Abstract
This study investigated the induction and in vitro alkaloid yield of calluses and protocorm-like bodies (PLBs) from Pinellia ternata (Thunb.) Berit (Araceae). We planned to use this material in future studies related to the mass production of medicinally valuable compounds and regulation of alkaloid metabolism. Different combinations of 2,4-dichlorophenoxyacetic acid (2,4-D), 6-benzyladenine (6-BA), kinetin (Kin), and α-naphthaleneacetic acid (NAA) were used to induce callus and PLB formation from P. ternata tuber explants. The results showed that three physiologically distinct calluses were induced by different combinations of 2,4-D, 6-BA, and Kin used in this study. The calluses differed in color, texture, differentiation status, and alkaloid content. The alkaloid content of the three calli types ranged from 0.0175% to 0.0293%. In comparison, the alkaloid content of field-grown tubers was 0.0072%. Many reports have indicated that 2,4-D suppresses the biosynthesis of secondary metabolites; however, our results show that 2,4-D promoted alkaloid production in Pinellia calluses. The combination of NAA + 6-BA induced PLB formation. The PLB alkaloid content of 0.0321% was 1.1 to 1.8 times higher than the alkaloid content of the calluses and 4.5 times higher than the field-grown tubers. In conclusion, the induction of calluses and PLBs with alkaloid content greater than that of field-grown tubers indicates the potential use of these tissue culture materials for bioprocessing alkaloids from P. ternata and for the study of alkaloid metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants synthesize a diverse array of secondary metabolites. These chemicals help plants survive and flourish in the natural environment. Secondary metabolites in plants are a major source of bioactive compounds, some of which may have value as pharmaceuticals and nutraceuticals. Plant tissue culture (including cell and organ culture) can provide useful experimental models for the study of plant secondary metabolism and efficient bioprocesses for the mass production of secondary metabolites, particularly from rare and slow-growing plant species (Lucumi et al. 2001; Magdi et al. 2004).
Pinellia ternata (Thunb.) Berit. (Araceae) is a well-known herbal plant and its tuber, which is known as banxia in Chinese. It has been widely used in Chinese medicine for its antiemetic, analgesic, and sedative effects. Recent studies have shown that P. ternata also has antianxiety, anticancer, and anti-inflammation effects as well as the ability to induce abortion in early pregnancy (Han et al. 2006; Hu et al. 2008). Alkaloids are generally regarded as the main biologically active compounds in P. ternata. Ephedrine, trigonelline, guanoline, and inosine are among the alkaloids isolated from the herb (Zhang et al. 2005).
Chemical methods have been developed for the extraction of alkaloids from P. ternata; however, alkaloid yield from these methods is low (Zeng et al. 2003). Several researchers have investigated clonal propagation of the herb (He et al. 1994, 1996; Xu et al. 2005). Other studies have investigated embryogenesis and organogenesis in P. ternata (Shoyama et al. 1992; He et al. 1996; Suk et al. 2005). However, no studies have investigated the use of tissue-culture-derived P. ternata material to produce alkaloids or to investigate the regulation of alkaloid metabolism.
A notable character of P. ternata is that highly differentiated tissue known as PLBs can be induced with the proper combination of hormones. Shoyama et al. (1992) proposed that these PLBs were an immediate stage between calluses and regenerated plants and may contain secondary metabolites. However, the authors were unable to detect secondary metabolites in either the calluses or PLBs. Tsay et al. (1989) tested the acute toxicity of PLBs, but did not attempt to isolate medicinally useful compounds. They hypothesized that PLBs may have potential value in organ culture for secondary metabolite production because PLBs are highly differentiated and also able to be propagated in a way similar to suspension cultures. Although organ culture has been applied to many plant species, it has not been tried with P. ternata.
Model materials such as calluses or PLBs are needed to investigate the regulation of alkaloid metabolism in P. ternata. The objective of this study was to investigate the induction and in vitro alkaloid yield of calluses and PLBs from P. ternata.
Materials and Methods
Plant material.
Tubers, 0.5–1.5 cm in diameter, were collected in August and September 2007 from P. ternata grown in experimental fields at Northwest Agriculture and Forestry (A&F) University, Yangling, People’s Republic of China. (P. ternata is identified by Vice-Professor Yuejin Zhang. A voucher specimen of the plant is deposited at the Department of Life Science, Northwest A&F University.) The tubers were washed and the epidermis was removed. The epidermis-free tubers were put under running tap water for about 2 h, then surface-sterilized in 75% (v/v) ethanol for 30 min followed by 0.1% (w/v) mercuric chloride solution for 15 min with occasional agitation. The tubers were then rinsed five times with sterile distilled water. The center part of the tubers was sliced longitudinally into approximately 5 × 5 × 2 mm3 explants on axenic filter paper.
