Abstract
Purpose
Osteoporosis increases the risk of fracture and is often considered a late effect of breast cancer treatment. We examined the prevalence of compromised bone health in a sample of exclusively African-American (AA) breast cancer survivors since bone mineral density (BMD) varies by race/ethnicity in healthy populations.
Methods
Using a case–control design, AA women in a weight loss intervention previously diagnosed and treated for stages I–IIIa breast cancer were matched 1:1 on age, race, sex, and BMI with non-cancer population controls (n = 101 pairs) from National Health and Nutrition Examination Survey (NHANES). Questionnaires and dual-energy x-ray absorptiometry (DXA) scanning were completed, and participants were categorized as having normal bone density, low bone mass, or osteoporosis using the World Health Organization (WHO) definition for femoral neck T-scores.
Results
The majority of these overweight/obese survivors were 6.6 (±4.7) years post-diagnosis, had stage II (n = 46) or stage III (n = 16) disease, and treated with chemotherapy (76 %), radiation (72 %), and/or adjuvant hormone therapies (45 %). Mean femoral neck BMD was significantly lower in cases vs. matched non-cancer population controls (0.85 ± 0.15 vs. 0.91 ± 0.14 g/cm2, respectively; p = 0.007). However, the prevalence of low bone mass and osteoporosis was low and did not significantly differ between groups (n = 101 pairs; p = 0.26), even when restricted to those on adjuvant hormone therapies (n = 45 pairs; p = 0.75). Using conditional logistic regression, controlling for dietary factors and education, the odds of developing compromised bone health in AA breast cancer survivors was insignificant (OR 1.5, 95 % CI 0.52, 5.56).
Conclusions
These null case–control findings challenge the clinical assumption that osteoporosis is highly prevalent among all breast cancer survivors, providing foundational evidence to support differences by race/ethnicity and body weight.
Implications for Cancer Survivors
Routine bone density testing and regular patient–provider dialogue is critical in overweight/obese AA breast cancer survivors to ensure that healthy lifestyle factors (e.g., ideal weight, regular weight-bearing exercises, dietary adequacy of calcium and vitamin D) support optimal skeletal health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advances in breast cancer diagnosis and treatment have resulted in improved survival from this potentially devastating disease [1]. Consequently, this progress has highlighted the need to address the detection and management of multiple chronic disease conditions as these survivors age. Maintaining skeletal integrity has long been a priority in older populations, since major osteoporotic fractures are associated with significant increases in morbidity and mortality [2]. Based on recent data from the United States (US) census and the National Health and Nutrition Examination Survey (NHANES), it is estimated that 99 million adults in the US are ≥50 years of age and of these, 53.6 million (~54 %) have osteoporosis or low bone mass (previously known as osteopenia) [3]. The occurrence of osteoporosis and low bone mass increases with age, especially after the fifth decade of life [4]; however, it is also more frequent among women and varies by race/ethnicity. The prevalence of osteoporosis and low bone mass for older US women (≥50 years of age) is 15.4 and 51.4 %, respectively, which is highest for Mexican American women (20.4 and 47.8 %, respectively) and lowest for African-American (AA) women (7.7 and 36.2 %, respectively.) [3] Women treated for breast cancer often experience premature ovarian failure, are prescribed estrogen deprivation therapies, and receive cytotoxic chemotherapies that result in higher bone turnover and bone loss [5, 6]. As a result, osteoporosis is prevalent in 20–30 % of breast cancer survivors [7–10] and considered a late-effect of treatment. However, these studies included predominantly European-American women and analyzed AA women in aggregate, despite the known differences in osteoporosis by race/ethnicity in healthy populations [11]. Therefore, the bone health of AA breast cancer survivors remains largely unknown.
Vitamin D, a fat-soluble vitamin, is best recognized for its role in calcium absorption in the gastrointestinal tract and maintaining serum calcium and phosphate concentrations to support bone mineralization. Sunlight exposure constitutes the primary source of vitamin D3 since dietary sources are derived from foods with limited consumption (e.g., fortified milk, salmon, tuna, and mackerel) [12]. Because darker skin pigmentation is associated with decreased ability to absorb ultraviolet light, reports of vitamin D deficiency among AA breast cancer survivors are not surprising [13, 14]. When considered in the context of adjuvant hormonal breast cancer treatment, bone disease may be a highly prevalent yet underappreciated condition among AA breast cancer survivors. Therefore, the primary purpose of this case–control study was to examine the prevalence of compromised bone health in an exclusively AA group of breast cancer survivors and compare them to matched non-cancer population controls, taking into account important body weight, lifestyle factors, and the influence of breast cancer treatments. We hypothesized that osteoporosis would be greater in our breast cancer survivors, especially among women prescribed adjuvant hormonal therapies. A secondary purpose was to provide clinicians preliminary evidence to support and to tailor pharmacological and non-pharmacological care plans for bone health in this population.
