Abstract
The conditions for efficient tuber production from suspension cultures of Pinellia ternata cells which is one of medicinal herbs were established and succinic acid in tubers propagated in vitro was determined. Leaf explants formed white nodular structures and off-white calluses at a frequency of 90.6 % when cultured on Murashige and Skoog medium supplemented with 0.54 μM α-naphthaleneacetic acid (NAA); however, this frequency declined substantially with increasing NAA concentration, up to 16.2 μM, at which the frequency reached zero percent. In combination treatments with 4.44 μM 6-benzyladenine (BA) and NAA, however, the frequency of white nodular structure and off-white callus formation from leaf explants did not decrease, even at 16.2 μM NAA. Suspension cultures of P. ternata cells were established from leaf-derived off-white calluses in MS liquid medium containing 5.4 μM NAA and 4.44 μM BA. Upon plating onto MS basal medium, over 90 % of cell aggregates gave rise to microtubers and developed into plantlets. Regenerated plantlets were transplanted in potting soil and grown to maturity in a growth chamber, with a survival rate of >90 %. The highest succinic acid content in suspension culture-derived microtubers was 45 g/kg of extract. Compared with wild P. ternata medicinal tubers, the succinic acid content was very similar. The in vitro P. ternata microtuber proliferation system established in this study is thus an efficient alternative for the mass production of medicinal tubers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The medicinal herb Pinellia ternata is a toxic plant belonging to the Araceae family of flowering plants and grows as an invasive weed in parts of Europe and North America. The genus Pinellia consists of a few species in eastern Asia (Kitamura et al. 1980) that can be propagated by tubers and bulbils. The medicinal part of the plant is its root, or tuber, the outer surface of which is smooth and has a whitish or yellowish color. Although the chemical composition of Pinellia tubers has not been fully elucidated, several kinds of alkaloids occurring in small quantities have been identified, including ephedrine.
The chemical composition of the essential oil from Pinellia tubers was recently elucidated using capillary gas chromatography (GC) and GC-mass spectrometry. A total of 114 compounds has been identified in the oil obtained from Pinellia tubers, and β-cubebene was found to be the primary component (Iwasa et al. 2014). Pharmacologic studies revealed that Pinellia tuber extracts exhibit cytotoxic, antitumor, antiemetic, insecticidal, antitussive, antimicrobial, and anticonvulsant activities. Most of the biological activities and cytotoxicity can be attributed to various alkaloids and lectins (Ji et al. 2014). Pinellic acid, found in the tubers of P. ternata, is an effective oral adjuvant for nasal influenza vaccine (Nagai et al. 2002). Moreover, a glycoprotein fraction of Pinellia tubers reportedly exhibits notable antiemetic effects (Kurata and Tai 1998). In traditional Chinese medicine, P. ternata is considered effective for reducing obesity but is primarily used for treating indigestion, nausea, vomiting, gastritis, and ulcers (Lee and Cho 1987; Bown 1995). However, high doses of Pinellia extract have been shown to enhance thermogenesis and fatty acid oxidation in Zucker rats (Kim et al. 2006). Recently, Ji et al. (2014) reported that the components of some Pinellia species exhibit significant reproductive toxicity, mucosal irritation, and hepatotoxicity.
Plants are major sources of important therapeutic compounds used in treating human diseases. As understanding of the toxicity and adverse health effects associated with the indiscriminate use of antibiotics and synthetic drugs has increased, interest in medicinal plants and plant-based natural drugs has also increased. However, the potential pharmacologic value of many medicinal plants has yet to be investigated. The Asian pharmaceutical industry depends heavily on the supply of raw plants containing pharmacologically active components. However, the lack of proper cultivation practices, the absence of quality control standards for medicinal plants, and the illegal and indiscriminate overuse of agrochemicals during cultivation processes threaten the stability and safety of medicinal plant supplies. Therefore, the introduction of advanced biotechnological methods such as culturing of plant cells and tissues and scale-up of cell culture bioreactors could expand the potential for mass propagation of valuable medicinal plants (Kim et al. 2013). Furthermore, tissue culture techniques could be used for the clonal propagation of elite lines as a quality control application. Despite the medicinal importance of Pinellia tubers, only a few in vitro studies have thus far been reported, including investigations of protoplast culture and plant regeneration in P. ternata (He et al. 1996), callus induction and plant regeneration from tubers (Xu et al. 2005), the formation of microtubers and plantlet regeneration in P. ternata treated with thidiazuron (Yoo and Lim 1997) or different concentrations of auxin (Wang et al. 2009), and clonal propagation of P. ternata via bulbils (Chen et al. 1989; Tsay et al. 1989).
