Abstract
Purpose
Because many survivors do not receive recommended follow-up, we sought to characterize patterns and predictors of survivorship clinic attendance in a population-based sample of childhood cancer survivors.
Methods
Using the Connecticut Tumor Registry, we identified all patients diagnosed with cancer at age ≤ 18 years from March 1, 1998 to March 1, 2008, still in follow-up 5 years post-diagnosis, and living <100 miles from Yale. Survivorship clinic attendance, demographics, disease characteristics, and treatment exposures were ascertained. Vital status was confirmed with the National Death Index. The Kaplan-Meier curves and hazard ratios were calculated for survivorship clinic attendance.
Results
Four hundred eighty-nine eligible survivors currently 19.1 ± 6.2 years old were diagnosed at a mean age of 9.1 ± 5.8 years with leukemias/lymphomas (47.2 %), central nervous system tumors (16.4 %), sarcomas (11.2 %), thyroid cancers or melanomas (7.8 %), and other solid tumors (17.4 %). The 10-year post-diagnosis clinic attendance probability was 27.8 % (SE = 2.3) overall, and 36.9 % (SE = 4.4) and 40.8 % (SE = 3.8), in patients with radiation and anthracycline exposure, respectively. In adjusted analysis, patients with insurance (HR = 2.90; p < 0.01 for private and HR = 2.05; p = 0.02 for public assistance), treated with anthracyclines (HR = 3.05; p < 0.01), and treated with radiation (HR = 1.90; p < 0.01) were significantly more likely to attend clinic.
Conclusions
The majority of childhood cancer survivors in our population-based sample had not attended survivorship clinic, even among those with high-risk exposures. Health care access, as measured by insurance status, was an important predictor of clinic attendance.
Implications for Cancer Survivors
More research is needed to clarify the link between insurance status and survivorship care to increase appropriate late effects surveillance in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The population of childhood cancer survivors has grown because of advances in therapy and supportive care that have led to cure rates of over 80 % [1]. However, about 73 % of long-term survivors will develop at least one chronic health condition within 30 years of their diagnosis with 42 % of those conditions being severe, disabling, life-threatening, or life-ending. The risk of developing these severe conditions was eight times greater when compared to siblings without a history of cancer [2].
The Children’s Oncology Group (COG), a National Cancer Institute supported clinical trials group with over 200 member institutions, launched guidelines in 2004 for childhood cancer survivors to receive risk-based follow-up and screening [3]. One of the proposed models for delivering this type of care is the establishment of specialized, long-term follow-up clinics [4, 5]. The majority of COG member institutions provide some level of survivorship care, with more than half reporting doing so in a specialized late effects program [6]. Yale established a regional clinic (HEROS) in 2003 to screen for and provide preventative health education regarding potential late effects from treatment for pediatric cancer survivors of all ages. Although individual practices may differ, it is the consensus recommendation of the Yale section of Pediatric Hematology-Oncology for all off-therapy patients to be referred to HEROS Clinic 5 years after diagnosis or 2 years after end of therapy. Analysis of patient data from the HEROS clinic from 2003 to 2009 showed that new treatment-related health conditions were identified as a result of a clinic visit in 34 % of the attendees [7].
Available estimates of childhood cancer survivors receiving appropriate risk-based survivorship care are limited to much older cohorts or general oncology follow-up in the short term. In adult survivors of childhood cancer survivors treated from 1970 to1986, only 31.5 % of survivors reported receiving any follow-up care focusing on their prior cancer with just 17.8 % reporting care that included consultation about risk reduction and screening tests [8]. An acknowledged limitation of a more recent analysis of the frequency of survivors receiving survivorship care is its inability to distinguish general recurrence monitoring follow-up from surveillance for late effects. In addition, this study only ascertained follow-up at a range of 0–5 years from diagnosis and did not collect detailed treatment exposure data [9].
