Abstract
In this paper, we evaluate the influence of various micromixer designs on the mixing efficiency of passive micromixers. We analyze the designs of various passive micromixers to identify the most efficient micromixer. Among them, the toroidal micromixer and 3D zig-zag micromixer demonstrated the highest mixing efficiency. We investigated the key factors influencing mixing in the toroidal and 3D zig-zag micromixer, identifying and confirming optimal designs. Ultimately, when comparing the mixing efficiency of the two micromixers, the 3D zig-zag micromixer achieved full mixing in a very short time of 0.8 ms. Through this research, it is anticipated that a benchmark will be provided for micromixer design in microfluidic devices when manufacturing micromixers of various forms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microfluidic systems play an important role in various application fields. In impacted on industries, such as environmental science, drug delivery, and biochemistry [1,2,3,4,5,6,7,8]. Recently, with the global pandemic of COVID-19, there has been active development of mRNA vaccines. Notably, the utilization of lipid nanoparticles (LNPs) in RNA delivery technology has a crucial role in the application of mRNA vaccines [9,10,11]. Macroscale LNP formation technologies are commonly used for convenience. However, achieving precise control over the size and polydispersity of liposomes poses inherent challenges for these techniques [12]. On the other hand, within microfluidic devices, the formation of LNPs using micromixers efficiently utilizes the surface and structure of microchannels for effective mixing, resulting in LNPs with an exceptional particle-size distribution. This methodology has attracted considerable attention from the biomedical industry and the fundamental biology and chemistry research area fields [13, 14].
In microfluidic systems, the microchannels exhibit a high surface area-to-volume ratio, leading to enhanced mass density and heat transfer and faster reaction rates. Furthermore, the handling of small fluid volumes provides the advantage of reduced reagents and energy consumption [15, 16]. The rapid and uniform mixing of two or more samples within these microchannels is crucially utilized in various applications, including biomedical analysis and synthesis [17].
To investigate the transient events occurring during chemical/biological processes on a microscale or to achieve optimal synthesizing results, rapid mixing within milliseconds before the reaction proceeds is essential [18]. However, the fluid flow in microchannels typically cannot generate turbulent flow and exhibits a laminar flow, resulting in a low Reynolds number (\(Re)\) [19]. \(Re\) is defined as
where \(\rho\) is the density of the fluid, \(V\) is the velocity of the fluid, \(D\) is the characteristic length, and \(\mu\) is the fluid dynamic viscosity.
In microfluidic device, the characteristic length (\(D)\) and the fluid velocity (\(V\)) are relatively small. Consequently, mixing in microfluidic devices occurs through molecular diffusion based on concentration gradients. This leads to an increased mixing time and increases the microchannels length [20]. Therefore, in numerous studies, various designs of micromixers have been developed to enhance the mixing efficiency in a short mixing channel [21]. In general, micromixers can be classified into active and passive, depending on the application of external forces. Active micromixers utilize external energy sources, such as electric force, magnetic force, and acoustic waves, to enhance mixing efficiency [22,23,24,25,26]. In microfluidic system, external energy sources are employed to control fluid flow and facilitate mixing, achieving faster mixing rates and increased efficiency. However, active micromixers, requiring connection to an external energy source, exhibit a relatively intricate structure. The device fabrication process is complex and involves high costs [27]. In contrast, passive micromixers utilize the inherent properties of fluid flow at the microscale for mixing without the need for external energy inputs. This is achieved by extending fluid interfaces with low-pressure drops and short mixing lengths or inducing chaotic flow, attracting significant interest [28,29,30,31,32]. Passive micromixers offer the advantages of simplicity in structure, ease of device fabrication, and independence from external energy sources. These mixers are typically designed in both 2-dimensional (2D) and 3-dimensional (3D) structures.
However, previous studies on passive micromixers focused on the improving mixing efficiency through unique geometric configurations, surface treatment, or the integration of obstacles within the fluidic pathways [33,34,35,36,37]. Thus, a notable gap persists in previous studies, and it is difficult to find a comprehensive comparison of these various designs under standardized conditions to determine the most effective approach for passive mixing in microfluidic devices. This lack of comparative analysis makes it difficult for researchers to select the optimal design for their specific applications, thereby impeding progress in the development and application of microfluidic technologies.
