Abstract
In the current study, an extracellular thermostable lipase from Bacillus sp. strain L2 was produced and purified through two-steps purifications, including ammonium sulfate precipitation and Heparin-Sepharose affinity chromatography. Then, the optimum pH, optimum temperature, thermostability, the effect of metal ions and inhibitors, and substrate specificity towards the natural oils were investigated. Extracellular L2 lipase showed a purification fold of 2.74 and specific activity of 3.54 U/mg towards olive oil as substrate. Furthermore, the purified extracellular L2 lipase had the optimum temperature and pH of 80 °C and pH 7, respectively. The half-lives (t1/2) of L2 lipase at 80 and 85 °C were 150 and 13.43 min, respectively. Moreover, the SDS-PAGE analysis illustrated the single band with a molecular mass of 43 kDa. Moreover, metal ions, including 10 mM concentrations of the Ba2+, Mn2+, Zn2+, Fe3+, Cu2+, and Sr2+, demonstrated inhibitory effects on the L2 lipase activity by decreasing the lipase activity by 100, 18.8, 4.16, 18.86, 100, and 6.25 times. However, the 5 mM concentration of Ca2+ metal ions improved the lipase activity by 1.2 fold. Furthermore, the results after 30 min incubation of L2 lipase with pCMB, PMSF, EDTA, and DTT illustrated that L2 lipase retained 4, 5.3, 5.5, and 26.6% of its initial activity, respectively. The substrate specificity results also illlustrated relative lipase activities of 200, 66.66, 44, 40, 11.33, 9.3, and 13.33% towards sesame oil, coconut oil, rice bran oil, corn oil, sun floweroil, soybean oil, and canola oil, respectively, compared to olive oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial lipases (EC 3.1.1.3) are widely distributed enzymes with significant physiological function and potential for application in industry. Unlike esterases, lipases catalyze the hydrolysis of triacylglycerols to glycerol and free fatty acids (Chandra et al. 2020; Alias et al. 2023). Lipases are activated only when adsorbed to an oil-water interface and do not hydrolyze dissolved substrates in the bulk fluid (Geoffry and Achur 2018). Furthermore, Lipases are serine hydrolases and display little activity in aqueous solutions containing soluble substrate (Ben Salah et al. 2007; Oyedele et al. 2019). Microbial lipases are mostly applicable in the industrial sectors, including biofuel, animal feed, detergents, textiles, leather, cosmetics, and paper (Raveendran et al. 2018; Vishnoi et al. 2020). Thermostability is one of the most important factors for industrial application of lipases. The thermostability of lipases can either be improved through protein engineering approaches or by discovering the lipases from thermophilic microorganisms from extreme and harsh regions such as hot springs (Contesini et al. 2020; Hamdan et al. 2021, 2023). Several investigations have illustrated the isolation of thermostable lipases from different microorganisms, including Brevibacillus thermoruber (Atanasova et al. 2023), thermo-halophilic bacterium (Febriani et al. 2020), Pseudomonas moraviensis M9 (Yang et al. 2015), and Thermus aquaticus (Febriani et al. 2013) that showed their maximum activities at temperatures 55 °C, 70 °C, 65 °C, and 65 °C, respectively. The features of enzymes, such as optimum pH and temperature, are sometimes changed through heterologous expression. For instance, the phytase PhyA from Aspergillus niger showed the two optimum pH of 2.5 and 5.5 through homologous expression (Dotsenko et al. 2022). Meanwhile, the expression of phytase PhyA in the E. coli expression system illustrated the optimum pH of 6.5 (Ushasree et al. 2014). The previous investigation isolated a thermophilic lipolytic Bacillus sp. strain L2 from a hot spring (Shariff et al. 2007). Then, its lipase gene was cloned and expressed in the Pichia pastoris expression system for characterization of the purified L2 lipase. The results demonstrated that the L2 lipase has the maximum activity at pH and temperature of 8.0 and 70 °C, respectively (Sabri et al. 2009). Therefore, the current study aims to purify the lipase from Bacillus sp. strain L2 through two-step purifications, including the ammonium sulfate precipitation and Heparin-Sepharose affinity chromatography, respectively. Subsequently, the current study investigated the optimum pH, optimum temperature, and thermostability, the effect of metal ions and inhibitors, and substrate specificity towards the natural oils to discover the differences between purified heterologous recombinant L2 lipase in P.pastoris and the purified extracellular lipase from the Bacillus sp. strain L2.