The Yale HEROS Clinic is an informative sample to examine patterns and predictors of survivorship clinic attendance accounting for all eligible survivors over a 10-year period, from 5–15 years post diagnosis. Our aims for this study are to (1) determine the cumulative incidence rate of survivorship clinic attendance and (2) identify cancer, treatment, and sociodemographic factors associated with increased risk of non-attendance.
Methods
Study population
Included in this retrospective study were patients diagnosed with cancer at age ≤ 18 years at Yale New Haven Hospital from March 1, 1998 to March 1, 2008. Patients additionally met the following inclusion criteria: residence within 100 miles from Yale at 5 years from diagnosis, primary oncologic care at Yale, and completion of all therapy within 5 years from the date of diagnosis. A cohort of 694 patients diagnosed with cancer at age ≤ 18 years from March 1, 1998 to March 1, 2008 and treated at Yale New Haven Hospital was identified through the hospital tumor registry, which contributes to the Connecticut (CT) Tumor Registry. The CT Tumor Registry is the oldest population-based tumor registry in the country, having collected case histories of all newly diagnosed cancer patients in the state of Connecticut and lifelong follow-up records from these patients since 1935 [10]. Last known address and change of address for each patient was ascertained from Yale medical records, which are uniformly updated by clinic registration staff at each encounter. After review of these charts, we excluded those patients who were deceased prior to 5 years from diagnosis (N = 111), transferred care prior to 5 years from diagnosis (N = 45), had moved >100 miles from Yale at 5 years from diagnosis (N = 28), were on active treatment as of March 1, 2013 (N = 1), and whose medical records were incomplete (N = 20). The final sample included 489 patients as displayed in Fig. 1. The Yale University Human Investigation Committee approved the current study.
Procedures
All eligible patients in our cohort were first identified as either “attenders” (having attended Yale’s survivorship clinic at least once) or “non-attenders” (never having attended Yale’s survivorship clinic), based on HEROS clinic records of all patient encounters from March 1, 2003 to March 1, 2013. Over this time period, clinic practices have consistently included medical abstraction to prepare a treatment summary and survivorship care plan, and multidisciplinary care from a team, which includes a physician, nurse practitioner, psychologist, and nurse educator. HEROS is located at a dedicated time in the same space as the general pediatric oncology outpatient clinic. There have been no concerted media or advertising campaigns, but the clinic is described in an oncology patient family manual given to patients and their families at the time of diagnosis since its inception.
For attenders of clinic, all sociodemographic and treatment variables were obtained from HEROS clinic electronic database. For non-attenders, these same sociodemographic and treatment variables were abstracted from both electronic and paper charts in the Yale medical record.
Sociodemographic variables abstracted included: gender, parent-reported race/ethnicity (White, Black, Hispanic, and other), type of insurance (none or self-pay, private, and having some form of public assistance), travel time from the hospital, and household income (based on quartiles). The last known address was entered into the Google Maps website to calculate shortest driving time to Yale New Haven hospital [11]. This address was also entered into the Federal Financial Institutions Examination Council’s (FFIEC) Geocoding system to obtain median household income for the census tract where the address is located based on data from the 2013 US census [12].
Treatment variables abstracted included: age at diagnosis, cancer diagnosis (leukemias, lymphomas, tumors of the central nervous system, sarcomas, thyroid cancers or melanomas, and all other solid tumors), surgery (yes/no), radiation (yes/no), radiation site (cranial, chest, abdomen and pelvis, limb, full body), stem cell transplant (yes/no), chemotherapy (yes/no), alkylating agent exposure (yes/no), anthracycline exposure (yes/no), lung toxic therapy exposure (yes/no), radioactive iodine (yes/no), relapse (yes/no), secondary malignancy (yes/no), and enrolled on clinical study (yes/no). For cancer diagnosis, we decided to group thyroid cancers and melanomas together based on the fact that these two diagnoses tend to be treated with surgical resection only and/or are not typically referred or followed by pediatric oncology at Yale. We identified treatment with alkylating agents as exposure to busulfan, carmustine, carboplatin, chlorambucil, cisplatin, cyclophosphamide, dacarbazine, ifosfamide, lomustine, mechlorethamine, melphalan, procarbazine, thiotepa, or temozolomide [13]; anthracyclines as exposure to doxorubicin, daunorubicin, idarubicin, epirubicin, mitoxantrone [13]; and lung-toxic agents as exposure to busulfan, carmustine (BCNU), lomustine (CCNU), or bleomycin [13].