In this paper, we perform a systematic investigation into the mixing efficiency of various micromixers. By employing a consistent set of experimental conditions across all designs tested, this study is to provide a clear and objective comparison of their performance, thereby offering valuable into the most effective design principles for passive mixing in microfluidic device. This study not only contributes to the advancement of microfluidic technology but also aids in the optimization of its applications.
Materials and Methods
Device Fabrication
In this experiment, various micromixers were designed using AutoCAD (Autodesk). Subsequently, the designs were fabricated through photolithography and soft lithography techniques. Through photolithography, we create a master mold with a design pattern on a silicon wafer, allowing for continuous and repeated use in subsequent experiments. To obtain a microfluidic device from master molds, soft lithography technology is applied using polydimethylsiloxane (PDMS). An elastomer (Sylgard 184, Dow Corning) and curing agent were mixed at a ratio of 10:1 (w/w) to make PDMS. After pouring PDMS onto the fabricated master mold, a thermal curing process was conducted on a hot plate at 65 °C for over 4 h. To fabricate the 2-dimensional microfluidic device, the cured PDMS was then separated from the master mold and bonded to a bare PDMS substrate through oxygen plasma treatment. We construct all four sides of the channel using PDMS, so we utilized PDMS to form the bottom part of the microchannel. The PDMS substrate was formed by curing PDMS in a petri dish, and it was subsequently peeled off for use. In contrast, the 3-dimensional microfluidic device is configured by stacking 2D structures to create layers. During the photolithography process, two separate 2D structures are produced. Subsequently, for the fabrication of the 3-dimensional device, PDMS is demolded from each silicon wafer, and after oxygen plasma treatment, the PDMS structures are aligned and bonded at the junctions.
Experiment in Mixing
To conduct comparative experiments, we utilized Alexa Flour 568 and Deionized water. Alexa flour 568 was dissolved in deionized water to create a 1 μM solution. For injecting solutions into the microfluidic device, we used a syringe pump (Harvard Apparatus PHD-2000 Advance Syringe pump) to inject fluid at a rate of 1 mL/min into each inlet. Fluorescence images for mixing efficiency analysis were captured using an optical microscope (Nikon ECLIPSE TE200-U) and a CCD camera (CoolSNAP™ cf Monochrome). We obtained fluorescent images of each microchannel. Furthermore, cross-sectional mixing images were acquired using a confocal microscope (Nikon A1 confocal, Japan) to observe fluid mixing patterns within the microchannels when interacting solutions into the 3D zig-zag micromixer.
Analysis of Mixing Efficiency
The analysis of mixing efficiency aimed to utilize the average fluorescence intensity and standard deviation obtained from analyzing the region of interest (ROI) in each micromixer. To quantitatively assess the mixing efficiency within our passive micromixer designs, we employed a fluorescence-based the equation [38]
\(\overline{{\varvec{I}} }\) represents the average fluorescence intensity measured in the region of interest (ROI), and \({\varvec{\sigma}}\) is the standard deviation value of fluorescence intensity measured in the ROI.
Furthermore, to provide a more accurate representation of mixing performance, we normalize the mixing efficiency using the equation [39]
In this formula, \({M}_{n}\) represents the normalized mixing efficiency, \({M}_{k}\) is the calculated mixing efficiency, \({M}_{max}\) is the maximum observed mixing efficiencies, and \({M}_{min}\) is the minimum observed mixing efficiencies, respectively. By normalizing the mixing efficiency, we effectively scale the values, such that 0 indicates no mixing, and 1 indicates complete mixing, facilitating a clearer comparison between different micromixer designs.
Results and Discussion
Design of Passive Micromixers
In previous studies, various passive micromixers have been proposed. In this study, to determine which design among passive micromixers achieves the most efficient mixing, we designed a total of 5 passive micromixers with different channel geometries (Table 1).
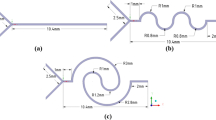
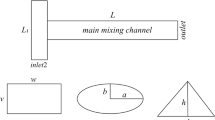
For passive micromixers with a 2D structure, we developed designs for straight, serpentine, herringbone [40], and toroidal [41] micromixers. For the 3D structure, we designed a 3D zig-zag micromixer [42, 43]. Additionally, we compared the Mn values for each micromixer design. The inlet and outlet of each micromixer were designed to be identical. To compare the Mn based on the structure of the micromixer, we designed them with variations only in the mixing region. Figure 1 shows the detailed structure of the designed micromixers. After designing each micromixer, we injected fluid with the same Reynolds number into each micromixer and compared the Mn at the same time.