The demand for Pinellia tubers in the medicinal herb market continues to increase. Simultaneously, concerns regarding the potential presence of agrochemicals in dried medicinal herbs are also increasing. However, industrialization and viral diseases have had an adverse impact on the stability of tuber yield and quality. Thus, a safer and more stable supply of raw medicinal plants is needed to meet market demands. To address this need, in this study, we established a plant tissue culture-based system for the propagation of P. ternata microtubers. We then quantitatively analyzed the content of succinic acid which is used as marker chemical for standardization of P. ternata tuber quality in in vitro-generated and field-grown tubers.
Materials and methods
Plant materials
Mature P. ternata (Thunb.) Breit grown in the field in Chungcheongnamdo Province in Korea were collected. Leaf and petiole explants were surface-sterilized by immersion in 70 % (v/v) ethanol for 1 min followed by 0.4 % (v/v) sodium hypochlorite solution for 20 min, with occasional agitation. The explants were then rinsed four times with sterile distilled water. Leaves (approximately 5 × 5 mm2) and petioles (approximately 2–3 mm in length) were dissected with a forceps and scalpel. Leaf and petiole explants were placed onto culture medium in a plastic Petri dish (90 × 15 mm).
Callus formation
To establish an efficient system for P. ternata micropropagation, we first evaluated the effects of various concentrations and combinations of α-naphthaleneacetic acid (NAA) and 6-benzyladenine (BA) on callus and tuber formation. Leaf and petiole explants were placed onto Murashige and Skoog (MS) medium (Murashige and Skoog 1962) containing various concentrations of NAA (0, 0.54, 1.62, 5.4, and 16.2 μM). Leaf and petiole explants were also placed onto MS medium containing 4.44 μM BA and various concentrations of NAA (0, 0.54, 1.62, 5.4, and 16.2 μM). Each experiment consisted of 10 explants per dish, with three replicates. The culture medium used throughout the experiments consisted of MS inorganic salts, 100 mg l−1 myo-inositol, 0.4 mg l−1 thiamine-HCl, 3 % (w/v) sucrose, and 0.4 % (w/v) Gelrite. The pH of all media was adjusted to 5.8 before autoclaving. Aliquots of the medium (25 ml) were dispensed into plastic Petri dishes (90 × 15 mm). Unless otherwise indicated, all cultures were incubated at 25 °C in the dark. After 4 weeks of culture, the mean percentage of explants producing white nodular structures and off-white compact calluses was determined for each treatment.
Establishment of cell suspension culture
To establish a cell suspension culture, the initial off-white compact calluses were transferred into MS liquid medium supplemented with 5.4 μM NAA and 4.44 μM BA (MS1N1B). The initial calluses maintained on culture medium containing BA were not suitable for cell suspension culture because they became rigid. To initiate the cell suspension culture, approximately 1 g of calluses maintained on MS1N1B solid medium were carefully disrupted with sterile forceps and transferred to a 250 ml Erlenmeyer flask containing 10 ml of liquid MS1N1B medium. The culture was then incubated on a gyratory shaker (100 rpm) at 25 °C in the dark. After 2 weeks of culture, 20 ml of liquid MS1N1B was added. After 4 weeks of culture, 5 ml of the cell suspension was transferred to a 250 ml Erlenmeyer flask containing 50 ml of liquid MS1N1B medium. Thereafter, cell suspension cultures were subcultured at 4-week intervals.
Plant regeneration from cell suspension cultures
To regenerate whole plants from the suspension cultures, aggregates of cells (1–2 mm in size) were collected from 2-week-old cell suspension cultures using a stainless steel mesh (pore size: 1 mm) and then rinsed with liquid MS basal medium. A total of 20 cell aggregates was plated onto solid MS basal medium in each Petri dish. After 4 weeks of culture under 30 μmol m−2 s−1 irradiation from cool-white fluorescent lamps, with a 16 h photoperiod, the regeneration frequency was determined by counting the number of plantlets regenerated in 5 Petri dishes. Plantlets that developed from microtubers were rooted well without any additional treatments. Rooted plantlets were transplanted to potting soil and maintained in a growth chamber (25 °C day/22 °C night; 70 μmol m−2 s−1 irradiation from cool-white fluorescent lamps, with a 16 h photoperiod).