Culture medium and culture conditions.
The medium used in this study consisted of MS basic salts (Murashige and Skoog 1962) supplemented with 3% sucrose and 0.7% agar. Plant growth regulators were added to the medium as described in the section below. The pH of the medium was adjusted to 5.8 before autoclaving. Medium (20 mL) was dispensed into each 100 mL Erlenmeyer flask. The cultures were maintained at 25°C with a photoperiod regime of 16 h light (135 μE/m2 s, fluorescent light) and 8 h dark cycle.
Induction of calluses and PLBs.
To examine the effect of different combinations and concentrations of hormones on callus and PLB induction, tuber explants were placed on MS medium supplemented with the following hormone combinations: (a) 0.2 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D) + various concentrations of 6-benzyladenine (6-BA; 0.2, 0.5, 1.0, 1.5, 2.0, and 3.0 mg/L); (b) 0.5 mg/L 2,4-D + various concentrations of kinetin (Kin; 0.2, 0.5, 1.0, 1.5, 2.0, and 3.0 mg/L); (c) 1.0 mg/L Kin + various concentrations of 2,4-D (0.2, 0.5, 1.0, 1.5, 2.0, and 3.0 mg/L); and (d) 1.0 mg/L or 2.0 mg/L 6-BA + various concentrations of α-naphthaleneacetic acid (NAA; 0.2, 0.5, and 1.0 mg/L). Each treatment consisted of 30 explants with three replicates.
The frequency of callus formation was determined after 30 d using the formula:
where:
- F :
-
Frequency of callus formation
- E :
-
Number of explants producing callus
- E 0 :
-
Number of explants inoculated
Pinellia calluses and PLBs growth studies.
Callus induction frequency of nearly 100% was observed from three hormone combinations: 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA, 0.5 mg/L 2,4-D + 1.0 mg/L Kin, and 2.0 mg/L 2,4-D + 1.0 mg/L Kin. The fresh weight of calluses from these three hormone treatments was recorded after 30 d (30 explants per treatment). After weighing, the calluses were placed on fresh growth medium. The calluses were cultured for 20 d under the same conditions as described before and then weighed again. This process was repeated two more times. Callus yield was defined as callus fresh weight per explant on day 30 (30 explants per treatment). The growth rate (GR) of established calluses was determined by measuring the change in callus weight over three 20-d periods according to Gibson et al. (1993)
\( {\hbox{GR}} = \frac{{\left( {W - {W_0}} \right)}}{{{W_0} \times D}} \) where:
- W :
-
final callus weight
- W 0 :
-
initial callus weight
- D :
-
days in culture
Preliminary studies showed that PLB induction was greatest in the 1.0 mg/L 6-BA + 0.5 mg/L NAA treatment. The fresh weight and number of PLBs per explant in this treatment were measured after 50 d (30 explants per treatment).
Histological examination.
After 30 d of culture, callus tissue was transferred onto clear glass slides with forceps and suspended in a drop of water. The tissue was triturated with forceps to separate cells, then covered with a coverslip and examined under a light microscope. After 50 d of culture, the PLBs were embedded on a potato, and freehand sections (20–40 µm) were excised. Freehand sections were also excised from 1- to 2-yr-old field-grown tubers. The sections were transferred onto clear glass slides, suspended in a drop of water, covered with a coverslip, and observed under a light microscope.
Determination of alkaloid content of calluses and PLBs.
The calluses and PLBs were harvested, rinsed with tap water to remove culture medium, and dried in an oven at 60°C. After drying, the calluses and PLBs were ground in a mortar. Alkaloid content was determined using the protocol of Yu et al. (2004) with the following modification. A 0.1000 g sample of dried powder was added to 0.5 mL dense ammonia and 10 mL chloroform. The solution was allowed to equilibrate at room temperature for 3 h, then extracted ultrasonically for 1 h. The solution was passed through a separatory funnel. The solid residue was collected and rinsed 3 times with a total of 10 mL chloroform. The filtrate from the initial extraction and all 3 rinses was combined and the chloroform was removed by boiling under reduced pressure at 80°C. The filtrate was transferred to a separatory funnel with 10 mL chloroform. A 5.0 mL aliquot of citric acid–sodium citrate buffer solution with pH of 5.4 and a 1 mL aliquot of 0.1% bromothymol blue solution was gradually added to the funnel. The solution was shaken for 1 min, then placed on a shelf for 12 h to allow for the separation of the water and chloroform layers. The aqueous layer was removed, then the alkaloid content of the chloroform solution was determined at a wavelength of 414 nm using a UV-visible spectrophotometer. The sample concentration was determined using the standard curve in Eq. 1.