The schematic drawing of each micromixer. a Straight micromixer. The channel height (H) and width (W) are 100 μm. The total length of the channel is 28 mm. b Serpentine micromixer. Both the height and width are 100 μm. The total length of the channel is 22 mm, and the number of repeat units is 4. c Herringbone micromixer. The channel has a height of 77 μm and a width of 100 μm. In the herringbone structure, height (h) is 23 μm, and the width d is 50 μm. The total length of the channel is 28 mm, and the number of repeat units is 8. d 3D zig-zag micromixer. Both the height and width are 100 μm. The total length of the channel is 23 mm, and the number of repeat units is 7. e Toroidal micromixer. Height (H) is 90 μm and width (W) is 110 μm. The inner circle of the toroidal micromixer (a) has a 330 μm, while the outer circle (b) has a 550 μm. The total length of the channel is 25 mm, and the number of repeat units is 8
Comparison and Analysis of Mixing Efficiency in Various Micromixers
In this study, we aimed to identify the most efficient design among micromixers with different designs. Figure 2 illustrates the Mn results of the five micromixers. To assess the Mn value in each micromixer, we measured the distance from the point where the two fluids meet to the ROI within the micromixer. This distance was then converted into time units for comparative analysis. In Fig. 2, the 3D zig-zag micromixer reached an Mn value of 1.0 at around 1.3 ms mixing time. In contrast, other micromixer designs did not reach within the same time. Even upon reaching the endpoint at 1.6 ms, the Mn did not attain a high value. This demonstrates that the Mn value is influenced by the microchannel design of each micromixer.
The straight micromixer relies on molecular diffusion at the fluid interface due to the absence of structural elements. However, this process is generally slow; the Mn value is observed to be low, around 0.01 [44]. The herringbone micromixer differs from the straight micromixer in that it features an asymmetric herringbone structure repeated on the bottom surface of the microchannel.
The herringbone structure consists of two connected channels of different lengths, one relatively long and the other short, designed asymmetrically. This herringbone structure indicates a helical flow pattern of the fluid within the microchannel, facilitating effective mixing [45, 46]. As a result, the Mn at the endpoint increased to around 0.54, indicating improved mixing performance compared to a straight micromixer. However, the Mn value did not reach or come close, even at the endpoint.
In the serpentine micromixer, as the two injected fluids traverse through a curved channel, secondary flow driven by centrifugal forces induces a dean flow pattern, causing the fluid to move from the inner side of the channel toward the outer wall [47]. During this process, the fluids experience an increased interfacial area due to the curved geometry, leading to enhanced mixing efficiency. This results in more efficient mixing compared to the previous micromixer, with a confirmed Mn value of approximately 0.81 at the termination point. However, the Mn value exhibits a monotonic increase toward the later part of the microchannel due to the absence of obstacle within the microchannel, evenly saturating.
In the toroidal micromixer, fluid undergoes splitting induced by the toroidal shape, followed by recombination at the neck, resulting in asymmetric collisions that promote effective mixing [48]. Additionally, the toroidal micromixer, characterized by its repetitive pattern of spit and recombining, exhibits a substantial increase in the interfacial area where the fluids come into contact. This unique structure contributes to more effective mixing, resulting in an efficiency of approximately 0.88 at the outlet. Based on these results, the toroidal micromixer has been confirmed to exhibit the most efficient mixing among the micromixers with a two-dimensional structure.
Finally, the 3D zig-zag micromixer features a three-dimensional structure in contrast to the previous micromixers. The 3D structure induces also primary flow in the x–y plane and secondary flow in the vertical z-axis. This secondary flow enhances mixing by increasing the contact surface of the fluids. With this improved mixing, the 3D zig-zag micromixer achieves a Mn of approximately 0.98 at 1.3 ms, earlier than the endpoint at 1.6 ms.