Materials and methods
Production and purification of the lipase from Bacillus sp. strain L2
The isolated thermophilic lipolytic Bacillus sp. strain L2 from a hot spring (91 °C) in Slim River, Perak was used in the current investigation, and the optimized condition of production of lipase that was obtained in the previous study was used in this study (Shariff et al. 2007). The crude lipase was obtained by centrifugation of growth culture at 10,000 x g, 4 °C for 10 min. The L2 lipase was precipitated at 0–20% ammonium sulphate saturation. Then, the precipitated lipase was collected by centrifugation at 14,000 x g for 15 min and dissolved in 20 mM Tris-HCl buffer (pH 7.4). Afterward, the suspension was dialyzed against the 20 mM Tris-HCl buffer (pH 7.4) for 18 h at 4 °C with regular changing buffer every 6 h. Then, the dialyzed lipase was loaded into the Heparin-Sepharose affinity chromatography column that was previously equilibrated with the PBS buffer (pH 7.4) with a flow rate of 0.5 mL/min. The unbound protein was washed with PBS buffer (pH 7.4). Finally, the gradients of NaCl from 0 to 1.5 M with a flow rate of 1 mL/min were applied to elute the bounded protein and followed by lipases assay for each fraction. The protein concentration of the samples was determined using the Bradford assay method with bovine serum albumin as a standard (Bradford 1976). The analysis of the molecular mass of purified protein was carried out using 12% SDS-PAGE.
Lipase assay
The amount of released free fatty acid was determined using a modified colorimetric approach using olive oil as a substrate (Kwon and Rhee 1986). The reaction mixture was prepared by combining 50 µL of purified lipase with the concentration of 0.5 mg/mL, 950 µL of 50 mM Tris-HCl buffer (50 mM, pH 7), 2.5 mL olive oil emulsion (1:1 50 mM Tris-HCl (pH 7) buffer and olive oil) and then followed by incubation at 80 °C and 200 rpm for 30 min. Afterwards, the reaction was subsequently halted by adding 5 mL of isooctane and 1 mL of 6 N HCl, followed by adding 1 mL of cupric acetate pyridine (Masomian et al. 2018). Finally, the optical density (OD) of the sample was measured at a wavelength of 715 nm to determine the free fatty acids. One unit (U) of lipase activity was defined as the amount of the enzyme that liberated one µmol of fatty acid per minute under standard test conditions (Borkar et al. 2009).
Detection of pH and temperature profiles of L2 lipase
The pH profile of L2 lipase was detected through lipase assay toward olive oil at 80 °C, with pH ranging from 4 to 12 with 1 interval. In this regard, different buffers were utilized at a concentration of 50 mM, including sodium acetate buffer (pH 4.0-5.5), phosphate buffer (pH 6.0–8.0), Tris-HCI (pH 8.0–9.0), glycine-NaOH (pH9.0-10.0), and disodium hydrogen phosphate (pH 10.0–12.0) (Masomian et al. 2018). Moreover, the temperature profile of lipase was detected at the range of 30 to 85 °C with 5 °C intervals.
Thermostability investigation of L2 lipase
The thermostability of L2 lipase was investigated by pre-incubation of the purified enzyme at 80 and 85 °C from 30 to 180 min with 30 min intervals. Then, the enzyme was cooled on ice and followed by lipase assay at 80 °C and pH 7 towards olive oil. The residual lipase activity was recorded at each 30 min interval to show the thermostability of the enzyme. The thermostability graph was plotted between incubation time and the percentage of the residual activity. The half-lives of L2 lipase were evaluated at temperatures 80 and 85 ˚C by fitting the experimental data to an exponential decay equation to indicate the first-order decay (Pulido et al. 2020).