where:
- C :
-
sample concentration
- ABS:
-
absorbance of sample
The alkaloid content was calculated using Eq. 2.
where:
- X :
-
alkaloid content
- W :
-
sample weight (0.1000 g)
- F :
-
dilution factor
Statistical analysis.
All data presented in this paper are the average of three replicates. Statistical analysis was confirmed by Duncan’s multiple range test using SAS 8.0 software for Windows.
Results
Induction of calluses and PLBs.
For the culture medium which contained 0.2 mg/L 2,4-D + varying amounts of 6-BA, no calluses were formed within 30 d at the level of 0.2 mg/L 6-BA (Fig. 1 a). Callus formation frequency gradually increased as the amount of 6-BA increased. Callus formation frequency reached 100% on 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA and then declined. The calluses at all levels of 6-BA were compact and green on top and white on the bottom (Fig. 2 a).
Effect of various phytohormone combinations and concentrations on callus formation from tuber explants of P. ternata. Each treatment consisted of 30 explants with three replicates. Vertical bars represent means ± SD. a 0.2 mg/l 2,4-D + various concentrations of 6-BA (0.2, 0.5, 1.0, 1.5, 2.0, and 3.0 mg/L), b 0.5 mg/L 2,4-D + various concentration of Kin (0.2, 0.5, 1.0, 1.5, 2.0, and 3.0 mg/L), and c 1.0 mg/L Kin + various concentrations of 2,4-D (0.2, 0.5, 1.0, 1.5, 2.0, and 3.0 mg/L).
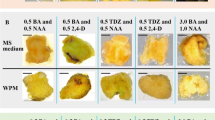
Calluses, PLBs, field-grown tubers of P. ternata, and their microstructures. a, Callus induced on medium containing 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA with a culture period of 30 d. b, Callus induced on medium containing 0.5 mg/L 2,4-D + 1.0 mg/L Kin with a culture period of 30 d. c, Callus induced on medium containing 2.0 mg/L 2,4-D + 1.0 mg/L Kin with a culture period of 30 d. d, PLBs induced on medium containing 1.0 mg/L 6-BA + 0.5 mg/L NAA with a culture period of 50 d. e, 1- to 2-yr-old field-grown tuber. f, Basic histological structure of PLBs. g, basic histological structure of field-grown tubers. h, Microstructure of callus cultured on 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA × 400. i, Microstructure of callus cultured on 2.0 mg/L 2,4-D + 1.0 mg/L Kin × 400 (bars equal 1 cm in all figures).
For the culture medium which contained 0.5 mg/L 2,4-D + varying amounts of Kin, explants began to generate compact, yellow calluses after 2 wk (Fig. 1 b). A floss-filled protuberance appeared on most calluses as culture time increased (Fig. 2 b). Later, this protuberance turned into roots. Nearly all explants produced this type of rooting callus at Kin concentrations between 1.0 and 3.0 mg/L. The number of roots increased as culture time increased, but root growth did not surpass callus growth. Calluses on 0.5 mg/L 2,4-D + 1.0 mg/L Kin were fast growing and 100% of the explants produced calluses within 1 mo.
For the culture medium which contained 1.0 mg/L Kin + varying amounts of 2,4-D, explants turned green, and bud-like dots appeared on their surface when 2,4-D concentrations were between 0.2 and 0.5 mg/L 2,4-D. No calluses were formed, however, until 2,4-D concentrations were ≥1.0 mg/L. At 2,4-D concentrations of 2.0 mg/L, 100% of explants produced calluses with 25 d (Fig. 1 c). Calluses on 1.0 mg/L Kin + 2.0 mg/L 2,4-D were yellow or white and fragile (Fig. 2 c). They grew quickly when subcultured.