Therefore, this paper, we aimed to modify the design parameters affecting Mn in the toroidal and 3D zig-zag micromixers. We optimized the design of toroidal and 3D zig-zag micromixer designs to confirm the most efficient one.
Experimental Assessment of Mixing in the Toroidal Micromixer with Various Designs
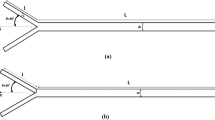
We anticipated that the neck angle (\(\theta\)) and width ratio (W1/W2) in the toroidal micromixer would influence Mn, as illustrated in Fig. 3. Consequently, we modified each parameter to investigate the correlation with the parameters and Mn. Ultimately, we aimed to design the most efficient micromixer within the toroidal micromixer. As shown in Table 2, to investigate the influence of neck angle on Mn, we designed different neck angles (90°–180°), and we fixed the width ratio at 1.0. In designs with various width ratios, we fixed the neck angle at 120° and adjusted the width ratio (1.0–4.0).
To analyze Mn, we defined each section of the neck in the toroidal micromixer as a Region of Interest (ROI) and captured fluorescent images. The Mn was calculated using the average fluorescence intensity and standard deviation within the designated ROI.
First, the Mn of each micromixer with different neck angle was compared. Comparing the Mn values when the mixing time reached 1.4 ms, we observed respective values of 0.89, 0.78, 0.65, 0.5, and 0.25, respectively. At the endpoint (2.6 ms), the Mn values were calculated as 0.95, 0.95, 0.91, 0.83, and 0.5 for each design, respectively. Through these results, it was observed that as the neck angle approached 90°, the graph of Mn exhibited a rapid increase in values within a short time, saturating quickly (Fig. 4a). At the endpoint, a comparison of Mn at the smallest neck angle of 90° and the largest angle of 180° revealed that the Mn at a neck angle of 90° was approximately 90% higher than at 180°. In addition, as shown in Fig. 4b, an increase in the neck angle leads to a more distinct boundary between the two fluids. When fluid is injected into the toroidal micromixer, it undergoes split and recombination due to the structure of the microchannel. This process results in imbalanced collision and mixing. This is attributed to the fluid recombining at the neck section and causing collisions, which become more imbalanced when the neck angle approaches 90° compared to grades closer to 180°. Therefore, chaotic flow occurs at the neck section, increasing interfacial area and facilitating mixing. It suggests that the difference in neck angles influences Mn.
Investigation of various designs to evaluate the influence of neck angle and width ratio on a toroidal micromixer. a Comparison of normalized mixing efficiency in toroidal micromixer with different neck angle. b Fluorescence images illustrating variations in mixing across different toroidal mixers with different two neck angles. (i) and (iii) are the fluorescence images at 1.4 ms. (ii) and (iv) are the fluorescence images at 2.6 ms. c Comparison of normalized mixing efficiency in toroidal micromixer with different width ratio. d Fluorescence images showing variations in mixing across different toroidal mixers with different width ratios. (v) and (vii) are the fluorescence images at 1.4 ms. (vi) and (viii) are the fluorescence images at 2.6 ms. e Schematic diagram of the measurement points in toroidal micromixer. The closed triangles represent 1.4 ms, while the open triangles indicate 2.6 ms (scale bar size = 100 μm)
To investigate the correlation between Mn and the width ratio of the microchannel, we conducted mixing experiments and compared the Mn in toroidal micromixers with varying width ratios. In the toroidal micromixer with width ratios of 3.0 and 4.0, Mn values achieving 0.99 were observed at approximately 2.0 ms. However, mixing was not complete in the toroidal micromixers with width ratios of 1.0 and 2.0, with Mn values of 0.89 and 0.96, respectively. For both designs, even at the endpoint, the Mn values were not reached 1.0 (Fig. 4c). In particular, with a width ratio of 4.0, the mixing of the two fluids was confirmed at 1.4 ms, while the ratio of 1.0 did not mix, and the boundary between the two fluids was visible (Fig. 4d). This indicates that, as the width ratio increases, the Mn reaches 1.0 within a short time. In the toroidal micromixer, when the width ratio is varied, one fluid enters the wider side of the channel with the other fluid, increasing the contact area between the fluids. This leads to enhanced mixing as the interface area increases, and there is an increase in imbalanced collisions at the recombination neck section, resulting in improved Mn. In contrast, when the width ratio of the channel is the same, the two fluids symmetrically enter the channel, leading to reduced imbalanced collisions and limiting the Mn [45, 49].
