Abstract
Lasioglossum marginatum sp. is the most efficient pollinator of stone fruit crops (such as peach, plum, and cherry). Its proportional contribution to the total visitation is significantly higher than the conventional visitation. This species is polylectic, polyandrous, and an efficient pollinator that is endogeic in nature. Its nesting behavior has been studied earlier. Various nest soil physical characteristics like tumulus length, turret height, nest depth, nest density, nest diameter, nest length, and the height from the plane have been determined across the experimental sites and found to be statistically significantly higher than the normal range. Various parameters were found to be most significant in Budgam (Experiment location-I) located at an altitude of 1700 m above sea level (a. s. l.) higher than Srinagar (Experiment location-II) which is situated at an altitude of 1400 m a. s. l. The current study investigated some of the parameters like the total number of individuals per nest, cell length, cell diameter, cell area, sex ratio per nest, and the weight of pollen mass. Based on the Kolmogorov–Smirnov analysis outcomes, it was found that the species prefers clay loam soil. Further, various soil gravimetrical characteristics such as gravel/sand, slit, and clay in soils at different locations were significantly higher than the normal range. In addition to these, the Chi-square results regarding various nest soil chemical characteristics like the hydrogen ion concentration (pH), electric conductivity (dSm−1), organic matter (%), organic carbon (%), nitrogen (kg/ha), porosity (%) and soil moisture (%) content at different locations were significantly higher than the normal range. The maximum number of males emerged from the final brood in the 2nd—4th week of April; followed by the emergence of other workers and gynes, till the 4th week of May. The maximum flight activity was recorded from 15th April till the 1st week of May. The number of individuals per nest varied from 6 to 27 with a sex ratio in the range of 0.24 to 0.5. The minimum number of males and females per nest was found to be 1.0 and 5 respectively; while the maximum numbers were 9 and 18, respectively. The pollen-masses were found in short-side tunnels that were terminated with cells, in which the eggs were present and it sustained in the brood cells during the long overwintering periods. The current study helps in the conservation of bees by preserving their foraging plants and providing them with suitable and pollution-free breeding and nesting habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interaction that occurs between the insect pollinators and plants remains the foundation for underpinning and encouraging a vast biodiversity (Crowley et al. 2021) through various forms of services that are essential for ecosystem maintenance and its functioning. Pollination facilitates the development of diverse crops that finally nourish animals and human beings for their survival on earth. In this background, it is crucial to conserve the insect pollinators and understand how multiple pressures, especially the floral resources and nesting characteristics, affect bee diversity and its abundance across the globe. The existence of uninterrupted floral resources and favorable nesting sites and habitats are the most important factors for both survival and the enhancement of the bee populations in farmlands (Vercelli et al. 2012). The prerequisites for the restoration of the bee population in farmlands of different elevations and varied weather conditions are to ensure the availability of rich floral sources and good nesting habitats (Angelella et al. 2021). Arbitrary pollination by insects is the only suitable option for the pollination of crops in general. Taking this phenomenon into consideration, the research works conducted across the globe highlighted the importance of bees belonging to the family, Helictidae, especially from the genus Lasioglossum Curtis, 1833 (Family: Halictidae); the species from this family are the most important and dominant pollinators of diverse crops in urban as well as rural areas of Kashmir valley (Dar et al. 2018).
The genus Lasioglossum is one of the most evolved genera in the family and exhibits unique features like eusociality, overlapping generations, cooperative brood care, and reproductive division of the labor (Brady et al. 2011). This genus is subjected to cleptoparasites by some species from the genera Sphecodes Latreille, 1804 and Leucophora Robineau-Desvoidy, 1830. On the other hand, some researchers found a fungal association with some species of the family Halictidae and genus Lasioglossum. The genus Lasioglossum exhibits solitary nesting (Wcislo 1993), communal nesting (Kukuk and Sage 1994), semi-sociality (Packer 2006), eusociality (Wyman and Richards 2003), cleptoparasitism and social parasitism (Michener et al. 1978; Dar et al. 2017). In line with this, the species, Lasioglossum marginatum Brullé, 1832 also exhibits intraspecific variations in its sociality with different social organizations (Plateaux-Quénu et al. 2000; Gibbs et al. 2013). The dominant ground-nesting species, i.e., L. marginatum exhibits eusocial organization (Rodrigo Gómez et al. 2016) and is perennial with the nest type-III (Stockhammer 1966; Sakagami 1982). Several research investigations have been conducted earlier to study its artificial nesting and domestication patterns. However, the efforts did not achieve the desired results. The nest architecture varies heavily in terms of habitat, such as the less-frequent vertical style to the most-oblique galleries with lateral tunnels that lead to terminal ovoid-shaped clustered cells, filled with pollen balls and space in which the egg is laid.