Vital status was ascertained for all patients using departmental lists of deceased patients, an Internet collection of obituaries on Legacy.com [14], as well as a search of records from the National Death Index (NDI) [15]. The NDI is a database run by the National Center for Health Statistics that tracks deaths and causes of death of US citizens occurring after 1978 in the USA [15]. Those patients found to be deceased less than 5 years from date of diagnosis were deemed ineligible for the study.
Statistical analysis
Descriptive statistics summarized patient characteristics for patients overall and stratified by survivorship clinic attendance. A Kaplan-Meier estimator was used to estimate the probability of attending survivorship clinic over time and to examine the proportional hazards assumption for each of our independent variables. A subsequent Cox proportional hazards regression was conducted adjusted for gender, age at diagnosis, and type of insurance. Type of insurance at the last documented medical visit in our study period was chosen as our best estimate of socioeconomic status, as income ascertained from census tracts was not significant in the unadjusted analysis. P < 0.05 was used to define statistical significance. All analyses were conducted in SAS software, Version 9.4 of the SAS system [16]. Copyright © 2013 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Results
Patient characteristics
Table 1 displays the characteristics of the 489 cancer survivors analyzed in this study. Patients were diagnosed at a mean age of 9.1 ± 5.8 years with leukemias/lymphomas (47.2 %), central nervous system tumors (16.4 %), sarcomas (11.2 %), thyroid cancers or melanomas (7.8 %), and other solid tumors (17.4 %). Of this group, 26.0 % were part of a racial minority group. In terms of their cancer treatment, 29.9 % had received conventional radiation, 45.5 % of patients had been exposed to alkylating agents, 45.6 % had been exposed to anthracyclines, and 13.1 % had been exposed to lung toxic chemotherapies. Over half (51.1 %) either had no insurance or some level of public assistance. The median age for clinic attendees when they first attended survivorship clinic was 13.6 years (interquartile range 10.2–19.8) with a normal distribution, results not shown.
Patterns
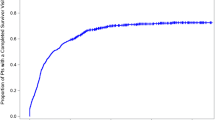
For all survivors, the Kaplan-Meier estimated 5-, 10-, and 15-year post-diagnosis survivorship clinic attendance probabilities were 4.5 % (SE = 0.9), 27.8 % (SE = 2.3), and 35.5 % (SE = 3.1), respectively. The 10-year attendance probabilities were 36.9 % (SE = 4.4) after radiation, 40.8 % (SE = 3.8) after anthracycline therapy, and 39.1 % (SE = 3.8) after alkylating agent therapy. If a patient had insurance, the attendance probability was 38.2 % (SE = 3.7) for private and 31.2 % (SE = 5.7) for public assistance at 10 years. The Kaplan-Meier curves for clinic attendance increased gradually over time with a period of leveling off. This general pattern remained true across diagnoses (Fig. 2a), as well as for significant predictors including anthracycline therapy (Fig. 2b), radiation (Fig. 2c), and insurance status (Fig. 2d). While clinic attendance increased with longer follow-up, the incidence rate of clinic attendance was not significantly different when compared across treatment periods (analyzed by 3-year subintervals based on date of diagnosis; log-rank = 0.43, p = 0.93), result not shown.