As a result, we confirmed that in the design of the toroidal micromixer, lower neck angles and larger width ratios lead to improved Mn. Among the toroidal designs, we identified the micromixer with a neck angle of 90° and a width ratio 4.0 as the most efficient design.
Comparison of Mixing Efficiency in 3D Zig-Zag Micromixer with and Without Junction
In Fig. 2, we confirmed that the 3D zig-zag micromixer is the most efficient. However, we observed a significantly lower Mn at 0.4 ms than other micromixers. We anticipated this due to the absence of junctions when entering the mixing region from the inlet in the 3D zig-zag structure. Therefore, we compared the 3D zig-zag micromixer with and without a junction at the inlet (Fig. 5a).
To evaluate the Mn, we obtained fluorescence images by designating the middle of each channel in the 3D mixer as an ROI. Then, we calculated the average fluorescence intensity and standard deviation from the fluorescence images.
In both designs, with and without a junction at the inlet, the Mn value increases sharply at the initial junction. However, the design with a junction was observed to mix more rapidly (Fig. 5b).
When compared the Mn value at 0.4 ms between the designs with and without a junction, the design without a junction exhibited approximately 0.27, while the design with a junction improved to approximately 0.75. This indicates that the design with a junction at the inlet achieved enhanced Mn value at the same time.
In addition, the design without a junction required approximately 1.3 ms to reach the Mn value of 1.0, while the design with a junction reached it at approximately 0.8 ms. The mixing time can be reached about 0.5 ms faster than the design without a junction, resulting in an improvement of approximately 38%. This demonstrates that the additional junction induced secondary flow, allowing for more efficient mixing. In conclusion, in the design of the 3D zig-zag micromixer, the configuration with an additional junction at the inlet proved to be the most efficient design.
Comparative Analysis of Mixing Efficiency in Toroidal and 3D Zig-Zag Micromixers
Ultimately, we compared the Mn of the toroidal micromixer and 3D zig-zag micromixer to identify the most efficient micromixer design (Fig. 6). The comparison of Mn between the two micromixers revealed that the 3D zig-zag micromixer is a more efficient design. The toroidal micromixer achieved a Mn value of 1.0 within about 2 ms. However, using the 3D zig-zag micromixer achieving an Mn value 1.0 in a short time of 0.8 ms. This indicates that the 3D zig-zag micromixer exhibited a significant improvement of about 60% compared to the toroidal micromixer, demonstrating superior mixing performance.
The difference in Mn is attributed to the toroidal micromixer having two-dimensional structure, while the 3D zig-zag micromixer has three-dimensional structure. In the case of the toroidal structure, mixing is confined to the x–y plane, whereas in the 3D zig-zag structure, in addition to the primary flow in the x–y plane, secondary flow occurs along the z-axis. This multi-dimensional fluid motion contributes to increased interfacial area, resulting in enhanced Mn compared to the toroidal micromixer.
Based on these results, we compare the Mn value of the 3D zig-zag micromixer with that of other micromixers. Table 3 presents a comparison of the Mn among five different micromixers. The toroidal micromixer features a design with a neck angle of 90° and a width ratio of 1.0, while the 3D zig-zag micromixer incorporates a design with junctions. When compared at a mixing tine of 1.0 ms, other micromixers, excluding the 3D zig-zag micromixer, did not reach the fully mixed value of 1.0. These results indicate that the comparison of Mn among different micromixers revealed that the mixing in the 3D zig-zag micromixer is the most efficient. And this attains an Mn value of 1.0 within a short time of 0.8 ms.
Estimation of Mixing in 3D Zig-Zag Micromixer with Different Reynolds Number
We investigated the Mn in the 3D zig-zag micromixer at different Reynolds numbers. Furthermore, we visualized the fluid flow along each junction using a confocal laser scanning microscope to observe the extent of fluid mixing.
Figure 7a shows the comparing normalized Mn in the 3D zig-zag micromixer at different Reynolds numbers. All variables except for the flow rate were fixed, and as a result, the Reynolds number increased with the flow velocity within the micromixer.