Though the nest density varies with elevation, the nest density of most of the species is at a slope angle of 5–7 degrees. The lateral tunnels vary in their length, diameter, and angle (Antoine and Forest 2021) of the attachment to the main vertical-to-oblique shaft. Some species construct a chamber-like structure with several cells that are clustered together (Packer et al. 1989), while some other species have a loop that reconnects the tunnel to the main galley or terminates it in the dead blind end. The variability, in terms of cell death and cell number per nest gallery, differs heavily among the species (Wcislo et al. 1993). The buffer zones in the crop areas function as a reservoir of bees. In line with this, the huge pollination of the flowers facilitates the higher yield of various crops. In literature, preliminary studies have been conducted on pollination efficiency and the nest behavior of L. marginatum. If the pollination efficiency and the nesting behavior are understood appropriately, it will support the domestication and conservation processes of the species. This would be an alternative way of using wild bees in place of honey bees under current prevailing conditions of colony calliopes disorder, climate change era, and shift cultivation (Lincoln and Blair 1977).
Material and methods
Study sites
All the experimental sites were on an average elevation of 10,000 feet (3000 m) with an average height of 1850 m above sea level (a. s. l.). The landscapes selected were the sloppy type with multiple fruit crops grown in and around experimental sites. Clay types of soils were preferred for nesting while the sandy areas were avoided as nesting cavities cannot be made in sand and therefore are not preferred for bee nesting. The selected sites were not sprayed by any chemical inputs. Insect pollinator visits per square meter branch length were observed continuously for ten minutes from commencement (1st week of April) till the end of the blooming season of stone fruit crops under open conditions. For this purpose, three branches of each plant species were selected and the total numbers of individual species were recorded by visual counting at the beginning of each hour for 10 min on all the days of observation. The periods accommodate the distinctive activity patterns exhibited by the different types of insect pollinator species. Before each observation period, the number of plants in the patch and the number of open flowers on each plant were registered. During an observation period, every individual pollinator/flower visitor entering a patch of flowers, the number of the flowers visited in succession per foraging bout, and the time spent visiting the flowers during the foraging bout was recorded. Observations were taken daily using a hand tally counter and chronometer (stopwatch) following the method given by Free (1993). The stopwatch with an accuracy of 0.01 s was used to record the time spend per flower and the number of visits per unit time by different insects on the three plant species. When the bee approaches the flower or enters the flower bout the stopwatch was switched on and as it leaves the flower or flower bout it was switched off. A foraging bout started when a pollinator entered and ends when the same pollinator left the patch. All pollinators were collected and later identified as per the procedure.
Efficient pollinator
The efficiency of the pollinator was determined through the species foraging behavior.
Relative abundance
The relative abundance of different insect pollinators was determined in terms of their visits per sq. meter branch length per 10 min. The percent relative abundance of each pollinator species and their visits were calculated to identify the most efficient pollinator of stone fruit species.
Rank abundance values
Rank abundance values for all species were calculated by taking the sum of individual species found throughout the crop blooming period and ranks were assigned based on the dominance of species falling into a particular genus. The curve displays the relative species abundance and data published (Dar et al. 2016).
K-dominance curve
The curve exhibits the cumulative percentage of the abundance of Kth most dominant species concerning species (K) rank and is expressed in the log value of (Kth) rank for that species. It was drawn for all species found during the study period and published previously (Dar et al. 2016).
Nesting behavior—nesting site location
A period of 30 min was taken to monitor nests in the selected sight. Surveys were performed on sunny dry days. Observations were taken between 09:00 h and 18:00 h of days of flowering (> 80%) period of Rosaceae plant species especially peach, plum, and cherry. At varying levels of slope degrees, the nest burrows were discovered in and around the fruit orchard. The foraging bees entering (setting foot in the burrow) and leaving (exiting from the burrow) the nests were recorded. The same bee samples were collected and identified as per the standard procedure. Different nesting substrates were observed as the nesting sites of the bees. Various characteristics of the live nests found in the habitat were determined. The nesting sites from various habitats that L. marginatum use to establish and construct the new brood cells were selected. The brood cells are small secondary to tertiary branch terminals used for the development of young and were observed and tagged near their good forging habitat of around 1/3–1/4 km away from fruit crops.