Predictors
Cancer factors
After adjusting for age at diagnosis, gender, and insurance, childhood cancer survivors with a history of leukemia (HR = 3.36 compared to central nervous system tumor; p < 0.01), lymphoma (HR = 3.99 compared to central nervous system tumor; p < 0.01), and sarcoma (HR = 3.30 compared to central nervous system tumor; p < 0.01) were significantly more likely to attend survivorship clinic. Relapse and secondary malignancy were not significant predictors.
Treatment factors
In the same adjusted analysis, patients with a history of chemotherapy (HR = 49.31; p < 0.01) and radiation (HR = 1.90; p < 0.01) were significantly more likely to attend survivorship clinic (Table 2). The elevated likelihood was found for all radiation sites except full body and limb radiation (cranial HR = 1.88, p < 0.01; chest HR = 2.72; p < 0.01; abdomen and pelvis HR = 1.78, p = 0.05). They were also significantly more likely to attend clinic if they were treated with alkylating agents (HR = 2.28; p < 0.01), anthracyclines (HR = 3.05; p < 0.01, and lung-toxic therapies (HR = 1.89; p < 0.01). Patients who underwent surgery without radiation or chemotherapy (HR = 0.02 compared to surgery combined with radiation and chemotherapy; p < 0.01) were significantly less likely to attend survivorship clinic. Clinical trial enrollment was not a significant predictor. Of the seven patients who received limb radiation and the six patients who experienced subsequent malignancies, none attended our survivorship clinic so we are unable to estimate a hazards ratio for these factors.
Sociodemographic factors
In the adjusted analysis, patients with insurance (HR = 2.90; p < 0.01 for private and HR = 2.05; p = 0.02 for public assistance) were more likely to attend clinic. A stratified analysis demonstrated that these findings remained significant in patients age < 18 years at 5 years from diagnosis (HR = 2.42; p < 0.01 for private and HR = 2.32; p = 0.02 for public assistance). For survivors ≥18 years at 5 years from diagnosis, patients with private insurance (HR = 4.60; p < 0.01) were more likely to attend clinic. Income, travel time to clinic, and race were not significant predictors.
Discussion
Despite the availability of a specialty survivorship clinic and guidelines recommending risk-based surveillance care, the majority (72.2 %) of childhood cancer survivors in our population-based sample did not attend survivorship clinic even at 10 years post-diagnosis. This includes high-risk patients who had received radiation, alkylating agents, anthracyclines, or lung-toxic agents as part of therapy. These groups of patients are known to be particularly susceptible to late effects including gonadal dysfunction, secondary malignancies, pulmonary fibrosis, and cardiomyopathies [13]. Our data showed that these rates have not significantly changed over time, in spite of published standards for all pediatric cancer centers to offer long-term follow-up survivorship care, as well as efforts by the Institute of Medicine and various advocacy groups to increase education and awareness of late-effects surveillance [3, 13, 17–19]. While past studies have looked at physician-perceived barriers to care and patterns of follow-up care for pediatric cancer patients in the period immediately after therapy, this is the first study to our knowledge that has examined specific disease and health service factors associated with long-term survivorship care [9, 20].
The existing literature is inconsistent in its analysis of childhood cancer survivors’ attendance patterns for general follow-up, with different studies linking increased attendance to female sex, insurance status, older age at diagnosis, increased treatment exposure, past history of relapse, shorter time off treatment, and living a shorter distance from the hospital [20–22]. The most recent of these analyses by Barakat et al. raised interesting questions regarding standard oncologic follow-up in the period immediately following treatment, but the study was limited in its ability to comment on survivorship care in the long-term as their study period only extended to the first 5 years after diagnosis [9]. Many of these earlier visits are focused on relapse monitoring rather than late effects screening and education. In contrast to the previous work, we did not find any of the sociodemographic variables except for insurance status as being predictive of survivorship clinic attendance, and we found that survivorship clinic attendance actually increased with longer time off treatment (27.8 % at 10 years post diagnosis and 35.5 % at 15 years post diagnosis).