Methods
Breast cancer participants
Participants comprised a subsample of overweight/obese AA women recruited as part of a larger, ongoing randomized controlled trial (R01CA154406) targeting AA breast cancer survivors from lower-income communities within Chicago, IL, a regional area prone to health disparities and higher breast cancer mortality [15]. The overarching goal of the larger trial was to determine the effectiveness of a behavioral weight loss program developed by and for AA breast cancer survivors. Women were recruited from 2011 to 2013 through the University of Illinois (UIC) Hospital and Health Sciences Cancer registry, oncologist referrals, community events, neighborhood leaflet distributions, posted and online advertisements and word of mouth. Eligible adult women were those who satisfied the following criteria: (1) self-identified as Black or AA females, (2) were diagnosed with stage I–III invasive breast carcinoma, (3) completed treatment (surgery, chemotherapy, and/or radiation) at least 6 months prior to recruitment (ongoing adjuvant hormonal therapies acceptable), and (4) were overweight (body mass index (BMI) 25.0–29.9 kg/m2) or obese (BMI ≥30.0 kg/m2). Women were excluded if they were (1) planning on moving out the community during the study; (2) deemed unable to engage in physical activity by their primary care physician (e.g., history of emphysema, extreme dyspnea on exertion, wheelchair or walker dependent); (3) currently pregnant, less than 3 months postpartum, or anticipating pregnancy; (4) taking prescribed weight loss medications; (5) enrolled in a formal weight loss program requiring special foods; or (6) suffering from psychiatric conditions that precluded study participation. The study was approved by the UIC Institutional Review Boards.
Women interested in the weight loss intervention were screened for initial eligibility over the telephone. Eligible women were then scheduled for a baseline interview, where the study was described in further detail, informed consent was obtained, and questionnaires were completed. Approximately, 1 month within the baseline interview, women returned for anthropometric measures and dual-energy x-ray absorptiometry (DXA) completion. For the cases, all laboratory and bone density information was collected at baseline prior to participating in the weight loss intervention.
Matched non-cancer population controls
A dataset of women was created from the 2007 to 2010 National Health and Nutrition Examination Surveys (NHANES) for comparison by using 1:1 matching on sex, age, race, and BMI [16].
Eligibility and matching
Women in the 2007–2010 NHANES datasets who were free of cancer, had complete data on age, height, weight, and femoral bone mineral density (BMD), and had 24-h dietary recall data and information on disease history (hypertension, diabetes), education, and insurance were identified. Matching criteria included age (within 1 year), sex, race, and BMI (within 2 kg/m2), based on their known relationship to bone health. To increase precision and to maximize sample size, BMI was matched within 2 kg/m2 vs. matched by weight categories (e.g., overweight, class I, II, and III obesity). All records that met the matching criteria were selected. The record having the same sex, race, and minimum age and BMI difference with a breast cancer case was selected as the control. To guarantee no duplication of non-cancer controls, the non-cancer record was deleted after being selected from the NHANES dataset and no longer available for further matching. The dataset with non-cancer population controls was vertically merged with the breast cancer dataset prior to analyses.
Study variables
The following variables were collected for all cases and controls, unless otherwise specified.
Demographics
Demographic parameters included age, sex, race, level of education (high school or less, some college, college graduate, or graduate degree), employment (paid work, not working, out of work, or other), and health insurance (none, public, private, or other). Self-reported co-morbid conditions were also collected, including hypertension and diabetes.
Additionally, cases of self-reported menopausal status, breast cancer stage, date of diagnosis and breast cancer treatments [e.g., chemotherapy (yes/no), radiation (yes/no), current or previous endocrine therapies (serum estrogen receptor modulators (SERMs) or aromatase inhibitors (AIs))], types and doses of chemotherapy, as well as length of time on adjuvant endocrine therapies were not ascertained. If a breast cancer participant was unsure of her breast cancer stage, her oncologist was contacted to verify that she was not stage 0 or IV, which precluded further participation.