Effect of Reynolds numbers on mixing efficiency. a Comparison of normalized mixing efficiency in 3D zig-zag micromixer at different Reynolds numbers. b 3D confocal fluorescence images for visualizing cross-sections of a 3D zig-zag micromixer (\({\varvec{R}}{\varvec{e}}\) = 332). Scale bar size = 100 μm
The results showed that as the Reynolds number decreased, the Mn also reduced. Conversely, an increase in the Reynolds number correlates with an upward trend in Mn. This indicates that at low Reynolds numbers, the mixing of fluids in the micromixer occurs slowly, decreasing in Mn. In contrast, as the Reynolds number increases, turbulence is induced in the fluid, resulting in an upward trend in Mn. As shown in the figure, except the case of \(Re\) = 17, we observed that the Mn value reached 1.0 at all other Reynolds numbers. Particularly, at \(Re\) = 332 and \(Re\) = 449, the Mn value reached 1.0 at 2.7 mm. These results imply the impact of flow velocity on Reynolds number.
Additionally, we visualized the mixing at the middle of each channel in a 3D zig-zag micromixer, and the fluid velocity fixed at \(Re\) = 332 (Fig. 7b). In the first cross-section (1.2 ms), we observed that the fluids remained unmixed. However, starting from the second cross-section (2.2 ms), mixing occurred, resulting in a uniform mixture.
In conclusion, in the 3D zig-zag micromixer, there was a trend of rapid increase of Mn at higher Reynolds numbers. When examining the cross-sectional area, it was confirmed that the two fluids are uniformly mixed.
Conclusions
Efficiently achieving high mixing efficiency within a short time in microfluidic devices is a critical technology in chemistry and biological reactions. To address this challenge, numerous researchers have proposed various designs for micromixers. In this paper, we compare various passive micromixer designs and propose a micromixer design that effectively achieves rapid mixing quickly. To confirm the most efficient design in a passive micromixer, designs for straight, serpentine, herringbone, toroidal and 3D zig-zag micromixers were developed and compared for Mn. As a result, it was confirmed that toroidal and 3D zig-zag micromixer achieved more efficient mixing compared to other micromixers. Additionally, we identified the parameters influencing the Mn of the toroidal and 3D zig-zag micromixer. The most effective designs for each micromixer were determined, and the Mn was compared among the final designs of each micromixer.
Ultimately, the 3D zig-zag micromixer was identified for exhibiting the highest Mn, with secondary mixing flows occurring along the z-axis. This result demonstrates the capability of achieving effective mixing in a short time, approximately 0.8 ms. Through this study, the proposed 3D zig-zag micromixer is expected to find valuable applications, particularly in efficiently conducting chemical reactions with small amounts of reagents or promoting reactions by mixing reagents in medical diagnostics and testing. Especially for the mRNA-LNP vaccines, aimed at relieving the world from a tragic global pandemic, where the uniform formation of nanoparticles and nanostructures is crucial, a rapid mixing process is essential. The 3D zig-zag micromixer introduced in this paper is expected to be highly beneficial, as it can achieve a high Mn quickly, making it suitable for various applications.
References
J.-C. Leong, C.-H. Tsai, C.-L. Chang, C.-F. Lin, L.-M. Fu, Rapid microfluidic mixers utilizing dispersion effect and interactively time-pulsed injection. Jpn. J. Appl. Phys. 46, 5345 (2007)
T.H. Schulte, R.L. Bardell, B.H. Weigl, Microfluidic technologies in clinical diagnostics. Clin. Chim. Acta 321, 1–10 (2002)
S.Z. Razzacki, P.K. Thwar, M. Yang, V.M. Ugaz, M.A. Burns, Integrated microsystems for controlled drug delivery. Adv. Drug Deliv. Rev. 56, 185–198 (2004)
Y. He et al., Numerical investigation of the mixing process in a Twin Cam Mixer: influence of triangular cam height-base ratio and eccentricity. Korean J. Chem. Eng. 38, 552–564 (2021)
A.G. Niculescu, C. Chircov, A.C. Birca, A.M. Grumezescu, Fabrication and applications of microfluidic devices: a review. Int. J. Mol. Sci. 22(4), 2011 (2021).