Nest structure of L. marginatum
We found that L. marginatum prefers loose, well-drained soil in sunny spots facing the southern aspect. Sloppy, flat, and earthen banks were used for nesting. Most preferred sloppy to flat areas, while some bee proportion prefer earthen banks for nesting. So different landscape areas with different slopes facing the south and having higher exposure to sun radiations were chosen. The farmlands with healthy and friable soil were selected and the vegetation was removed several yards across to observe the nests. Different ground conditions from vertical banks to well-drained flat ground draw different results, so in the present investigation, the flat ground of slope 5 was preferably selected for studying the nesting behavior of L. marginatum.
Nest structure
Depending on the environmental conditions various parameters were recorded after the nest was discovered by following the transect walk of one square meter in the orchard from each location.
Tumulus
Most female bees of L. marginatum construct their nest in the soil of the upland areas revealed by the presence of an ant mound-like clump of excavated soil at the surface called a tumulus. The tumuli were best seen in the early morning when the fresh moist soil pellets were excavated and pushed to the surface by the female during the night is still damp. These circular piles of the earth dry and may be eroded by wind and rain. The tumuli may be clustered in areas of suitable soil, or scattered over the surface. The closed tumuli indicate that the female is in her burrow constructed, while the open tumuli indicate that it had once left the burrow and has gone for foraging (Woodcock et al. 2018).
Turret
The long and intricate entrance of the nest above the ground surface made of soil and lined with the sticky secretion from the inner surface is called a turret. The turret is thought to function as a defense mechanism against parasites, invading bees, or insect predators. The bees construct the turret entrance tube to prevent the eggs and the offspring from ants.
Wall fineness
It is the smoothness of the internal walls of the nest tumulus and was determined by visual observation of the nest excavated.
Nest depth
It is the vertical depth to which the nest of the species L. marginatum can penetrate to lay eggs and deposit pollen for the next generation. It was measured by the graduated scale. Different nests of the same species varied in their depth depending on the slope, soil type, and foraging crop in the field.
Diameter of the nest
Burrow diameter is generally determined by the diameter of the adult bee. However, the Helictidae excavate a main burrow that is somewhat larger than normal; therefore, the diameter was determined by putting the graduated tape over the exterior of the nest hole.
Length of the nest
It was determined by digging the nest tunnel in an apparent attempt to avoid plant roots. After excavating the nest cavity carefully, ribbon tape was used to record its length.
Height of the nest
It is the height of the nest from ground level and was measured by measuring tape.
Nest density
It was determined by counting the number of live nest burrows per square meter area in an orchard (nests/m2). Square transects of one meter were established in the study site and the number of nests was recorded.
Cell characteristics
Lycoperdon marginatum bee and nest cell characteristics like the number of individual bees per nest, the sex ratio of bees, cell size, cell length, cell diameter, and pollen mass per cell were recorded after excavation of the nesting tunnel.
Physical properties of the soils
Soil texture: It was determined by International Pipette Method given by Pipper (1966).
Soil porosity: It was calculated by the formula:
Chemical properties of nest soils
Nitrogen: Kjeldahl method (Kjeldahl 1883).
Organic matter: It was estimated by Walkey Black method (1934).
pH (Power of H+ ion concentration): It was observed by pH meter.
Statistical analysis
Design of the experiment | : | Stratified Random Sampling (SRS) |
Locations | : | 03 |
Ultimate sample size (Plant) | : | 3 trees/species/experimental location |
Ultimate sample size (Nest) | : | 3 nests/ location |
Replications | : | 03 |
Treatment | : | Pollinator |
Factor | : | 02 |
The sample size (pollinators) within every three plots of the one experimental location varies; therefore each plot was sampled independently. And the stratification was done homogenously before sampling. The plots of every experimental location (strata) were mutually exclusive. The strata are collectively exhaustive, and no population element was excluded. Stratified Random Sampling was applied within each stratum. Further, each population per stratum was representative. And the arithmetic means of the population were done to determine the variability/exp. location (strata). Data were entered, manipulated, and analyzed in the appropriate statistical software. Further appropriate ecological statistical methods were also used.