For the culture medium which contained 1.0 or 2.0 mg/L 6-BA + various amounts of NAA, explants produced PLBs within 1 to 2 mo (Fig. 2 d). The physiological characteristics of the PLBs varied depending on the hormone concentration. Root number increased as the NAA concentrations increased. Higher concentrations of 6-BA induced the PLBs to generate shoots and grow into plants. As an intermediate stage in the formation of plantlets, PLBs easily generate shoots, but we wanted to preserve the PLBs in their tubercle state so that we could use them in organ culture. In this way, we could use the PLBs to produce secondary metabolites and also study the regulation of alkaloid metabolism. For this purpose, we found that 1.0 mg/L 6-BA + 0.5 mg/L NAA was optimum.
Calluses and PLBs growth studies.
The frequency of callus formation was 100% on the following hormone combinations: (1) 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA; (2) 0.5 mg/L 2,4-D + 1.0 mg/L Kin; and (3) 2.0 mg/L 2,4-D + 1.0 mg/L Kin. A comparison of these three growth media showed that 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA had the highest callus initiation rate (Table 1). Caulogenesis was earliest from explants growing on 2.0 mg/L 2,4-D + 1.0 mg/L Kin; however, callus initiation rate in this treatment was lowest among the three types of growth media. Callus growth rate was highest on growth medium containing 2.0 mg/L 2,4-D + 1.0 mg/L Kin. The growth rate in this treatment was 0.21 ± 0.02 g/d, which was equivalent to a yield of 5.2 g/g inoculated callus/subculture or a 420% increase in fresh weight.
The combination of NAA + 6-BA showed little ability to induce callus formation from tuber explants, but it was able to induce differentiated tissue. On medium containing 0.5 mg/L NAA + 1.0 mg/L 6-BA, three to seven PLBs were produced per explant after 50 d (Table 1). These PLBs remained in their organ stage without generating shoots for a relatively long time.
Alkaloid content of calluses, PLBs, and field-grown tubers.
The alkaloid content of PLBs was highest among all the samples (Table 2). A comparison among different types of calluses showed that calluses grown on 2.0 mg/L 2,4-D + 1.0 mg/L Kin had the greatest alkaloid content. Calluses grown on 0.5 mg/L 2,4-D + 1.0 mg/L Kin had the lowest alkaloid content. The alkaloid content of field-grown tubers was much lower than the alkaloid content of calluses or PLBs.
Microstructures of calluses, PLBs, and field-grown tubers.
The morphology and histology of PLBs were similar to field-grown tubers (Fig. 2 d, e, f, g). The microstructure of calluses grown on 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA was different than the microstructure of calluses grown on 2.0 mg/L 2,4-D + 1.0 mg/L Kin. Calluses on the latter growth medium consisted of loosely organized falciform cells. There were no inclusions observed in cells (Fig. 2 i). In contrast, calluses grown on 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA consisted of rotund cells which were closely packed. Granulose, a kind of inclusion, could be observed in the cells (Fig. 2 h).
Discussion
Dias et al. (2000) pointed out that hormones could be used to increase both the variety and quantity of secondary metabolites in calluses. Xu et al. (1995) also reported that the yield of medicinally useful compounds varied depending on callus color, shape, texture, and degree of differentiation. In this study, we cultured explants on growth medium containing different phytohormone combinations. Our objective was to investigate the induction and in vitro alkaloid yield of calluses and PLBs from P. ternata. We hoped to obtain tissue culture material which could be used in future studies related to the mass production of medicinally valuable compounds.
Three types of calluses were eventually selected in this study. Calluses grown on 2.0 mg/L 2,4-D + 1.0 mg/L Kin were fast growing but not inclined to differentiate. In contrast, we observed bud differentiation on the green portion of calluses grown on 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA and rhizogenesis on calluses grown on 0.5 mg/L 2,4-D + 1.0 mg/L Kin. Our data showed that calluses cultured on 2.0 mg/L 2,4-D + 1.0 mg/L Kin had the highest alkaloid content, while calluses cultured on 0.5 mg/L 2,4-D + 1.0 mg/L Kin had the lowest alkaloid content. From a morphological point of view, this was surprising, since the degree of differentiation was greater for calluses grown on 0.5 mg/L 2,4-D + 1.0 mg/L Kin, compared to calluses grown on 2.0 mg/L 2,4-D + 1.0 mg/L Kin. Since the two types of growth medium contained the same Kin concentration, differences in callus alkaloid content must be related to the 2,4-D concentration in the growth medium. The general viewpoint is that 2,4-D has a negative effect on the synthesis of secondary metabolites (Kutchan 1995; Zhao et al. 2001). However, there are conflicting reports. Rhodes et al. (1994) found that flavone yield increased 30 times in suspension cultures of Morinda citrifolia L. (Rubiaceae) when 2,4-D was substituted for NAA. Xu et al. (1995) reported that increasing the 2,4-D content of the growth medium resulted in a larger increase in the salidroside content of Rhodiola sachalinesis A. Bor calli than increasing the NAA content of the growth medium. The difference in the alkaloid content of calluses in our study also indicated that 2,4-D promoted alkaloid biosynthesis in P. ternata.