Quantitative HPLC analysis of succinic acid in in vitro-generated and field-grown P. ternata microtubers
Microtubers derived from P. ternata cell suspension cultures were collected, washed with distilled water, freeze dried, and stored at −70 °C. Some of field-grown P. ternata microtubers were purchased at a medicinal herb market and the others were supplied form Korea Institute of Oriental Medicine (KIOM). Field- and in vitro-generated microtubers were disrupted using a laboratory grinder. The resulting Pinellia tuber powders (2 g) were placed in separate 50 ml Falcon tubes, to which 15 ml of 100 % methanol was added. The tubes were capped and mixed for 1 h at 25 °C in a rotary shaker operated at 200 rpm. The extracts were then filtered (0.45 μm syringe filter) and dried under a stream of nitrogen. The dried extracts were re-dissolved in methanol (10 mg extract/mL methanol) before HPLC analysis. Samples of each extract (20 μl) were analyzed on an Agilent 1200 series HPLC (Agilent Technologies Inc., Santa Clara, CA, USA). The HPLC conditions were as follows: Agilent Hi-Plex H column (300 × 7.7 mm packed); 20 μl injection volume; 0.5 ml/min flow rate with quaternary pumps; column oven temperature of 65 °C; variable wavelength detector (VWD) operated at 210 nm; isocratic elution with solvent A (trifluoroacetic acid in water, pH 2.5). Succinic acid standard was purchased from Sigma Chemical Co. (USA) and used to prepare calibration curves. Succinic acid in samples was identified by comparison of the chromatographic retention time with that of the authentic standard, and the concentration of succinic acid was calculated by extrapolation from a standard curve of peak area versus concentration.
Results and discussion
Effect of NAA and BA on formation of microtubers from P. ternata leaf and petiole explants
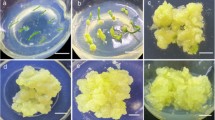
After 1 week of culture, the cut edge of leaf explants began to expand (Fig. 1a). After 2 weeks of culture, white globular structures and off-white calluses began to form along the cut edge of the leaf explants (Fig. 1b). After 4 weeks of culture, the number of white globular structures and off-white compact calluses formed on the cut edge of the leaf explants increased substantially (Fig. 1c). Similar structural responses were observed with petiole explants, such as the simultaneous formation of numerous white globular structures and off-white compact calluses on the explants after 4 weeks of culture (Fig. 1d–f).
Formation of white nodular structures and off-white compact calluses from P. ternata leaf (a–c) and petiole (d–f) explants. a Leaf explant after 1 week of culture; b initial formation of white nodular structures and off-white compact calluses from leaf explant cultured on MS medium containing 5.4 μM NAA and 4.44 μM BA; c formation of multiple white nodular structures and off-white compact calluses from leaf explant after 4 weeks of culture; d petiole explant after 1 week of culture; e initial formation of white nodular structures and off-white compact calluses from petiole explant cultured on MS medium containing 5.4 μM NAA and 4.44 μM BA; f formation of multiple white nodular structures and off-white compact calluses from petiole explant after 4 weeks of culture. Scale bars 1 mm
When transferred to solid MS basal medium, the white globular structures from leaf and petiole explants developed into plantlets after 4 weeks of culture, indicating that the globular structures were an early stage of microtubers. The off-white compact calluses were subcultured on the same medium in order to assess their embryogenic potential. After 4 weeks of culture, white globular structures formed on the surface of proliferating calluses, indicating that the off-white compact calluses also had the potential to form microtubers.
The effects of various concentrations of NAA and BA on the formation of white nodular structures and off-white compact calluses from P. ternata leaf and petiole explants are summarized in Fig. 2. The highest formation frequency of these structures was 90.6 % when leaf explants were cultured on MS medium supplemented with 0.54 μM NAA (Fig. 2a). The frequency decreased to zero percent when NAA at a concentration of 16.2 μM was added as the sole growth regulator. These results indicate that high concentrations of NAA inhibit the formation of calluses on P. ternata leaf explants. However, a very different pattern of callus formation from leaf explants was observed in combined treatment with 4.44 μM BA and various concentrations of NAA. The frequency of callus formation was 61.3 % in leaf explants cultured in the presence of 4.44 μM BA alone. In contrast to treatment with NAA alone, the frequency of formation of white nodular structures and off-white compact calluses from leaf explants was not affected when the explants were cultured in the presence of both BA and NAA, even at an NAA concentration of 16.2 μM (Fig. 2a). These results clearly show that the cytokinin BA plays a primary role in the formation of the white nodular structures and off-white compact calluses from P. ternata leaf explants.
