/2018) for the funding to the laboratory.
Authors Contribution
RK, NRN and AK conceived the manuscript. AM and TKT gave the technical support during the MS preparation. All authors read and approve the final MS.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kumar, R., Nizampatnam, N.R., Alam, M., Thakur, T.K., Kumar, A. (2021). Genome Editing Technologies for Plant Improvement: Advances, Applications and Challenges. In: Kumar, A., Kumar, R., Shukla, P., Pandey, M.K. (eds) Omics Technologies for Sustainable Agriculture and Global Food Security Volume 1. Springer, Singapore. https://doi.org/10.1007/978-981-16-0831-5_10
Download citation
DOI: https://doi.org/10.1007/978-981-16-0831-5_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0830-8
Online ISBN: 978-981-16-0831-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)