Effect of metal ions and inhibitors on L2 lipase
The effect of chloride salt solutions of metal ions, including Ca 2+, Cu2+, Fe2+, Mn2+, Co2+, Sr2+, Ba2+, and Zn2+ on L2 lipases was investigated by pre-incubation of lipase for 30 min in 50 mM Tris-HCl buffer (pH 7) including concentrations of 2, 5 and 10 mM of metal ions. Furthermore, the effect of the different inhibitors, including ethylenediaminetetraacetic acid (EDTA), Dithiothreitol (DTT), Phenylmethanesulfonyl fluoride (PMSF), and P-Chloromercuribenzcic acid (pCMB) on L2 lipase activity was investigated by pre-incubation of purified lipase for 30 min in 50 mM Tris-HCl buffer (pH 7) with the 10 mM of each reagent and followed by standard lipase assay with incubation at 80 °C for 10, 20, and 30 min to obtain the relative activity.
Substrate specificity of L2 lipase towards natural oils
The substrate specificity of L2 lipase was investigated towards natural oils, including coconut oil, rice bran oil, olive oil, sesame oil, canola oil, soybean oil, corn oil, and sunflower oil. The natural oils were emulsified in 50 mM Tris-HCl buffer in a 1:1 ratio at pH 7 and then utilized as substrates for L2 lipase assay. The lipase assay was carried out in 50 mM Tris-HCl buffer at pH 7 and 80 °C for 30 min.
Data analysis
GraphPad Prism 9 (GraphPad Software Inc., CA, USA) was used to analyze the gathered numerical data. The data was presented as the mean ± standard deviation. The data were initially compared using one-way ANOVA and Tukey’s multiple comparison test. The P-values below 0.05 were deemed to indicate statistical significance.
Results and discussion
Production and purification of thermostable lipase
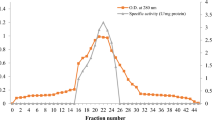
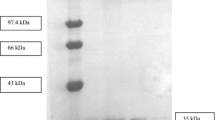
The promoted production of lipase from Bacillus sp. strain L2 was carried out by adding 1% casamino acid (w/v), 1.5% Tween (v/v), 5 mM Ca2+, and 1% trehalose (w/v) and then followed by incubation at 70 °C and 150 rpm for 28 h (Shariff et al. 2007). The two steps of purification, including the ammonium sulfate precipitation and Heparin-Sepharose affinity chromatography, were performed to obtain the single band of L2 lipase. The purification profile of L2 lipase is summarized in Table 1. The extracellular L2 lipase was purified with a purification fold and yield of 2.74 and 34.88%, respectively. Purification of L2 lipase increased the lipase activity from 0.125 U/mL to 0.44 U/mL with increases in specific lipase activity from 1.3 U/mg to 3.54 U/mg after two steps of purification. The SDS-PAGE analysis of extracellular L2 lipase showed a single band with a molecular mass of 43 kDa (Fig. 1) that was the same molecular mass as the reported ones of the recombinant L2 lipase (Shariff et al. 2011; Abd Rahman et al. 2012).