Frequency of white nodular structure and off-white compact callus formation from P. ternata leaf (q) and petiole (b) explants. Symbols indicate treatment with NAA only (filled circle) and combination treatment with NAA and 4.44 μM BA (filled square). Each experiment consisted of 10 explants with three replicates. Vertical bars standard deviation
The effects of NAA and BA on the formation of white nodular structures and off-white compact calluses from petiole explants were similar to those observed with leaf explants (Fig. 2b). The highest frequency of white nodular structure and off-white compact callus formation was 88.9 % when the petiole explants were cultured on MS medium supplemented with 0.54–1.62 μM NAA (Fig. 2b). The frequency decreased to 38.9 % when 16.2 μM NAA was used as the sole growth regulator. However, the frequency of white nodular structure and off-white compact callus formation from petiole explants was >95 % with combined treatment with 4.44 μM BA and NAA, regardless of NAA concentration (Fig. 2b).
The frequency of white nodular structure and off-white compact callus formation from petiole explants was slightly higher than that observed with leaf explants. These results were similar to those of Tsay et al. (1989), who reported no significant difference with regard to bulbil formation with leaves and petioles serving as the original explants. Recently, Liu et al. (2010a) reported that treatment with a combination of 0.2 mg/L NAA and 1.0 mg/L BA is optimal for inducing the formation of undifferentiated protocorm-like bodies (PLBs) from P. ternata leaf and petiole explants. Similar to these reports, we found that the frequency of white nodular structure and off-white compact callus formation was significantly higher when explants were treated with both NAA and BA compared with only NAA. Wang et al. (2009) also reported that treatment with BA alone effectively induces tuber formation from P. ternata petioles and leaves. Considering these results, we hypothesize that cytokinins are the primary effectors of white nodular structure and off-white compact callus formation from P. ternata leaf and petiole explants, whereas auxins play a secondary role in this process. Furthermore, in the absence of BA, concentrations of NAA >5.4 μM did not further enhance tuber formation.
Plant regeneration and tuber production from cell suspension cultures
An efficient system for regenerating plants from P. ternata cell suspension cultures was established, as shown in Fig. 3. Leaf-derived off-white calluses were transferred into liquid MS1N1B medium to establish P. ternata cell suspension cultures (Fig. 3a). After the second subculture in the same medium, a stable cell suspension culture was established (Fig. 3b). The suspension cultures were primarily composed of round, isodiametric cells containing a prominent nucleus and dense cytoplasm. When transferred to MS basal solid medium, over 80 % of 2-week-old cell aggregates (over 1 mm in diameter) developed into microtubers after 4 additional weeks of culture (Fig. 3c). Single, slender roots developed from these microtubers without any root induction treatment. In addition, green shoots developed when the root-bearing microtubers were transplanted and exposed to light (Fig. 3d). Upon further incubation in the presence of light, numerous roots developed at the base of the green shoots that had developed on the microtubers (Fig. 3e). The root-bearing green tubers developed into plantlets at a frequency of approximately 90 %. Over 90 % of the plantlets transplanted to potting soil survived and grew to maturity (Fig. 3f).
Efficient plant regeneration from P. ternata cell suspension cultures. a Off-white compact calluses from leaf explant cultured on MS medium containing 5.4 μM NAA and 4.44 μM BA; b establishment of cell suspension cultures from off-white compact calluses; c development of microtubers from cell suspension cultures; d development of green shoots from microtubers; e root formation from regenerated plantlets; f transfer of mature plants regenerated from P. ternata microtubers to soil. Scale bars 1 cm
In most studies of Pinellia species published to date, in vitro propagation was achieved through microtuber formation (Chen et al. 1989; Tsay et al. 1989; Yoo and Lim 1997; Wang et al. 2009) or somatic embryogenesis (Kim et al. 2005). In this study, we established an efficient system for regenerating P. ternata plants from cell suspension cultures. We also confirmed that microtubers could be produced from P. ternata cell suspension cultures. Therefore, the production of leaf- and petiole-derived tuber-forming calluses as described in this study could be used to regenerate P. ternata plants for the mass propagation of medicinal tubers.