A comparison of the morphology and microstructure of the two types of calluses indicated that calluses grown on 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA were more differentiated and their cells were more tightly packed than calluses grown on 2.0 mg/L 2,4-D + 1.0 mg/L Kin. Many reports have indicated that cell aggregation and differentiation at the cellular, tissue, or organ level are essential to the production of secondary metabolites (Holden 1988; Dörnenburg and Seydel 2008). The fact that calluses grown on 0.2 mg/L 2,4-D + 2.0 mg/L 6-BA had a lower alkaloid content than calluses grown on 2.0 mg/L 2,4-D + 1.0 mg/L Kin may also be related to differences in the 2,4-D content, though we cannot confirm this.
Research by other scientists has shown that phytohormones can induce the formation of calluses, large tissue masses, PLBs, and direct seedlings from P. ternata explants. The morphology and histology of the PLBs in this study were similar to field-grown tubers, but the alkaloid content of PLBs was greater than the alkaloid content of either induced calluses or field-grown tubers. He et al. (1994) pointed out that all seedlings go through the PLB stage, regardless of whether they are direct seedlings or seedlings formed from calluses. This conclusion was similar to that of Shoyama et al. (1992) who speculated that PLBs were an intermediate stage between calluses and regenerated plantlets. Our results showed that with the correct combination of phytohormones, we could slow the differentiation process in PLBs and preserve them in their tubercle stage. This could have application in biochemical studies of P. ternata.
In conclusion, the induction of calluses and PLBs with high alkaloid content may present a novel and effective strategy for bioprocessing alkaloids from P. ternata as well as a convenient method for studying alkaloid metabolism. This makes our work different from previous studies which investigated PLBs primarily from the standpoint of plant regeneration. In regard to bioprocessing alkaloids from P. ternata calluses, Niamh et al. (1993) reported that suspension cultures of Ephedra lost the ability to produce alkaloids with successive subcultures. There were also reports that dissociated cells produced little or no secondary metabolites in suspension culture (Rokem and Goldberg 1985; Holden 1988). To investigate this further, we have transferred P. ternata calluses to suspension cultures and are currently working to induce the formation of the critical cell mass required for alkaloid production. In addition, we are testing PLBs from P. ternata explants to determine their feasibility for organ culture. We plan to use both cell suspension culture and organ suspension culture to study the regulation of alkaloid metabolism in the future through methods such as precursor and elicitor feeding.
References
Dias A. C. P.; Seabra R. M.; Andrade P. B. Xanthone biosynthesis and accumulation in calli and suspended cells of Hypericum androsaemum. Plant Sci 150: 93–101; 2000.
Dörnenburg H.; Seydel P. Effect of irradiation intensity on cell growth and kalata B1 accumulation in Oldenlandia affinis cultures. Plant Cell Tissue Organ Cult 92: 93–99; 2008.
Gibson D. M.; Ketchum R. E. B.; Vance N. C.; Christen A. A. Initiation and growth of cells lines of Taxus brevifolia (Pacific yew). Plant Cell Rep 12: 479–482; 1993.
Han M. H.; Yang X. W.; Zhang M.; Zhong G. Y. Phytochemical study of the rhizome of Pinellia ternata and quantification of phenylpropanoids in commercial Pinellia tuber by RP-LC. Chromatographia 64: 647–653; 2006.
He Y. K.; Liu G.; Lu T. G.; Sun J. S. Stem apex culture and quality improvement of tubercles of Pinellia ternata. Acta Bot Sin 36: 39–44; 1994.
He Y. K.; Zhu C. F.; He M. Y.; Hao S. Protoplast culture and plant regeneration of Pinellia ternata. Plant Cell Rep 16: 92–96; 1996.