Our study corroborates self-reported cross-sectional data from an older cohort in the Childhood Cancer Survivor Study (CCSS). Of over 8000 childhood cancer survivors surveyed, only 17.8 % reported receiving risk-based, survivor-focused care in the preceding 2 years [8]. We similarly found that an overwhelming majority of childhood cancer survivors had not attended survivorship clinic and that insurance status was a significant predictor. The differences in our results compared to the CCSS papers, including our study not finding age or race to be a significant predictor, may be due to fundamental differences between our two samples. The CCSS represents an older, more racially homogenous cohort (treated from 1970 to 1986, 13.5 % racial minority) compared to our study sample (treated from 1998 to 2013, 26.0 % racial minority).
Another CCSS study found that in general, only 41.9 % reported a cancer-related visit in the last 2 years with even fewer (19.2 %) reporting a visit at a cancer center [23]. Cross-sectional post-hoc analysis of our cohort at the end of the study period revealed that 75 and 54 % of patients were still in follow-up with the treating clinic at 5 and 10 years post diagnosis, respectively. These proportions were 85 and 67 %, respectively, for any Yale encounter. These data suggest that there is a contribution from general institutional loss of follow-up, as well as from a specific failure to attend survivorship clinic. Our findings are consistent with a body of literature highlighting the deficiency of successful transition of young adult survivors of pediatric cancer from their treating oncologist to more long-term follow-up [24, 25]. We do not have the data to know exactly what drives either general loss of follow-up or specific failure to attend survivorship clinic. Further research might be able to identify specific system-, physician-, or patient-driven factors as potential targets for intervention.
Our analysis benefits from the ability to examine ten consecutive years of patient records both from the Connecticut Tumor Registry, as well as survivorship clinic data. Because the CT Tumor Registry documents all incident cancers diagnosed in the state including those at our institution during the 10-year study period, we are able to track all eligible patients in a population-based sample [10]. Strict inclusion criteria allowed us to accurately identify a sample that should have had access to our survivorship clinic—namely patients that were alive, lived in close proximity to the clinic (<100 miles), and were being followed in the same health care system for their oncologic care. Verification of vital status within our study sample with the gold standard measure of the National Death Index further increases the validity of the findings [15]. Our study is additionally strengthened by the fact that we were able to identify particular individuals at elevated risk for late effects based on high-risk treatments of radiation and specific chemotherapeutic agents.
Certain potential limitations must be taken into account when interpreting our findings. Principal among them is that we looked at associations in this study but we do not have descriptive information on why patients did or did not come to clinic. For example, attendance patterns could be strongly linked to referral rates from different oncologists or parent/patient perception of the need for survivorship care. Past work has found that even attenders of a survivorship clinic have a low perceived likelihood of developing a late effect from cancer treatment and that uninsured survivors in particular minimize their need for care [26, 27]. We additionally cannot say with certainty whether any of our non-attenders attended a different survivorship clinic not at Yale, but preliminary unpublished data from a randomized clinical trial at our center suggest that fewer than 2 % do. Lastly, we are unable to comment on any causal relationship between our significant predictors, such as insurance status and clinic attendance. However, these findings suggest critical future areas of inquiry.
Our work builds on past literature demonstrating that lack of insurance was associated with survivors not reporting a general physical examination, a cancer-related visit, or a cancer center visit in the last 2 years [23, 28]. Previous work has also shown that compared with siblings, adult survivors of childhood cancer had both significantly lower rates of health insurance coverage as well as more difficulty obtaining coverage [29]. Nearly a third of our sample did not have insurance, proportions that are consistent with past US census data from 2008 to 2011 for young adults ages 19 to 25 [30]. However, our study largely took place before the Affordable Care Act (ACA) went into a full effect, a period in which it was standard for young adults to be excluded from their parents’ policies when they graduated from high school or college and for those insured through Medicaid or the Children’s Health Insurance Program to be excluded at age 19 [31]. One report identified five specific provisions of the ACA with implications for adult survivors of pediatric cancer. These provisions include prohibition of discrimination on the basis of health status, extended coverage on parents’ insurance, change in minimum income eligibility for Medicaid, no annual or lifetime coverage limits, and state-based exchanges [32]. It is difficult to make specific conclusions about the impact of the ACA on our data since these different provisions came into effect gradually during the last 3 years of our study period. Early follow-up demonstrates there is a higher proportion of young adults age 19 to 25 receiving coverage since the implementation of the first provisions of the ACA from 68.1 to 73.6 % [33]. However, further research is needed to know if and how health care access remains a potential barrier to survivorship care.