Anthropometrics
Height and weight were measured by trained personnel using standardized, calibrated equipment and quality assurance measures were employed in each study. Measurements were recorded to the nearest 0.1 cm or 0.1 kg, respectively. Height and weight were used to calculate BMI (kg/m2) which was used to classify overweight and obesity.
Diet assessment
Breast cancer participants completed the Block 2005 Food Frequency Questionnaire (FFQ), a validated [17], 110-item dietary assessment tool administered by trained personnel to assess dietary intake and supplement use. FFQs were processed by Nutrition Quest (Berkeley, CA) to procure habitual dietary intakes of energy, calcium, and vitamin D. Calcium and vitamin D intake were quantified from food and beverage sources and in the form of dietary supplements. For the matched non-cancer population controls, two 24-h diet recalls were collected by NHANES interviewers trained in the USDA’s automated multiple-pass method. Data were collected in five standardized steps to accurately capture detailed descriptions of the amount, type, time, and location of food consumption, including intake of specific dietary supplements [18].
Bone density
Quantifications of femoral neck BMD were obtained using DXA, the reference standard for describing osteoporosis [19]. The left hip was scanned on all participants, unless otherwise specified. BMD was ascertained on all breast cancer participants using the iLunar device (GE Healthcare, software version 13.6), while NHANES participants completed femur scans using a Hologic QDR 4500A fan-beam densitometer (Discovery, software version 12.4). For quality control purposes, DXA machines were regularly calibrated and scans were performed and analyzed by trained technicians and study personnel blind to study outcomes. Although DXA devices from all manufacturers have inherently high accuracy and precision in quantifying bone density, BMD quantifications will not be identical across manufacturers due to differences in calibration techniques [20]. Therefore, to account for differences in femoral neck BMD between DXA manufacturers, the conversion equations set forth by Lu et al. [21] and endorsed by the GE Healthcare manufacturer (personal communication) for femoral neck and femoral total femur were applied.
Statistical analyses
Means and standard deviations (SDs) were used to describe normally and non-normally distributed variables. Frequencies were used as descriptors for categorical variables. A paired t test or Wilcoxon signed rank test was used for continuous variables, and the McNemar’s test or a Mantel–Haenszel matched-pairs analysis test was used for categorical variables. The following World Health Organization (WHO) diagnostic criteria were used to classify bone health for all participants: osteoporosis [a BMD value that lies 2.5 SDs or more below the average value for young healthy women (a T-score of <−2.5 SDs)], low bone mass [a BMD value between 1.0 and 2.5 SDs below mean of young adult reference group (T-score −1.0 to −2.5 SDs)], and normal bone mass (T-score >−1.0 SD). As recommended by the WHO, femoral BMD values of 20–29-year-old healthy, non-Hispanic White women from NHANES III 1988–1994 were used to calculate femoral neck T-scores for all breast cancer cases and matched non-cancer population controls [22, 19].
Conditional logistic regression was used to assess whether having breast cancer was a risk factor for osteoporosis and low bone mass (a T-score of ≤−1.0), controlling for the well-known confounders of age, sex, race, and BMI (through matching). In addition, dietary intakes of calcium (calcium intake ≥1200 mg/day) and vitamin D (vitamin D intake ≥600 IU/day) were included since these specific cut-points are considered sufficient to support bone health for ~98 % of the population by the Institute of Medicine [12]. The potential confounding effect of educational level (college degree yes/no) was also explored. Based on previous studies demonstrating adverse relationships with adjuvant hormone therapies and bone health [23, 24], women who had been prescribed AIs at any time (current or prior use) and received tamoxifen prior to menopause or tamoxifen prior to 45 years of age were compared to their sex-, age-, race-, and BMI-matched counterparts. Conditional logistic modeling for this subanalysis was approached similarly to the primary outcome analyses.
Although strong correlations between nutrient data obtained from FFQs and 24-h dietary recall have been reported, inconsistencies remain [25]. To address concerns regarding differences in nutrient intake by dietary assessment methodology, linear trends in femoral BMD were assessed across quartiles of total calcium and vitamin D intake using FFQ and 24-h recall dietary data. All statistical analyses were performed using SAS (version 9.2, 2009, SAS Institute, Cary, NC.) and a p value ≤0.05 was used to denote statistical significance.