Holden M. A. Elicitation of cell cultures. In: Robins R. J.; Rhodes M. J. C. (eds) Manipulating Secondary Metabolism in Culture. Cambridge University Press, Cambridge, pp 57–65; 1988.
Hu X. F.; Ying F. X.; He Y. B.; Gao Y. Y. Characterization of Pectobacterium carotovorum subsp. carotovorum causing soft-rot disease on Pinellia ternata in China. Eur J Plant Pathol 120: 305–310; 2008.
Kutchan T. Alkaloid biosynthesis—the basis for metabolic engineering of medicinal plants. Plant Cell 7: 1059–1070; 1995.
Lucumi E.; Luczkiewicz M.; Vera A.; Hallard D.; Heijden R. V. D.; Verpoorte R. Alkaloid formation in cell suspension cultures of Tabernaemontana divaricata after feeding of tryptamine and loganin. Biotechnol Lett 23: 1691–1696; 2001.
Magdi E. S.; Young H. C.; Frederich M.; Sittiruk R.; Robert V. Alkaloid accumulation in Catharanthus roseus cell suspension cultures fed with stemmadenine. Biotechnol Lett 26: 793–798; 2004.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–479; 1962.
Niamh A. O. D.; Patrick G. M. C.; David H. S. R.; Graham W. Callus production, suspension culture and in vitro alkaloid yields of Ephedra. Plant Cell Tissue Organ Cult 34: 149–155; 1993.
Rhodes M. J. C.; Parr A. J.; Giulietti A. Influence of exogenous hormones on the growth and secondary metabolite formation in transformed root culture. Plant Cell Tissue Organ Cult 38: 143–151; 1994.
Rokem J. S.; Goldberg I. Secondary metabolites from plant cell suspension cultures: methods for yield improvement. Adv Biotechnol Process 4: 241–274; 1985.
Shoyama Y.; Nishioka I.; Hatano K. In: Bajaj Y. P. S. (ed) Biotechnology in Agriculture and Forestry 19, High-tech and micropropagation Ш. Springer-Verlag, Berlin, Germany, pp 464–480; 1992.
Suk W. K.; Dong S. I.; Kyoung H. T.; Jang R. L. Somatic embryogenesis and plant regeneration in leaf and petiole explant cultures and cell suspension cultures of Pinellia tripartite. Plant Cell Tissue Organ Cult 80: 267–270; 2005.
Tsay H. S.; Gau T. G.; Chen C. C. Rapid clonal propagation of Pinellia ternata by tissue culture. Plant Cell Rep 8: 450–454; 1989.
Xu J. F.; Zhao Y.; Han A.; Feng P. Induction and culture of calli from Rhodiola sachalinesis A. Bor. Chin J Appl Environ Biol 1: 19–25; 1995.
Xu T. F.; Zhang L.; Sun X. F.; Tang K. X. Efficient in vitro plant regeneration of Pinellia ternata (Thunb) Breit. Acta Biol Crac Ser Bot 2: 27–32; 2005.
Yu C.; Zhang M.; Wang Y.; Luo Q. Determination of alkaloids content of cultivated and wild Pinellia ternata from various areas. Chin Mater Med 29: 583–584; 2004.
Zeng J. H.; Peng Z. S.; Mao Z. C.; Wei S. H. Study on optimum extration conditions of alkaloids from Pinellia ternata. Chin Mater Med 26: 361–363; 2003.
Zhang K. W.; Wu H.; Li W. Determination of inosine and guanosine in Rhizoma Pinellia ternata. Chin J Pharm Anal 25: 487–489; 2005.
Zhao J.; Zhu W. H.; Hu Q. Effects of light and plant growth regulators on the biosynthesis of vindoline and other indole alkaloids in Catharanthus roseus callus cultures. Plant Growth Regul 33: 43–49; 2001.
Acknowledgments
This work was supported by a grant to Prof. Liang from the Knowledge Innovation Project of Chinese Academy of Sciences (no. K2 CX2-XB1-05). We gratefully acknowledge Associate Professor Xu Hong and the technical staff in the Department of Bioscience for their assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: F. A. Krens
Rights and permissions
About this article
Cite this article
Liu, Y., Liang, Z. & Zhang, Y. Induction and in vitro alkaloid yield of calluses and protocorm-like bodies (PLBs) from Pinellia ternata . In Vitro Cell.Dev.Biol.-Plant 46, 239–245 (2010). https://doi.org/10.1007/s11627-009-9268-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-009-9268-9