Results
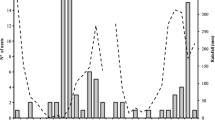
The total number of visits, visitation rate, and the visitation percent of 4.24, 1.002, and 16.08 visits/bout (m2) × 100, performed by the species L. marginatum, on peach, plum, and cherry flowers are shown in Figs. 1, 2 and 3, respectively. The time duration spent on peach, plum, and cherry flowers varied significantly as observed from the figures (Figs. 1, 2, 3). The relative abundance and the percent relative abundance contributed by L. marginatum on peach, plum, and cherry flowers were found to be significantly different as shown by the ANOVA results. A considerable variation was found in all the pollinators in terms of total visits, visitation rate, and percent visitation during the period 2013–2014 (Figs. 1, 2, 3).
It is to be noted that not all the pollinator species exhibit the same nesting requirements whereas the nesting habitats of L. marginatum were found to be located nearby the good foraging habitat in and around the fruit orchards. The species L. marginatum constructs its nest in soil and prefers exclusive areas like bare or sparsely-vegetated soil for nesting and movement. Generally, loose and well-drained soil in a sunny spot and sloppy areas facing the eastern direction at an angle of 5–10 degrees are preferred by the species L. marginatum for constructing its nests. The species was found to be the most efficient pollinator and its contributions to total visitation were 13.69%, 12.86%, and 11.99% upon peach, plum, and cherry flowers, respectively (Fig. 3). Therefore, based on their high abundance, the contribution to total pollination efficiency and the nesting behavior of this species were studied.
Nest architecture
Physical characters
Tumulus length (cm)
The species L. marginatum digs central tunnels while the excavated soil is pushed towards the nest entrance and its surroundings during the late hours of the night or/and in the daytime (morning or afternoon), especially in the wet season. Thus, it constructs the nest with a mound-like soil structure in the name of ‘tumuli’. The maximum number of soil pellets got excavated during the night hours, which indicates that the nest is constructed during the night time. The maximum tumulus length i.e., 3.58 cm was recorded in the district of Budgam, followed by Pulwama and Srinagar districts which recorded the tumulus lengths such as 2.98 and 2.88 cm, respectively (Table 1). In the Pulwama district, it was observed that the tumulus got washed away by heavy rainfall, particularly on the sloppy banks. On the other hand, in deep areas where the water gets stagnated, there was no tumulus observed at all though the researchers were able to observe the nest tunnels and entrances. However, in plain areas, the tumuli were found to be highly spread, less spread or sometimes completely spread on the plain ground surfaces. However, on the vertical banks and shady areas of the orchard, no tumulus was found. Similarly, in dense vegetation areas too, no tumuli were found. In the sloppy areas of Budgam district that are located at 40–45 degrees and are exposed to sun, broad and well-developed tumuli were found. Exceptionally, in the plains of Budgam, one of the nests showed only a turret and no tumulus at all. In terms of nest tumulus length, a statistically significant difference was found across the three experimental locations (p = 0.002).
Turret height (cm)
The species L. marginatum builds earthen entrance tubes (turrets) during the night time onto its subterranean nest burrows. These tubes prevent the burrows from getting filled with dirt or debris. Generally, a single turret is observed for a nest, though, at times, two nests are also found nearby (3.0 cm apart). Such turrets are found connected on the sides. The lengths of the turrets, recorded in the current investigation, were in the range of 8.88 cm in the Budgam district to 4.58 cm in the Srinagar district. The turret generally has a broader base that gets narrowed down as it reaches the top and this structure supports the height of the turret. In plain banks of the Srinagar district, the turrets were found to be minimum in height due to which it gets washed away by heavy rains. However, in the sloppy areas of the Budgam district, the turret heights were found to be considerably higher. Since the species L. marginatum prefers grass-free open sloppy banks for the construction of nests, the turrets were found to be highly visible even from a distance of 10–20 m. In the landscape in the Budgam district nearby the stone fruit orchards, some nests were found with rough and newly-constructed turrets to which loose soil pellets were found to be attached to it. In plain areas, the turrets were found to be weak and minimum compared to the sloppy areas in which the turrets were strong and thick with rough side walls. Generally, in the elevated soil banks, the turrets were found to be at high altitudes than the plain grasslands. The statistically-significant variations were found in the turret lengths across the three experimental sites (p ≤ 0.05) (Table 1).