Results
A total of 141 women were screened for the weight loss intervention. Of these, 8 withdrew, 10 did not complete the initial interview, 11 were interviewed but unable to participate, and 1 did not receive physician approval, leaving 111 breast cancer participants for inclusion in this ancillary study. When all matching criteria were applied to obtain population controls, data on 101 pairs were available. A general description of the matched participant characteristics is reported in Table 1. Breast cancer survivors had achieved higher levels of education (p = 0.0002), were more likely to be out of work (p = 0.03), but were more often had insurance coverage (p = 0.01). The average breast cancer survivor was diagnosed at 47.7 (±9.6) years of age and was 6.6 (±4.7) years post-diagnosis at the time of enrollment. The majority of the women had stage II (n = 46) or stage III (n = 16) disease, and 76 and 72 % had received chemotherapy and radiation therapy, respectively.
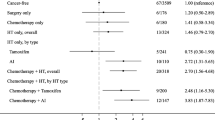
Table 2 describes the bone health characteristics between the breast cancer survivors and their matched non-cancer population controls (n = 101 pairs.) Breast cancer survivors had significantly lower femoral neck BMD (p = 0.007) and femoral neck T-scores (p = 0.007) than controls. Despite these differences, no significant differences were observed in the prevalence of low bone mass or osteoporosis (p = 0.263) between groups. Specifically, the prevalence of osteoporosis was 1 vs. 0 %, while the prevalence of low bone mass was 16 vs. 10 % in cases vs. controls, respectively. Energy intake and dietary intake of calcium were significantly higher among the breast cancer survivors compared to the matched non-cancer population controls (p = 0.007 and p = 0.05, respectively.) While supplemental vitamin D intake was significantly higher in the controls (p = 0.01), there were no differences in the average total vitamin D intake between groups. Conditional logistic regression modeling showed that AA women who had breast cancer were not more likely to have compromised bone health compared to age, sex, race, and BMI matched non-cancer population controls (crude OR 1.86, 95 % CI 0.74, 4.66). This relationship did not change after controlling for calcium intake >1200 mg/day and vitamin D intake >600 IU/day (OR 1.86, 95 % CI 0.72, 4.84), nor did it change after controlling for education (college degree; OR 1.53, 95 % CI 0.52, 5.56).
Table 3 reflects the subanalyses conducted on women who had received adjuvant hormone therapies (n = 45 pairs.) No differences between groups were found for femoral neck BMD, total femur BMD, or femoral neck T-scores. Specifically, the prevalence of osteoporosis was 0 vs. 0 %, while the prevalence of low bone mass was 18 vs. 13 % in cases vs. controls, respectively (p = 0.754). Total calcium intake was higher in the breast cancer survivors vs. the matched non-cancer population controls (p = 0.03) due to significantly higher intakes of supplemental calcium intake (p = 0.01). Similar trends were seen in the breast cancer cases for vitamin D intake. Conditional logistic regression modeling showed that AA women breast cancer survivors on adjuvant hormone therapies were not more likely to have compromised bone health compared to age-, sex-, race-, and BMI-matched non-cancer population controls (crude OR 1.50, 95 % CI 0.42, 5.32). The relationship remained insignificant after controlling for calcium intake >1200 mg/day and vitamin D intake >600 IU/day (OR 1.16, 95 % CI 0.24, 5.59) and after additionally controlling for education (college degree; OR 3.25, 95 % CI 0.28, 37.58).