(a) Elution profile of the lipase on Affinity column Heparin. (b) SDS-PAGE analysis of L2 lipase purification at which lane M illustrates the protein ladder (Cat. No. 26,610), lane 1 demonstrates the crude enzyme, and lane 2 and 3 demonstrate purified L2 lipase after two steps of purification with the molecular mass of 43 kDa
Determination of temperature and pH profiles, and thermostability of L2 lipase
The purified L2 lipase illustrated the optimum activity at pH 7, while it showed more than 60% activity at pH 5, 6, and 8. The lipase activity was decreased to about 47% at pH 9 followed by a drastic decrease to 25% at pH 10. The lipase also showed 20 and 5% activity at pH 11 and 12, respectively (Fig. 2a). In the previous investigation, expressed recombinant L2 lipase in P.pastoris showed the optimum activities at pH 8 (Sabri et al. 2009). This difference in optimum pH can be derived from the post-translational modification in the P. pastoris expression system (Karbalaei et al. 2020). To detect the temperature profile of L2 lipase, the lipase assays were carried out at a temperature range of 50 to 85 °C with 5 °C intervals. The results demonstrated the optimum temperature at 80 °C, while the lipase activity was started at 50 °C and followed by a dramatic increase until 75 °C with 95% of its optimum activity. L2 lipase at 70 and 85 °C showed activity around 75% compared to the optimum temperature (Fig. 2b). However, the current investigation demonstrated the maximum lipase activity at pH 7 and 80 °C. However, the previous study on the expressed recombinant L2 lipase in P. pastoris demonstrated the optimum activity at pH 8 and 70 °C (Sabri et al. 2009). Protein expression in the P. pastoris likely affected the optimum pH and temperature of the L2 lipase due to the post-translational modifications that usually occur in the P. pastoris expression system (Karbalaei et al. 2020). The optimum temperature of purified L2 lipase in the current study showed 25, 10, and 22.5 °C higher optimum temperature compared to recently investigated thermostable lipases from Brevibacillus thermoruber (Atanasova et al. 2023), thermo-halophilic bacterium (Febriani et al. 2020), and Anoxybacillus sp. ARS-1 (Sahoo et al. 2020), respectively. Concerning thermostability, the L2 lipase retained 93% of its initial activity after 120 min incubation at 80 °C. It retained only 6% of its initial activity after 30 and 60 min incubation at 85 °C. Concerning half-life (t1/2), the L2 lipase illustrated 150 and 13.43 min at 80 and 85 °C, respectively (Fig. 2c).
Effect of metal ions on L2 lipase
Metal ions play a significant role in the functions of numerous enzymes (Riordan 1977). Enzyme activity can be increased or inhibited in the presence of different metal ions. For instance, it has been illustrated that Ca2+ ions increased the activity and folding of lipase from Antarctic Pseudomonas fluorescens strain AMS8 (Ali et al. 2020). Therefore, the effects of the metal ions on L2 lipase were investigated in the current study. The results illustrated that 5 mM concentration of Ca2+ ions improved the lipase activity by 1.2 fold. While, other metal ions, including 2 mM concentrations of the Ba2+, Mn2+, Zn2+, Fe3+, Cu2+, and Sr2+, showed inhibitory effects on the L2 lipase activity by decreasing the lipase activity by 3.4, 2.7, 0.16, 0.165, 12.5, 18.86 folds. Furthermore, metal ions, including 5 mM concentrations of the Ba2+, Mn2+, Zn2+, Fe3+, Cu2+, and Sr2+, demonstrated inhibitory effects on the L2 lipase activity by decreasing the lipase activity by 9.43, 1.7, 3.125, 1.38, 15, and 2.68 folds, respectively. Moreover, metal ions, including 10 mM concentrations of the Ba2+, Mn2+, Zn2+, Fe3+, Cu2+, and Sr2+, demonstrated inhibitory effects on the L2 lipase activity by decreasing the lipase activity by 100, 18.8, 4.16, 18.86, 100, and 6.25 times (Fig. 3). In the current study, only the 5 mM Ca2+ could improve lipase activity (Fig. 3). Ca2+ improved lipase activity of a thermostable lipase from a thermo-halophilic bacterium, while Zn2+ decreased lipase activity (Febriani et al. 2020). Furthermore, investigating the effect of metal ions on a lipase from Chromohalobacter japonicus BK-AB18 also demonstrated that Ca2+ was the only metal ion that increased the lipolytic activity of lipase, while Zn2+and Ba2+ metal ions diminished the lipase activity (Hertadi and Widhyastuti 2015).