Nest depth (cm)
The nest depths recorded in the districts such as Budgam, Pulwama, and Srinagar were 28.66, 18.33, and 10.87 cm, respectively. Table 1 shows the depth of the nest on the plane bank. However, in sloppy areas, the depth of the nests was found to be higher. Those slopes that faced the direct sun had deeper nests than the nests in shady and vertical areas. The nest, covered by grassy surroundings, was generally shallow because the dense roots hinder the construction of the nests. Statistically-significant differences were found in the depth of the nests across the three experimental locations (t-test: p ≤ 0.005) (Table 1).
Nest density (nests/m2)
The maximum nest density was recorded in the district of Budgam (16.67 nests/m2) whereas the minimum value was found in the district of Pulwama (10 nests/m2) (Table 1). The nest density values varied from plains to vertical and sloppy banks. Generally, in sloppy areas, the nest density was the maximum compared to the plains. Further, the slopes exposed to the direct sun had the highest nest density volume compared to those nests in shady and deep areas. Due to heavy water stagnation in plains and low-lying areas, the species L. marginatum did not prefer nesting in those areas as continuous water stagnation decomposes the pollen, which in turn causes larval death. Therefore, this species prefers sloppy sites for its nest construction. Statistically significant differences were found in the nest density of L. marginatum (t-test: p = 0.013) across the three experimental locations.
Nest diameter (cm)
The maximum nest diameter of L. marginatum was 1.06 cm in the Budgam district whereas the minimum nest diameter was found to be 0.77 cm in the district of Srinagar (Table 1). In plains, the nest diameter was higher compared to the nests in sloppy areas. Generally, the internal diameter of the nest is higher than the outer diameter. This is attributed to the fact that the bees move around and in an up-to-down fashion as well inside the cavity, which demands a wide internal space for movement. The study found that the nest diameters varied significantly (t-test: p = 0.037).
Nest length (cm)
The maximum nest length of 18.34 cm was recorded in the district of Budgam whereas the minimum of 5.98 cm was recorded in the Srinagar district. The nest length was bigger in plain areas than in the sloppy areas and vertical banks. In sloppy areas and vertical banks, the nest cavity went a little deep inside, for the first 10–12 cm, later on, it went in an oblique direction. In the case of plains, it moved laterally to avoid water stagnation near the cells. Overall, a significant difference (t-test: p = 0.019) was observed in the length of the nests (Table 1).
Height from the plain (m)
The height of the nesting site from the plain was found to be statistically significant (t-test: p = 0.021); the maximum height (of 2.72 m) was recorded in the dry lands of Budgam whereas the minimum height (of 1.47 m) was recorded in the plains of Srinagar (Table 1). Since the greater height of the nesting sites generally corresponds to the maximum slope, the study found the preference of L. marginatum in terms of height for its nest construction.
Nest cell parameters
The analyses outcomes of various characteristics of the L. marginatum nests are given in Table 2. For the physical characteristics of the nest cell, Wilcoxon t-test was conducted and the results are as follows; N = 8, H1 = 1.04, p = 0.0039. The regression analysis achieved significance at 1% with r2 = 0.141 and the regression coefficient rc = 0.31. The ANOVA outcomes of the nest soil physical features are given in Table 2. The mean values of the L. marginatum population, its nest cell characteristics, and the dry weight of the pollen mass are given in Table 2.
Gravimetrical characteristics
The variations among the soil characteristics of the three selected experimental locations were lesser. The soil samples, collected in the study, possessed high percentages of silt, clay, and fine sand content (Table 1). The slit content was found to be the maximum in the Budgam district and the last one in the Srinagar district, as per the Kolmogorov–Smirnov test results; its statistics are as follows; 0.0012 < D < 0.136, n1 = n2 = 4, p ≤ 0.05. When conducting the post hoc analysis, various parameters of the nest characteristics were found to be significant. The soil preferred for building the nests was clay loam type in all three experimental locations.
Chemical characteristics
The pH value of the soil was found to be weakly alkaline i.e., ranging at 7.2 in Budgam to slightly acidic i.e., 6.78 in Srinagar (Table 1). The amount of organic matter also varied from low to moderate in the study locations. The EC, OC, OM, and nitrogen were found to be significantly different (p = 0.027). The highest value was found in the district of Srinagar. Following the Chi-square test (n = 7, H1 = 0.018, p ≤ 0.05; χ2 = 4.05) of various soil chemical characteristics, the differences were found to be significant across the sites.