In summary, this study demonstrates that the majority of childhood cancer survivors, including patients who have received radiation and high-risk chemotherapy, do not attend survivorship clinic. It is particularly concerning that these rates have not changed significantly even as the clinic has become a well-established and integrated part of our regional system. Even with providers’ increasing knowledge and experience on the importance of assessing patients for cancer treatment-related late effects, there continues to be a major deficit of care. Patients without insurance were significantly less likely to attend survivorship clinic, underscoring the impact of health care disparity in this patient population. Further work is needed to determine the link between these predictors and may help inform future interventions to increase the proportion of this population that receives appropriate late effects surveillance.
References
Pui C-H, Gajjar AJ, Kane JR, Qaddoumi IA, Pappo AS. Challenging issues in pediatric oncology. Nat Rev Clin Oncol. 2011;8(9):540–9.
Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. doi:10.1056/NEJMsa060185.
Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–90. doi:10.1200/JCO.2004.11.032.
Friedman DL, Freyer DR, Levitt GA. Models of care for survivors of childhood cancer. Pediatr Blood Cancer. 2006;46(2):159–68. doi:10.1002/pbc.20611.
Kinahan KE, Sanford S, Sadak K, Salsman JM, Koptik KD, Didwania A, editors. Models of cancer survivorship care for adolescents and young adults. Semin Oncol Nurs; 2015: Elsevier.
Eshelman-Kent D, Kinahan KE, Hobbie W, Landier W, Teal S, Friedman D, et al. Cancer survivorship practices, services, and delivery: a report from the Children’s Oncology Group (COG) nursing discipline, adolescent/young adult, and late effects committees. J Cancer Survivorship. 2011;5(4):345–57.
Staba Hogan MJ, Ma X, Kadan‐Lottick NS. New health conditions identified at a regional childhood cancer survivor clinic visit. Pediatr Blood Cancer. 2013;60(4):682–7.
Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26(27):4401–9.
Barakat LP, Schwartz LA, Szabo MM, Hussey HM, Bunin GR. Factors that contribute to post-treatment follow-up care for survivors of childhood cancer. Journal of Cancer Surviv. 2012;6(2):155–62.
Connelly RR, Campbell PC, Eisenberg H. Central registry of cancer cases in Connecticut. Public Health Rep. 1968;83(5):386.
Google Maps. Google, Google Maps. 2015. https://www.google.com/maps. 2015.
Council FFIE. Geocoding system based on 2013 tract definition of the 2013 US Census for Median Household Income. http://www.ffiec.gov/Geocode/default.aspx. 2014.
Group CO, Group CO. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Arcadia, CA: Children’s Oncology Group; 2008.
Legacy.com. Obitfinder. http://www.legacy.com/ns/obitfinder/obituary-search.aspx. 2014.
Bilgrad R. National Death Index user’s manual. US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Health Statistics; 1995.
Institute S. SAS Institute version 9.4. Cary NC; 2013.
Control CfD, Prevention, Lance Armstrong Foundation. A national action plan for cancer survivorship: advancing public health strategies. Atlanta, Ga: CDC and Lance Armstrong Foundation; 2004.
Stovall E, Greenfield S, Hewitt M. From cancer patient to cancer survivor: lost in transition. National Academies Press; 2005.