Substrates specificity of evaluation towards natural oils
The lipase activity was evaluated towards various commercially available natural oils, including sesame oil, coconut oil, rice bran oil, corn oil, sunflower oil, soybean oil, and canola oil and the olive oil was considered as control (100%). The results illlustrated relative lipase activities of 200, 66.66, 44, 40, 11.33, 9.3, and 13.33% towards sesame oil, coconut oil, rice bran oil, corn oil, sunflower oil, soybean oil, and canola oil, respectively, compared to olive oil (Fig. 4). The results demonstrated that L2 lipase has activity towards a range of the natural oils. However, it has a higher affinity towards long-carbon chain natural oils, including sesame and olive oils. A lipase from Aspergillus tamarii JGIF06 also showed a high substrate affinity towards sesame oil. This lipase showed the highest lipolytic activity of 48,333.33 U/mL towards peanut oil, while its optimum temperature was 37 °C (Das et al. 2016). However, the highest lipolytic activity of L2 lipase in the current investigation was 0.72 ± 0.011 U/mL towards the sesame oil, which has an optimum temperature of 80 °C. It has been demonstrated that thermostable enzymes have lower activities due to their rigid protein structure (Nezhad et al. 2022, 2023). Therefore, the low activity of the L2 lipase might be associated with its rigid structure.
Inhibitory effects of reagents on L2 lipase activity
The inhibitory effects of the pCMB, PMSF, EDTA, and DTT were investigated on the purified L2 lipase at 10, 20, and 30 min. After 10 min incubation of L2 lipase with pCMB, PMSF, EDTA, and DTT, the results illustrated that L2 lipase retained 14.6, 20, 73.3, and 56% of its initial activity, respectively. Moreover, the results after 20 min incubation of L2 lipase with pCMB, PMSF, EDTA, and DTT illustrated that L2 lipase retained 5.3, 13.3, 29.35, 37.6% of its initial activity, respectively. Furthermore, the results after 30 min incubation of L2 lipase with pCMB, PMSF, EDTA, and DTT illustrated that L2 lipase retained 4, 5.3, 5.5, and 26.6% of its initial activity, respectively (Fig. 5). The pCMB reagent showed the strongest inhibitory effect on the lipase L2 and decreased lipase activity by 96% after 30 min incubation. The pCMB is a thiol-binding reagent that reacts with the thiol groups in the enzymes and plays an inhibitory role in enzyme activity that their reactivity is dependent on thiol (Kundu et al. 1987). Therefore, it can be concluded that a thiol group of cysteine residue is probably close to the active site of the L2 lipase. Moreover, the PMSF showed the second strong inhibitory effect that decreased the lipase activity by 94.7% after 30 min incubation. The PMSF, a serine protease inhibitor, can interact with serine in the active site of lipase (Febriani et al. 2013, 2020). A previous investigation on the elucidation of L2 lipase structure unraveled the catalytic triad of Ser 113, His 358 and Asp 317 (Abd Rahman et al. 2012). Therefore, this lipase activity inhibition by PMSF is derived from the interaction between the PMSF and serine residue in the active site of L2 lipase. The EDTA decreased lipase activity by 94.5% and was the third strong inhibitor in the current investigation. Therefore, this lipolytic activity inhibition by EDTA showed that L2 lipase is a metalloprotein.
Conclusion
The extracellular thermostable lipase from Bacillus sp. strain L2 was produced and purified through two-step purifications, including ammonium sulfate precipitation and Heparin-Sepharose affinity chromatography. The optimum temperature and pH of lipase in this study were 80 °C and 7, respectively. Meanwhile, in the previous investigation, the expression of recombinant lipase from Bacillus sp. strain L2 in the P.pastoris showed the optimum temperature and pH of 70 °C and 8, respectively. The current study demonstrated that the production and purification of L2 lipase from its WT host can preserve the native lipase features, which is better than the heterologous expression.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abd Rahman RNZR, Shariff FM, Basri M, Salleh AB (2012) 3D structure elucidation of thermostable l2 lipase from thermophilic Bacillus sp. L2. Int J Mol Sci 13:9207–9217. https://doi.org/10.3390/ijms13079207