Porosity and soil moisture
Both soil porosity and the moisture content of the soil varied significantly among the experimental sites considered in the present investigation (Table 1). The highest soil porosity of 33 percent was recorded in the district of Budgam whereas the least 18 percent was found in the district of Pulwama. The maximum moisture content was recorded in the soil samples collected from the nests of L. marginatum in the Srinagar district whereas the minimum value was observed in the district of Budgam. However, differences in the soil porosity values across all three experimental locations were not significant (p ≥ 0.05%, t-test ≥ 0.491). However, the soil moisture content differed significantly across the locations (p < 0.05).
Correlation between the abundance of L. marginatum and various soil characteristics
In the current investigation, various nest soil characteristics (such as physical, chemical, and gravimetrical) were found to significantly affect the abundance of L. marginatum in the landscapes of all three experimental locations. The relative abundance of the species was found to be positively correlated with a few parameters such as nest density, nest length, nest depth, clay content, soil nitrogen, soil slope, soil porosity, and moisture content (Table 3). However, the bee abundance was found to have a weak correlation with the maximum number of physical characteristics of the soil. The abundance was minimum in sandy areas which implies the existence of a weak correlation with the sand content. In other terms, the species does not prefer to live in dunes or sandy slopes. Adding to the above, the field observation too confirmed that there exists a non-linear relationship between the bee abundance and the number of nests found. However, the nest characteristics are not the sole deciding factor for bee abundance in the habitats under investigation. Most of the nests were filled with fresh dirt by late morning and opened again in the early hours of the next day. So, to estimate the exact nest density was slightly obscure. It is usually obvious that an open nest hole is active because the hole is nearly circular, and is present in a fresh moist landscape. The fresh holes are generally moistened with round bits of soil mounted around the nest. In addition to various landscape level variables, various soil characteristics especially the moisture content was significant and positively correlated with L. marginatum abundance. The moisture content affects the bee abundance by making the soil easier to excavate. The soils, at various nesting sites, were of clay-rich type and were quite hard, when dry. The other nest soil features are correlated with the abundance of L. marginatum as given in Table 3.
Discussion
Pollination efficiency
The ‘most-effective pollinator principle’ implies that the floral characteristics often reflect the adaptation of the plant and its flowers to attract the insect pollinator as observed in the current studies i.e., almost the same pollinators visited all three fruit crop species in both years of investigation. The insect-mediated pollen transfer, due to a high rate of visitation to the flower and higher abundance, leads to increased total visitation and high pollination effectiveness. The dominant flower visitors of the three fruit plant species were found to be of the order Hymenoptera and are comprised of the overall maximum visits by the genus, Lasioglossum. The species L. marginatum was found to be efficient and represented 4.24, 6.50, and 7.55 of the total visits and 1.002, 1.094, and 1.243 of the total visitations on peach, plum, and cherry flowers, respectively. Likewise, in the US, the species L. marginatum was found to be the dominant fruit pollinator and comprised 93.0 percent of the total population (Tuell et al. 2014). It also performed the maximum number of visits to flowers. In the current investigation, a significantly higher proportion of the contribution was made by L. marginatum as per the Wilcoxon test (p ≤ 0.05) results than the rest of the flower-visiting species. The contribution to the total bee visitation recorded was 16.08, 13.96 and 11.74%, respectively, for the peach, plum, and cherry flowers. However, the abundance contribution to total efficiency by L. marginatum was 9.48, 9.10 and 7.36% on peach, plum, and cherry flowers, respectively. Generally, an efficient pollinator spends less time on the flowers, corresponding to L. marginatum. This notion is in agreement with the findings of Huda et al. (2015) who mentioned that the species with higher abundance, a high number of total visits, and higher visitation spend less time per flower and achieve high pollination efficiency. Therefore, L. marginatum can be considered an efficient stone fruit pollinator since it had the highest and the most significant flower visitation proportion and percent abundance.