Quantitative analysis of succinic acid in in vitro: generated and field-grown P. ternata tubers
As a first step in establishing an analytical method for the determination of standard compounds in P. ternata medicinal tubers, we first compared in vitro-generated and field-grown tubers with respect to morphologic variations. The morphology of microtubers derived from cell suspension cultures was similar to that of field-grown P. ternata tubers (Fig. 4). Recently, quantitative method using high performance liquid chromatography with a photodiode array detector (HPLC–PDA) was established for the quantitative analysis of the four main compounds for classification of Pinellia ternata (Jo et al. 2013). Although numerous studies examining the pharmaceutical applications of Pinellia tubers have been published, the standard and effective compounds found in Pinellia tubers have yet to be fully characterized. We therefore established a method for the quantitative analysis of succinic acid, which is known as a marker chemical for the standardization of Pinellia tuber quality in Chinese pharmacopoeia. As shown in Fig. 5a, succinic acid could be extracted from the tubers and detected using an HPLC and monitoring of the eluate at 210 nm using a VWD. The concentration of succinic acid in each Pinellia tuber sample was determined based on peak area using linear regression curves prepared from dilutions of pure succinic acid standard (Fig. 5b). Most field-grown Pinellia tubers showed a similar chromatographic pattern, regardless of cultivation origin (Fig. 5c). However, the succinic acid content of the Korea-17 Pinellia tuber sample was higher than that of the other samples, at 85.2 g/kg of extract (Table 1), a level approximately 10-times higher than that of Pinellia tuber samples obtained from China. The average succinic acid content in the samples of tubers from Korea was 34.5 ± 29.7 g/kg of extract. However, there was significant variation in the succinic acid content of the Pinellia tuber samples from Korea. We inferred from these data that the quality of medicinal herbs may be impacted by a number of agronomic and marketing practices, including cultivation origin, cultivation method, storage duration, and storage conditions. The significant variation we observed in the succinic acid content of Pinellia tuber samples, regardless of cultivation origin, highlights the need for quality standardization in the medicinal herb market.
Quantitative analysis of succinic acid in in vitro–generated and field-grown P. ternata tubers. a HPLC chromatogram of pure succinic acid standard; b linear regression analysis of peak area versus concentration of pure succinic acid standard solutions; c comparison of HPLC chromatograms of field-grown tubers and microtubers generated from P. ternata cell suspension cultures
Table 1 shows the succinic acid content in samples of callus, plantlet, and microtubers derived from cell suspension cultures. No succinic acid was detected in regenerated plants. However, calluses, which are capable of forming microtubers, contained succinic acid at levels similar to field-grown Pinellia tubers. Similarly, three different microtuber lines derived from cell suspension cultures contained succinic acid at levels similar to field-grown Pinellia tubers. Microtuber line-2 contained the highest level of succinic acid (45 g/kg of extract) (Table 1). These results suggest that microtubers produced from cell suspension cultures are metabolically equivalent to field-grown P. ternata tubers (at least with respect to standard compounds).
Tests with mice indicated that the acute toxicity of crude extracts of microtubers derived from tissue culture is about one-fourth that of extracts of tubers collected from field-grown plants (Tsay et al. 1989), suggesting that tissue culture could be employed for the clonal propagation of P. ternata plants for commercial use. Recently, Liu et al. (2010a, b) compared the content of major alkaloids in cell culture—derived microtubers and field-grown P. ternata tubers. The concentration of guanosine was tenfold higher in tuber-derived microtubers than field-grown tubers. In contrast, the concentration of inosine was slightly lower in PLBs than field-grown tubers (Liu et al. 2010a, b) reported that the alkaloid content of microtubers is 4.5-times higher than that of field-grown tubers. Our results suggest that microtubers generated from P. ternata cell suspension cultures are metabolically equivalent to field-grown tubers, at least with respect to standard compounds. Therefore, we conclude that the method we established in this study for the mass production of microtubers from cell suspension cultures could serve as an alternative means for producing Pinellia tubers. Furthermore, the method we describe for the quantitative analysis of succinic acid in Pinellia tubers is suitable for use as a quality evaluation tool.