Ali NSM, Salleh AB, Rahman RNZRA et al (2020) Calcium-induced activity and folding of a repeat in toxin lipase from antarctic Pseudomonas fluorescens strain AMS8. Toxins (Basel) 12:. https://doi.org/10.3390/toxins12010027
Alias, Nezhad NG, Normi YM et al (2023) Recent advances in overexpression of functional recombinant lipases. Mol Biotechnol 65:1737–1749. https://doi.org/10.1007/s12033-023-00725-y
Atanasova N, Paunova-Krasteva T, Kambourova M, Boyadzhieva I (2023) A thermostable lipase isolated from Brevibacillus thermoruber strain 7 degrades Ɛ-Polycaprolactone. Biotech 12:23. https://doi.org/10.3390/biotech12010023
Ben Salah R, Mosbah H, Gargouri Y, Mejdoub H (2007) Comparative study of kinetic and interfacial properties of a novel Rhizopus oryzae lipase and ROL29. OCL - oilseeds fats. Crop Lipids 14:361–365. https://doi.org/10.1051/ocl.2007.0144
Borkar PS, Bodade RG, Rao SR, Khobragade CN (2009) Purification and characterization of extracellular lipase from a new strain: Pseudomonas aeruginosa SRT 9. Brazilian J Microbiol 40:358–366. https://doi.org/10.1590/s1517-83822009000200028
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chandra P, Enespa, Singh R, Arora PK (2020) Microbial lipases and their industrial applications: A comprehensive review. BioMed Central
Contesini FJ, Davanço MG, Borin GP et al (2020) Advances in recombinant lipases: production, engineering, immobilization and application in the pharmaceutical industry. Catalysts 10:1–33. https://doi.org/10.3390/catal10091032
Das A, Shivakumar S, Bhattacharya S et al (2016) Purification and characterization of a surfactant-compatible lipase from aspergillus tamarii JGIF06 exhibiting energy-efficient removal of oil stains from polycotton fabric. 3 Biotech 6:1–8. https://doi.org/10.1007/s13205-016-0449-z
Dotsenko A, Rozhkova A, Zorov I et al (2022) Enhancement of activity and thermostability of Aspergillus Niger ATCC 10864 phytase A through rational design. Biochem Biophys Res Commun 634:55–61. https://doi.org/10.1016/j.bbrc.2022.10.010
Febriani I, Hertadi R et al (2013) Thermostable alkaline lipase isolated from Thermus aquaticus. Int J Integr Biol 14:104–112
Febriani AN, Kemala P et al (2020) Novel thermostable lipase produced by a thermo-halophilic bacterium that catalyses hydrolytic and transesterification reactions. Heliyon 6:e04520. https://doi.org/10.1016/j.heliyon.2020.e04520
Geoffry K, Achur RN (2018) Screening and production of lipase from fungal organisms. Biocatal Agric Biotechnol 14:241–253. https://doi.org/10.1016/j.bcab.2018.03.009
Hamdan SH, Maiangwa J, Ali MSM et al (2021) Thermostable lipases and their dynamics of improved enzymatic properties. Appl Microbiol Biotechnol 105:7069–7094. https://doi.org/10.1007/s00253-021-11520-7
Hamdan SH, Maiangwa J, Nezhad NG et al (2023) Knotting terminal ends of mutant T1 lipase with disulfide bond improved structure rigidity and stability. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-023-12396-5
Hertadi R, Widhyastuti H (2015) Effect of Ca2 + ion to the Activity and Stability of Lipase Isolated from Chromohalobacter japonicus BK-AB18. Procedia Chem 16:306–313. https://doi.org/10.1016/j.proche.2015.12.057
Karbalaei M, Rezaee SA, Farsiani H (2020) Pichia pastoris: a highly successful expression system for optimal synthesis of heterologous proteins. J Cell Physiol 235:5867–5881. https://doi.org/10.1002/jcp.29583
Kundu M, Basu J, Guchhait M, Chakrabarti P (1987) Isolation and characterization of an extracellular lipase from the conidia of Neurospora Crassa. J Gen Microbiol 133:149–153. https://doi.org/10.1099/00221287-133-1-149
Kwon DY, Rhee JS (1986) A simple and rapid colorimetric method for determination of free fatty acids for lipase assay. J Am Oil Chem Soc 63:89–92. https://doi.org/10.1007/BF02676129