I. Ji, J.W. Kang, T. Kim, M.S. Kang, S.B. Kwon, J. Hong, 3D printing-based ultrafast mixing and injecting systems for time-resolved serial femtosecond crystallography. Korean Chem. Eng. Res. 60(2), 300–307 (2022)
S.-H. Jang, I.-J. Kang, Drug delivery study on chitosan nanoparticles using iron oxide (II, III) and valine. Korean Chem. Eng. Res. 59, 514–520 (2021)
W.-H. Choi, B. Kim, Fabrication and characterization of dissolving microneedles containing lecithin for transdermal drug delivery. Korean Chem. Eng. Res. 59, 429–434 (2021)
M. Maeki, S. Uno, A. Niwa, Y. Okada, M. Tokeshi, Microfluidic technologies and devices for lipid nanoparticle-based RNA delivery. J. Control. Release 344, 80–96 (2022)
K.K.V. Canlas et al., Trends in nano-platforms for the treatment of viral infectious diseases. Korean J. Chem. Eng. 40, 706–713 (2023)
E. Hong, J. Jeon, H.U. Kim, Recent development of machine learning models for the prediction of drug-drug interactions. Korean J. Chem. Eng. 40, 276–285 (2023)
G. Zhang, J. Sun, Lipid in chips: a brief review of liposomes formation by microfluidics. Int. J. Nanomed. 16, 7391–7416 (2021).
M. Maeki, N. Kimura, Y. Sato, H. Harashima, M. Tokeshi, Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 128, 84–100 (2018)
M.S. Ali, N. Hooshmand, M. El-Sayed, H.I. Labouta, Microfluidics for development of lipid nanoparticles: paving the way for nucleic acids to the clinic. ACS Appl. Bio Mater. 6, 3566–3576 (2021)
M. Faryadi, M. Rahimi, S. Safari, N. Moradi, Effect of high frequency ultrasound on micromixing efficiency in microchannels. Chem. Eng. Process. 77, 13–21 (2014)
K.-I. Min, Fabrication of 3D multilayered microfluidic channel using fluorinated ethylene propylene nanoparticle dispersion. Korean Chem. Eng. Res. 59, 639–643 (2021)
B. Zhou et al., Design and fabrication of magnetically functionalized flexible micropillar arrays for rapid and controllable microfluidic mixing. Lab Chip 15, 2125–2132 (2015)
D. Ahmed, X. Mao, J. Shi, B.K. Juluri, T.J. Huang, A millisecond micromixer via single-bubble-based acoustic streaming. Lab Chip 9, 2738–2741 (2009)
K. Karthikeyan, L. Sujatha, Study of permissible flow rate and mixing efficiency of the micromixer devices. Int. J. Chem. React. Eng. 17, 20180047 (2018)
A. Cosentino et al., An efficient planar accordion-shaped micromixer: from biochemical mixing to biological application. Sci. Rep. 5, 17876 (2015)
A. Agarwal, A. Salahuddin, H. Wang, M.J. Ahamed, Design and development of an efficient fluid mixing for 3D printed lab-on-a-chip. Microsyst. Technol. 26, 2465–2477 (2020)
H. Lv, X. Chen, Novel study on the mixing mechanism of active micromixers based on surface acoustic waves. Ind. Eng. Chem. Res. 61, 10264–10274 (2022)
M. Bayareh, M.N. Ashani, A. Usefian, Active and passive micromixers: a comprehensive review. Chem. Eng. Process. Process Intens. 147, 107771 (2020)
J. Sun et al., Numerical and experimental investigation of a magnetic micromixer under microwires and uniform magnetic field. J. Magn. Magn. Mater. 551, 169141 (2022)
D. Bahrami, A.A. Nadooshan, M. Bayareh, Effect of non-uniform magnetic field on mixing index of a sinusoidal micromixer. Korean J. Chem. Eng. 39, 316–327 (2022)
S.-G. Jeong et al., Nanoliter scale microloop reactor with rapid mixing ability for biochemical reaction. Korean J. Chem. Eng. 35, 2036–2042 (2018)
B. Yin et al., Micromixer with fine-tuned mathematical spiral structures. ACS Omega 6, 30779–30789 (2021)
E. Nady, G. Nagy, R. Huszánk, Improvement in mixing efficiency of microfluidic passive mixers functionalized by microstructures created with proton beam lithography. Chem. Eng. Sci. 247, 117006 (2022)