Abbreviations
- BA:
-
6-Benzyladenine
- HPLC:
-
High performance liquid chromatography
- MS:
-
Murashige and Skoog
- NAA:
-
α-Naphthaleneacetic acid
References
Bown D (1995) Encyclopaedia of herbs and their uses. Dorling Kindersley, London
Chen CC, Gau TG, Tsay HS (1989) Studies on the callus formation and plant regeneration of Pinellia ternata. J Agric Res China 38:30–41
He Y, Zhu C, He M, Hao S (1996) Protoplast culture and plant regeneration of Pinellia ternata. Plant Cell Rep 16:92–96
Iwasa M, Iwasaki T, Ono T, Miyazawa M (2014) Chemical composition and major odor-active compounds of essential oil from Pinellia tuber (dried rhizome of Pinellia ternata) as crude drug. J Oleo Sci 63:127–135
Ji X, Huang B, Wang G, Zhang C (2014) The ethnobotanical, phytochemical and pharmacological profile of the genus Pinellia. Fitoterapia 93:1–17
Jo JE, Lee AY, Kim HS, Moon BC, Choi G, Ji Y, Kim HK (2013) Content comparative analysis and classification for Piniellia ternate, P. pedatisecta and Typhonium flagelliforme by HPLC-PDA analysis. Kor J Herbol 28:95–101
Kim SW, In DS, Tae KH, Liu JR (2005) High frequency plant regeneration from leaf-derived cell suspension cultures of Pinellia tripartita (Blume) Schott. Plant Cell Tiss Organ Cult 80:267–270
Kim YJ, Shin YO, Ha YW, Lee S, Oh JK, Kim YS (2006) Anti-obesity effect of Pinellia ternata extract in Zucker rats. Biol Pharm Bull 29:1278–1281
Kim JA, Moon HK, Choi YE (2013) Microtuber formation from in vitro Codonopsis lanceolata plantlets by sugar. J Plant Biotechnol 40:147–155
Kitamura S, Murata G, Koyama T (1980) Colored illustrations of herbaceous plants of Japan (Monocotyledon). Hoikusa Pub Co., Tokyo, p 196
Kurata K, Tai T (1998) Quantitative analysis of anti-emetic principle in the tubers of Pinellia ternata by enzyme immunoassay. Plant Med 64:645–648
Lee BK, Cho TS (1987) Experimental studies on pharmalogical action of Banhahubagtang, a combined preparation of oriental medicine. Kor J Pharmacogn 18:14–25
Liu Y, Liang Z, Liu J (2010a) Use of protocorm-like bodies for studying alkaloid metabolism in Pinellia ternata. Plant Cell Tiss Organ Cult 100:83–89
Liu Y, Liang Z, Zhang Y (2010b) Induction and in vitro alkaloid yield of calluses and protocorm-like bodies (PLBs) from Pinellia ternata. Alkaloid yield of in vitro tissues from Pinellia ternata. Vitro Cell Dev Biol Plant 46:239–245
Murashige T, Skoog F (1962) A reversed medium for rapid growth and bioassays with tobacco culture. Physiol Plant 15:473–497
Nagai T, Kiyohara H, Munakata K, Shirahata T, Sunazuka T, Harigaya Y, Yamada H (2002) Pinellic acid from the tuber of Pinellia ternata Breitenbach as an effective oral adjuvant for nasal influenza vaccine. Int Immunopharmacol 2:1183–1193
Tsay HS, Gau TG, Chen CC (1989) Rapid clonal propagation of Pinellia ternata by tissue culture. Plant Cell Rep 8:450–454
Wang J, Wang Q, Wang J, Lu Y, Xiao X, Gong W, Liu J (2009) Effect of different plant growth regulators on micro-tuber induction and plant regeneration of Pinellia ternate (Thunb) Briet. Physiol Mol Biol Plant 15:359–365
Xu T, Zhang L, Sun X, Tang K (2005) Efficient in vitro plant regeneration of Pinellia ternata (Thunb) Breit. Acta Biol Crac Ser Bot 47:27–32
Yoo CY, Lim HT (1997) Effect of thidiazuron on the formation of micro-tubers and plantlet regeneration of Pinellia ternata. Korean J Med Crop Sci 5:21–27
Acknowledgments
This work was supported by a grant from the KRIBB Research Initiative Program and by a grant (K14418) to SWK from the KIOM. We also greatly appreciate the supply of Pinellia tubers from the KIOM.
Author information
Authors and Affiliations
Corresponding authors
Additional information
E. Y. Jie and Y. B. Ryu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jie, E.Y., Ryu, Y.B., Choi, S.A. et al. Mass propagation of microtubers from suspension cultures of Pinellia ternata cells and quantitative analysis of succinic acid in Pinellia tubers. Plant Biotechnol Rep 9, 331–338 (2015). https://doi.org/10.1007/s11816-015-0369-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-015-0369-0