Hord J, Feig S, Crouch G, Hale G, Mueller B, Rogers Z, et al. Standards for pediatric cancer centers. Pediatrics. 2014;134(2):410–4.
Bowers DC, Adhikari S, El‐Khashab YM, Gargan L, Oeffinger KC. Survey of long-term follow-up programs in the United States for survivors of childhood brain tumors. Pediatr Blood Cancer. 2009;53(7):1295–301.
McBride ML, Lorenzi MF, Page J, Broemeling A-M, Spinelli JJ, Goddard K, et al. Patterns of physician follow-up among young cancer survivors Report of the Childhood, Adolescent, and Young Adult Cancer Survivors (CAYACS) research program. Can Fam Physician. 2011;57(12):e482–90.
Michel G, Kuehni CE, Rebholz CE, Zimmermann K, Eiser C, Rueegg CS, et al. Can health beliefs help in explaining attendance to follow-up care? The Swiss Childhood Cancer Survivor Study. Psycho-Oncology. 2011;20(10):1034–43.
Oeffinger KC, Mertens AC, Hudson MM, Gurney JG, Casillas J, Chen H, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2(1):61–70.
Ginsberg JP, Hobbie WL, Carlson CA, Meadows AT. Delivering long-term follow-up care to pediatric cancer survivors: transitional care issues. Pediatr Blood Cancer. 2006;46(2):169–73.
Oeffinger KC, Wallace WHB. Barriers to follow-up care of survivors in the United States and the United Kingdom. Pediatr Blood Cancer. 2006;46(2):135–42.
Cherven B, Mertens A, Meacham LR, Williamson R, Boring C, Wasilewski-Masker K. Knowledge and risk perception of late effects among childhood cancer survivors and parents before and after visiting a childhood cancer survivor clinic. Journal of Pediatric Oncology Nursing. 2014:1043454214532022.
Park ER, Kirchhoff AC, Zallen JP, Weissman JS, Pajolek H, Mertens AC, et al. Childhood Cancer Survivor Study participants’ perceptions and knowledge of health insurance coverage: implications for the Affordable Care Act. J Cancer Surviv. 2012;6(3):251–9.
Park ER, Kirchhoff AC, Perez GK, Leisenring W, Weissman JS, Donelan K, et al. Childhood Cancer Survivor Study participants’ perceptions and understanding of the Affordable Care Act. J Clin Oncol. 2015;33(7):764–72.
Park ER, Li FP, Liu Y, Emmons KM, Ablin A, Robison LL, et al. Health insurance coverage in survivors of childhood cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23(36):9187–97.
Rodean J. Health insurance coverage of young adults aged 19 to 25: 2008, 2009, and 2011. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2012.
Collins SR, Garber T, Robertson R. How the Affordable Care Act is helping young adults stay covered. Issue Brief (Commonwealth Fund). 2011;5:1–26.
Mueller EL, Park ER, Davis MM. What the Affordable Care Act means for survivors of pediatric cancer. J Clin Oncol. 2014;32(7):615–7.
Sommers BD, Buchmueller T, Decker SL, Carey C, Kronick R. The Affordable Care Act has led to significant gains in health insurance and access to care for young adults. Health Aff. 2013;32(1):165–74.
Acknowledgments
This publication was made possible by the Yale University School of Medicine Medical Student Research Fellowship at Yale University School of Medicine. NKL was supported by American Cancer Society RSGHP-10-107-01-CPHPS, Research Scholar Grant in Cancer Control.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We have no conflicts of interest to disclose. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Ethical approval
The Yale University Human Investigation Committee approved the current study.
Rights and permissions
About this article
Cite this article
Zheng, D.J., Sint, K., Mitchell, HR. et al. Patterns and predictors of survivorship clinic attendance in a population-based sample of pediatric and young adult childhood cancer survivors. J Cancer Surviv 10, 505–513 (2016). https://doi.org/10.1007/s11764-015-0493-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-015-0493-4