S. Hossain, M. Ansari, K.-Y. Kim, Evaluation of the mixing performance of three passive micromixers. Chem. Eng. J. 150, 492–501 (2009)
J. Choe, Y. Kwon, Y. Kim, H.-S. Song, K.H. Song, Micromixer as a continuous flow reactor for the synthesis of a pharmaceutical intermediate. Korean J. Chem. Eng. 20, 268–272 (2003)
M. Nimafar, V. Viktorov, M. Martinelli, Experimental comparative mixing performance of passive micromixers with H-shaped sub-channels. Chem. Eng. Sci. 76, 37–44 (2012)
V. Viktorov, M.R. Mahmud, C. Visconte, Comparative analysis of passive micromixers at a wide range of Reynolds numbers. Micromachines 6, 1166–1179 (2015)
P. Li, J. Cogswell, M. Faghri, Design and test of a passive planar labyrinth micromixer for rapid fluid mixing. Sens. Actuat. B Chem. 174, 126–132 (2012)
T. Tofteberg, M. Skolimowski, E. Andreassen, O. Geschke, A novel passive micromixer: lamination in a planar channel system. Microfluid. Nanofluid. 8, 209–215 (2010)
J.J. Chen, Y.S. Shie, Interfacial configurations and mixing performances of fluids in staggered curved-channel micromixers. Microsyst. Technol. 18, 1823–1833 (2012)
M.K. Parsa, F. Hormozi, D. Jafari, Mixing enhancement in a passive micromixer with convergent–divergent sinusoidal microchannels and different ratio of amplitude to wave length. Comput. Fluids 105, 82–90 (2014)
X. Chen, Z. Zhao, Numerical investigation on layout optimization of obstacles in a three-dimensional passive micromixer. Anal. Chim. Acta 964, 142–149 (2017)
S.O. Hong et al., Gear-shaped micromixer for synthesis of silica particles utilizing inertio-elastic flow instability. Lab Chip 21, 513–520 (2021)
S. Kim et al., Monolithic 3D micromixer with an impeller for glass microfluidic systems. Lab Chip 20, 4474–4485 (2020)
A.D. Stroock et al., Chaotic mixer for microchannels. Science 295, 647–651 (2002)
Wild, Andre, Timothy Leaver, and Robert James Taylor. "Bifurcating mixers and methods of their use and manufacture." U.S. Patent No. 10,076,730. (2018)
H. Kim et al., Submillisecond organic synthesis: Outpacing Fries rearrangement through microfluidic rapid mixing. Science 352, 691–694 (2016)
B. Lee et al., Characterization of passive microfluidic mixer with a three-dimensional zig-zag channel for cryo-EM sampling. Chem. Eng. Sci. 281, 119161 (2023)
C. Wang, Y. Hu, Mixing of liquids using obstacles in y-type microchannels. J. Appl. Sci. Eng. 13, 385–394 (2010)
M.A. Ansari, K.-Y. Kim, Shape optimization of a micromixer with staggered herringbone groove. Chem. Eng. Sci. 62, 6687–6695 (2007)
T.J. Kwak et al., Convex grooves in staggered herringbone mixer improve mixing efficiency of laminar flow in microchannel. PLoS ONE 11, e0166068 (2016)
P.B. Howell Jr., D.R. Mott, J.P. Golden, F.S. Ligler, Design and evaluation of a Dean vortex-based micromixer. Lab Chip 4, 663–669 (2004)
C. Webb et al., Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: a case study using protein-loaded liposomes. Int. J. Pharm. 582, 119266 (2020)
J. Li, G. Xia, Y. Li, Numerical and experimental analyses of planar asymmetric split-and-recombine micromixer with dislocation sub-channels. J. Chem. Technol. Biotechnol. 88, 1757–1765 (2013)
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C3004936 and 2021R1A5A8032895). ※ MSIT: Ministry of Science and ICT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oh, S.y., Lee, CS. Comparison and Analysis of Mixing Efficiency in Various Micromixer Designs. Korean J. Chem. Eng. 41, 2449–2458 (2024). https://doi.org/10.1007/s11814-024-00161-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00161-x