Masomian M, Rahman RNZRA, Salleh AB (2018) A novel method of affinity tag cleavage in the purification of a recombinant thermostable lipase from Aneurinibacillus thermoaerophilus strain HZ. Catalysts 8:1–23. https://doi.org/10.3390/catal8100479
Nezhad NG, Abd Rahman RNZ, Normi YM et al (2022) Thermostability engineering of industrial enzymes through structure modification. Appl Microbiol Biotechnol 106:4845–4866. https://doi.org/10.1007/s00253-022-12067-x
Nezhad NG, Abd Rahman RNZ, Normi YM et al (2023) Recent advances in simultaneous thermostability-activity improvement of industrial enzymes through structure modification. Int J Biol Macromol 232:123440. https://doi.org/10.1016/j.ijbiomac.2023.123440
Oyedele SA, Ayodeji AO, Bamidele OS et al (2019) Enhanced lipolytic activity potential of mutant Bacillus niacini EMB-5 grown on Palm Oil Mill Effluent (POME) and biochemical characterization of purified lipase. Biocatal Agric Biotechnol 18:101017. https://doi.org/10.1016/j.bcab.2019.01.055
Pulido IY, Prieto E, Pieffet GP et al (2020) Functional heterologous expression of mature lipase lipa from Pseudomonas aeruginosa psa01 in Escherichia coli shuffle and bl21 (De3): Effect of the expression host on thermal stability and solvent tolerance of the enzyme produced. Int J Mol Sci 21:1–19. https://doi.org/10.3390/ijms21113925
Raveendran S, Parameswaran B, Ummalyma SB et al (2018) Applications of microbial enzymes in food industry. Food Technol Biotechnol 56:16–30. https://doi.org/10.17113/ftb.56.01.18.5491
Riordan JF (1977) The role of metals in enzyme activity. Ann Clin Lab Sci 7:119–129
Sabri S, Rahman RNZRA, Leow TC et al (2009) Secretory expression and characterization of a highly Ca2+-activated thermostable L2 lipase. Protein Expr Purif 68:161–166. https://doi.org/10.1016/j.pep.2009.08.002
Sahoo RK, Das A, Gaur M et al (2020) Parameter optimization for thermostable lipase production and performance evaluation as prospective detergent additive. Prep Biochem Biotechnol 50:578–584. https://doi.org/10.1080/10826068.2020.1719513
Shariff FM, Leow TC, Mukred AD et al (2007) Production of L2 lipase by Bacillus sp. strain L2: Nutritional and physical factors. J Basic Microbiol 47:406–412. https://doi.org/10.1002/jobm.200610275
Shariff FM, Rahman RNZRA, Basri M, Salleh AB (2011) A newly isolated thermostable lipase from Bacillus sp. Int J Mol Sci 12:2917–2934. https://doi.org/10.3390/ijms12052917
Ushasree MV, Vidya J, Pandey A (2014) Gene cloning and soluble expression of Aspergillus Niger phytase in E. Coli cytosol via chaperone co-expression. Biotechnol Lett 36:85–91. https://doi.org/10.1007/s10529-013-1322-3
Vishnoi N, Dixit S, Mishra J (2020) Microbial Lipases and Their Versatile Applications. In: Microbial Enzymes: Roles and Applications in Industries. pp 207–230
Yang W, Cao H, Xu L et al (2015) A novel eurythermic and thermostale lipase LipM from Pseudomonas moraviensis M9 and its application in the partial hydrolysis of algal oil. BMC Biotechnol 15:1–15. https://doi.org/10.1186/s12896-015-0214-0
Funding
N/A.
Author information
Authors and Affiliations
Contributions
NGN, and TCL have conceived, designed, verified the data, and wrote the manuscript. NGN and ADMM conducted the research, tabulated, and analyzed the data. RNZRA, MB, and ABS contributed to the materials used in the preparation, expressed, and purified the protein.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest with regards to the experimental design, authorship, and publication of this work.
Ethical approval
N/A.
Informed consent
N/A.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nezhad, N.G., Mukred, A.D.M., Rahman, R.N.Z.R.A. et al. Purification and biochemical characterization of extracellular thermostable lipase from Bacillus sp. strain L2. Biologia 79, 1887–1894 (2024). https://doi.org/10.1007/s11756-024-01647-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-024-01647-z