Discussion
Osteoporosis is associated with age, hormone-related changes in bone microarchitecture, and decreased BMD. These increase the propensity for fracture resulting in long-term disability, morbidity, and mortality, especially among breast cancer survivors [26]. The widely cited investigation by Chen et al., which compared bone density and rate of changes in BMD between breast cancer survivors and women without cancer, showed that osteoporosis occurred in 27.2 % of the survivors vs. 19.4 % of women in the reference group over an average of 6.7 years. Among the survivors, bone loss was (1) most likely to occur within the first few years following breast cancer treatment, (2) significantly associated with chemotherapy, and (3) more prominent in women diagnosed premenopausally [7]. The prevalence of osteoporosis in other studies of female breast cancer survivors has been reported to be 20–22 % [8–10]. Our results indicate that despite suboptimal mean intakes of calcium and vitamin D and the frequent use of chemotherapy and adjuvant hormone therapies, the prevalence of osteoporosis was only 1 % which was similar to sex-, age-, race-, and BMI-matched non-cancer population controls. On average, our participants had completed their initial breast cancer treatment 6.6 years prior to the time of enrollment, a similar time period to Chen et al. [7]. Therefore, we believe this time frame was sufficient to observe the outcome of interest, if it were to occur. We speculate that the prevalence estimates are higher in other studies due to the inclusion of predominantly white women who had a substantially lower body weight and BMI. The average BMI of our study participants was 35.8 kg/m2, whereas participants in the Twiss et al. [9] and Lindsey et al. [8] studies had a BMI of 27.3 kg/m2. In the more recent investigation by Friedman et al. [10], 72 % of the participants had a BMI less than 30 kg/m2 and only 18 % of their study population was “nonwhite.” Further, although the mean calcium and vitamin D intake was considered “suboptimal” for the cases and controls in our study, a greater number of breast cancer survivors reported “optimal” calcium (>1200 mg/day) and vitamin D intake (>600 mg/day) compared to the non-cancer population controls (32 vs. 16 %, respectively; p = 0.02 and 20 vs. 12 %, respectively, p = 0.12). These nutrients are considered essential to optimal bone health [12] and may help to further explain the lower occurrence of compromised bone health in our study sample compared to others.
Although the mechanisms are not fully understood, breast cancer treatment can impact bone health in a multitude of ways, beginning with acute deteriorations in important lifestyle behaviors (e.g., diet and physical activity) that provide essential nutrients and favorable stress on the bone. In addition, women frequently experience temporary or permanent premature ovarian failure induced by chemotherapy, oopherectomy, or gonadotropin-releasing hormone (GnRH) agonists, resulting in prolonged periods of hypoestrogenemia. This estrogen deficiency leads to increased bone resorption and rapid bone loss [27]. Further, in women whose tumors express estrogen (ER) or progesterone (PR), adjuvant endocrine therapies are prescribed to induce estrogen blockade or inhibit estradiol production. In previous trials comparing the efficacy of AIs to tamoxifen in early hormone-receptor positive breast cancer, higher fractures rates were observed among women in the AI arms, revealing important negative implications of these drugs [28, 29]. Because fractures were reported as adverse events, no detailed information was provided. Subsequent bone sub-studies on these cohorts, however, revealed decreases in BMD in the lumbar spine (−6.08 vs. +2.77 %, p < .0001) and total hip (−7.24 vs. +0.74 %, p < .0001) from baseline to 5 years in patients treated with anastrozole (a non-steroidal AI) vs. tamoxifen, respectively. Changes were most pronounced within the first 2 years of therapy [23]. Additional reports found that anastrozole was associated with increases in bone turnover, whereas tamoxifen was associated with decreases in turnover [30]. Further, Lonning et al. evaluated the potential detrimental effects of exemestane (a steroidal AI) on BMD in 147 postmenopausal women with early breast cancer using a randomized (double-blind), controlled design [24]. Rates of BMD loss on an annual basis were 2.17 vs. 1.84 % in the lumbar spine (p = 0.56) and 2.72 vs. 1.48 % in the femoral neck (p = 0.02) in the exemestane and placebo arms, respectively. Similarly, Gonnelli et al. observed decreases in BMD in postmenopausal women randomized to the exemestane group vs. continuation of tamoxifen group [31]. Although these data strongly suggest that both steroidal and non-steroidal AIs detrimentally impact bone, body weight was inconsistently controlled for and race/ethnicity was not described. Conventionally, AA women are underrepresented in breast cancer trials; therefore, the implications of these therapies on overweight/obese AA breast cancer survivors cannot be readily determined. Our data continue to suggest that these therapies may have limited impact on the bone health of overweight/obese AA breast cancer survivors or that the negative impact of these therapies may be offset by the increased bone mass associated with higher body mass.
It is well accepted that race/ethnicity is an established mediator of bone health, and BMD is consistently higher in AA vs. white women and across the body weight spectrum [32–34, 4]. Previous investigators have theorized that greater muscle mass [35], resistance to bone-resorbing action of parathyroid hormone (PTH) [36], and lower concentrations of osteocalcin [37] reflective of reduced bone turnover may be responsible for these race/ethnicity differences. More recently, however, Powe et al. demonstrated that AA community-dwelling adults had significantly lower levels of vitamin D-binding protein compared to their white counterparts, which were largely explained by genetic polymorphisms in the vitamin D-binding protein genes (rs7041 and rs4588). Additionally, these authors suggested that despite significant differences in serum concentrations of 25(OH)D, bioavailable 25(OH)D levels were equivalent between races, and thus, these findings help to explain the lack of association between low 25(OH)D levels and compromised bone health in AAs in the general population [38]. It is well known that BMI is positively associated with higher BMD [39]; however, osteoporosis is considered a late effect of breast cancer treatment, regardless of BMI. Our results indicate that overweight and obese AA women are partially protected from this risk, making a unique contribution to this growing body of literature. Current prevalence estimates of skeletal health stratify by sex, race, and decade of life, not BMI or body weight [3]. As a result of matching BMI within 2 kg/m2, we may have artificially lowered the overall prevalence of compromised skeletal integrity in our study sample. More importantly, however, these study findings reinforce the importance of controlling for race/ethnicity and body weight for all future investigations examining bone health.
This case–control study is not without limitations, and several factors merit consideration. First, it is well known that body weight and body mass index are positively associated with BMD and this study did not include AA women with normal BMI (18.5–24.9 kg/m2). Considering that population estimates classify 77 % of AA women as overweight or obese [40] and that adverse body composition changes are associated with breast cancer treatment (e.g., weight gain and increased adiposity) [41], these findings likely have broad applicability for the majority of AA breast cancer survivors. Second, BMD was generated from two different DXA manufacturers in cases vs. matched non-cancer population controls, thereby requiring the application of translational equations for validated comparisons of femoral BMD and T-score calculations [21]. To further unify these BMD comparisons, all T-scores were calculated using the same referent population and BMD values were not standardized to avoid converting all data to a third metric that has limited clinical utility. Third, menopausal status, date of diagnosis, breast cancer treatments, and adjuvant hormonal therapy use was self-reported and, therefore, prone to the inherent biases and misclassifications of such reports. It is possible that women could have misreported dates or the use and type of these treatments. In addition, the length of time on various adjuvant therapies was not ascertained. Fourth, even though participants were recruited from neighborhoods of varying socioeconomic status, many of our participants were well educated, and all had the desire, motivation, and physical abilities to enroll in a weight loss program. While this may present confines on the generalizability of these findings for all AA breast cancer survivors, education (a strong proxy of socioeconomic status) was not a significant contributor in the primary outcome models. Fifth, the time of study recruitment (2011–2013) and the time controls which were ascertained from NHANES (2007–2010) do not directly align. Although significant shifts in the prevalence of obesity and osteoporosis could occur, adverse changes in these health conditions have not been observed in the previous decade [4, 42]. Sixth, the differences in macro- and micronutrient intake between groups may be explained by the use of two different dietary assessment methodologies. The failure to detect a linear trend for BMD across quartiles for calcium and vitamin D within these respective instruments minimizes the effects of these various methodologies on outcome. Finally, this study is only a “snap shot” assessment of bone health in these overweight/obese women. Studies of longer duration are needed.
Conclusions
Current evidence suggests that women who survive their breast cancer are at an elevated risk for bone disease; however, our case–control findings challenge these clinical assumptions and suggest potential differences by race/ethnicity and body weight. Despite several clinical factors that would predispose breast cancer survivors to bone disease, the prevalence of osteoporosis and low bone mass in this sample was relatively low overall and comparable to race-, sex-, age-, and BMI-matched non-cancer population controls from NHANES. Due to the lack of knowledge regarding the bone health of AA breast cancer survivors, this study makes a novel contribution to the survivorship literature and underscores several important clinical points. First, routine bone density screening is necessary to ensure bone integrity over time and to prevent unnecessary prescription and/or over-the-counter medications (e.g., bisphosphonates, dietary supplements). Similar to studies in healthy AA women [43], several of our participants stated that they had not undergone bone health screening, and some reported receiving bisphosphonates. These practices are not consistent with the recommendations of the National Comprehensive Cancer Network on bone health [44]. Second, future trials evaluating bone health endpoints need to intensify recruiting efforts to specifically include AA breast cancer survivors for appropriate risk stratification. Third, while bisphosphonates are efficacious for the prevention and/or treatment of osteoporosis [45], the importance of non-pharmacologic behaviors (e.g., regular weight-bearing exercises, physical activity, avoidance of tobacco products, limited alcohol consumption, and dietary adequacy of calcium and vitamin D) merit regular dialogue between health care providers and patients [46]. The preventative role of these behaviors should be explained in the context of bone health during the development of individually tailored survivorship care plans and should be appropriately considered (e.g., data collection and analyses) in future research efforts, especially as these minority women age.
Abbreviations
- AA:
-
African-American
- AIs:
-
Aromatase inhibitors
- BMI:
-
Body mass index
- BMD:
-
Bone mineral density
- DXA:
-
Dual-energy x-ray absorptiometry
- IOM:
-
Institute of Medicine
- IU:
-
International units
- SERMs:
-
Selective estrogen receptor modulators
- SD:
-
Standard deviation
- WHO:
-
World Health Organization
References
National Cancer Institute SEER Cancer Statistics Review 1975–2010, National Cancer Institute. http://seer.cancer.gov/csr/1975_2010/browse_csr.php?sectionSEL=4&pageSEL=sect_04_table.10.html. Accessed December 23 2013.
Sattui SE, Saag KG. Fracture mortality: associations with epidemiology and osteoporosis treatment. Nat Rev Endocrinol. 2014;10(10):592–602. doi:10.1038/nrendo.2014.125.
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6. doi:10.1002/jbmr.2269.
Looker AC, Melton 3rd LJ, Harris TB, Borrud LG, Shepherd JA. Prevalence and trends in low femur bone density among older US adults: NHANES 2005-2006 compared with NHANES III. J Bone Miner Res. 2010;25(1):64–71. doi:10.1359/jbmr.090706.
Brufsky AM. Cancer treatment-induced bone loss: pathophysiology and clinical perspectives. Oncologist. 2008;13(2):187–95. doi:10.1634/theoncologist. 2007-0152.
Winters-Stone KM, Schwartz A, Nail LM. A review of exercise interventions to improve bone health in adult cancer survivors. J Cancer Surviv. 2010;4(3):187–201. doi:10.1007/s11764-010-0122-1.
Chen Z, Maricic M, Pettinger M, Ritenbaugh C, Lopez AM, Barad DH, et al. Osteoporosis and rate of bone loss among postmenopausal survivors of breast cancer. Cancer. 2005;104(7):1520–30. doi:10.1002/cncr.21335.
Lindsey AM, Gross G, Twiss J, Waltman N, Ott C, Moore TE. Postmenopausal survivors of breast cancer at risk for osteoporosis: nutritional intake and body size. Cancer Nurs. 2002;25(1):50–6.
Twiss JJ, Waltman N, Ott CD, Gross GJ, Lindsey AM, Moore TE. Bone mineral density in postmenopausal breast cancer survivors. J Am Acad Nurse Pract. 2001;13(6):276–84.
Friedman CF, DeMichele A, Su HI, Feng R, Kapoor S, Desai K, et al. Vitamin D deficiency in postmenopausal breast cancer survivors. J Women's Health. 2012;21(4):456–62. doi:10.1089/jwh.2011.3009.
Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891–9. doi:10.1007/s11999-011-1863-5.
Institute of Medicine of the National Academies. Dietary reference intakes for calcium and vitamin D. Washington, DC. Report Brief; 2010. Available at: www.iom.edu. Accessed 23 March 2015.
Neuhouser ML, Sorensen B, Hollis BW, Ambs A, Ulrich CM, McTiernan A et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr. 2008;88(1):133–9. doi: 88/1/133.
Trukova KP, Grutsch J, Lammersfeld C, Liepa G. Prevalence of vitamin d insufficiency among breast cancer survivors. Nutr Clin Pract. 2012;27(1):122–8. doi:10.1177/0884533611431461.
Ansell D, Grabler P, Whitman S, Ferrans C, Burgess-Bishop J, Murray LR, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Causes Control. 2009;20(9):1681–8. doi:10.1007/s10552-009-9419-7.
Centers for Disease Control and Prevention/National Center for Health Statistics. National Health and Nutrition Examination Survey Data. http://www.cdc.gov/nchs/nhanes.htm. Accessed May 12, 2014.
Mares-Perlman JA, Klein BE, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr. 1993;123(3):489–501.
Centers for Disease Control and Prevention/National Center for Health Statistics. National Health and Nutrition Examination Survey http://www.cdc.gov/nchs/tutorials/dietary/surveyorientation/dietarydataoverview/info2.htm. Accessed December 16, 2014
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton 3rd LJ, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–75. doi:10.1016/j.bone.2007.11.001.
Bonnick S, editor. Bone densitometry in clinical practice. New York: Humana Press; 2010.
Lu Y, Fuerst T, Hui S, Genant HK. Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int J Established Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA. 2001;12(6):438–44. doi:10.1007/s001980170087.
Looker AC, Orwoll ES, Johnston Jr CC, Lindsay RL, Wahner HW, Dunn WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Mineral Res Off J Am Soc Bone Mineral Res. 1997;12(11):1761–8. doi:10.1359/jbmr.1997.12.11.1761.
Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(7):1051–7. doi:10.1200/JCO.2007.11.0726.
Lonning PE, Geisler J, Krag LE, Erikstein B, Bremnes Y, Hagen AI, et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(22):5126–37. doi:10.1200/JCO.2005.07.097.
Gibson R. Principles of nutrition assessment. 2nd ed. New York: Oxford University Press; 2005.
Kanis JA, McCloskey EV, Powles T, Paterson AH, Ashley S, Spector T. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer. 1999;79(7–8):1179–81. doi:10.1038/sj.bjc.6690188.
Abdel-Razeq H, Awidi A. Bone health in breast cancer survivors. J Cancer Res Ther. 2011;7(3):256–63. doi:10.4103/0973-1482.87006.
Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(5):486–92. doi:10.1200/JCO.2006.08.8617.
Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45–53. doi:10.1016/S1470-2045(07)70385-6.
Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the anastrozole, tamoxifen, alone or in combination (ATAC) trial (18233230). J Bone Miner Res. 2006;21(8):1215–23. doi:10.1359/jbmr.060508.
Gonnelli S, Cadirni A, Caffarelli C, Petrioli R, Montagnani A, Franci MB, et al. Changes in bone turnover and in bone mass in women with breast cancer switched from tamoxifen to exemestane. Bone. 2007;40(1):205–10. doi:10.1016/j.bone.2006.06.027.
Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20(2):185–94. doi:10.1359/JBMR.041007.
Cauley JA, Lui LY, Stone KL, Hillier TA, Zmuda JM, Hochberg M, et al. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American women. J Am Geriatr Soc. 2005;53(2):183–9. doi:10.1111/j.1532-5415.2005.53101.x.
Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93(3):861–8. doi:10.1210/jc.2007-1876.
Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr. 2000;71(6):1392–402.
Cosman F, Morgan DC, Nieves JW, Shen V, Luckey MM, Dempster DW, et al. Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res. 1997;12(6):958–66. doi:10.1359/jbmr.1997.12.6.958.
Finkelstein JS, Sowers M, Greendale GA, Lee ML, Neer RM, Cauley JA, et al. Ethnic variation in bone turnover in pre- and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87(7):3051–6.
Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. doi:10.1056/NEJMoa1306357.
Lloyd JT, Alley DE, Hawkes WG, Hochberg MC, Waldstein SR, Orwig DL. Body mass index is positively associated with bone mineral density in US older adults. Arch Osteoporos. 2014;9(1):175. doi:10.1007/s11657-014-0175-2.
Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults 1999-2010. JAMA. 2012;307(5):491–7. doi:10.1001/jama.2012.39.
Sheean PM, Hoskins K, Stolley M. Body composition changes in females treated for breast cancer: a review of the evidence. Breast Cancer Res Treat. 2012;135(3):663–80. doi:10.1007/s10549-012-2200-8.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–14. doi:10.1001/jama.2014.732.
Wilkins CH, Goldfeder JS. Osteoporosis screening is unjustifiably low in older African-American women. J Natl Med Assoc. 2004;96(4):461–7.
Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar RN, et al. NCCN task force report: bone health in cancer care. J Natl Compr Cancer Netw JNCCN. 2009;7(3):S1–32. quiz S3-5.
Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(6):967–75. doi:10.1200/JCO.2009.24.5902.
Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar R, et al. NCCN task force report: bone health in cancer care. J Natl Compr Cancer Netw JNCCN. 2013;11(3):S1–50. quiz S1.
Conflict of interests
The authors have no conflict of interests to disclose regarding the conduct and report of this work.
Funding
This study was funded by the National Institute on Aging, Midwest Roybal Center for Health Promotion and Translation (P30AG022849); National Cancer Institute, Moving Forward (R01CA154406); National Cancer Institute, Cancer Education and Career Development Program (R25CA057699).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheean, P., Liang, H., Schiffer, L. et al. Assessing the prevalence of compromised bone health among overweight and obese African-American breast cancer survivors: a case–control study. J Cancer Surviv 10, 21–30 (2016). https://doi.org/10.1007/s11764-015-0448-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-015-0448-9