Nesting behavior and architecture
The family Halictidae exhibits the most diverse gradation in social behavior from solitary to eusocial, based on the time of year, geographic location, and altitude (Michener 2000). The dominant species i.e., L. marginatum behaves eusocially with a high number of populations recorded per nest and square meter area. The species L. marginatum gregariously constructs its nest in the ground. In the current study, various nest features in terms of physical, chemical, and gravimetrical parameters across the landscapes of Kashmir valley were evaluated and were found to be significant. For all the physical characteristics of the nest under study, the Kruskal–Wallis test was conducted and the results were significant. So, it followed the alternate hypothesis with p = 0.03; and Chi-square value χ2 ≤ 4.050; in other terms, a high level of significance was found. Similarly, the regression analysis also achieved significance at 1% with r2 = 0.943 and a coefficient (r) of 0.92. The physical characteristics of the nests including tumulus height (cm) varied significantly. This finding is in agreement with the findings of the study conducted earlier by Wcislo-Luraniec (1985) who mentioned that the species Metopia campestris (Fallén, 1810) constructs the nests with a tumulus height of 3.0 cm. Jerome and Rozen (1989), from the American Museum, recorded a tumulus length of 1 to 4 cm by Panurgine spp. (bees). The turret height, recorded during the field experiment, was in the range of 4.58 to 8.88 cm. The turrets were erect, upright, vertical, externally rough, and pointed at the mouth of the nest on the surface. Frison (1992) observed that the nest turrets are smoother on the interior side and rough on the exterior side. The turrets are oriented in various directions with no obvious pattern and have an average length of approximately 8.0 cm. In this study, the turret was observed perpendicular to the surface/on the bank. This finding is corresponds with the findings of Wcislo (1992) who found that the nest entrance of the species Lasioglossum figueresi (Wcislo, 1990) is surrounded by chimney-like turrets. Such turrets are more or less perpendicular to the bank at varying lengths from 0 to 15 mm. Since the current study observed 2, 3, or even 4 turrets often together in groups, separated by a distance of one inch (2.54 cm), and was almost similar in height, this finding aligns with the results of Gess and Gess (2010). They observed that the turrets of the species Priscomasaris namibiensis (Gess, 1998) were constructed in groups and reached a maximum height of 13–17 mm. The nesting depth recorded in the study varied significantly, and similar results were also found by Rehan et al. (2013). They observed that the average nest depth of the species Halictus ligatus Latreille, 1804 was 13 cm from the ground surface. However, Michener (1964) observed that the nesting depth of the species Exoneurella lawso (Rayment, 1946) varies from 20 to 51 cm with an average depth of 33.33 cm. The results of the nesting depth observed in the current study contradict with the findings of Osgood (1972) who found that the maximum nest depth, excavated by solitary bees, was 6.09 cm.
The nest density, recorded in the present study, was found to be significantly high. This is in agreement with Eltz et al. (2003) who found that the nest densities of the solitary bees are generally higher (8.4 nests/ha) in forest areas than in plantations areas (nest density: 0–2.1 nests/ha). The results showed that the nest density was higher at the field borders followed by sloppy lands. This was confirmed by Kim et al. (2006) who mentioned that the nest densities of native bees were higher at the field borders than inside the fields (interior mean: 0.089; border mean: 0.31). The nest diameter of L. marginatum was significantly bigger (range 1.06 mm) in Budgam district than in the district of Srinagar (0.77 mm). These findings correlate with those of the Heard (1992)’s research outcomes. According to this research, Amegilla Friese, 1897 species make nests with a diameter of 10–15 mm. Barbosa et al. (2013) recorded the nest diameter to be 7.5 mm for Osmia lignaria (Say, 1837). The nest length was found to be significantly bigger, in the range of 5.96 to 18.34 cm, which is supported by Barbosa et al. (2013) who recorded varying nest length (for Proteus anguinus anguinus Laurenti, 1768) in the range of 18.7 to 70 cm. The height of the nesting sites from the plain varied statistically and was found to be abundantly available on sloppy areas compared to flat surfaces. This finding is supported by Osgood (1972) who found that the nesting sites were generally at high altitudes in the immediate areas on south-to-west slopes, compared to the control areas with relatively flat surfaces. The locations, chosen by L. marginatum for nesting, were found to be well-drained with a good surface flow. Further, such locations had a plant and weed stand of sparse-to-intermediate density. The bees were found to be constructing galleries in A-horizon though the O-horizon was relatively loose, pliable, and gets penetrated at first. The soil analysis results infer many variations in different gravimetrical characteristics of all three experimental locations. The slit and clay content were found to be significantly (Kolmogorov–Smirnov test: 0.0012 ≤ D ≤ 0.136, n1 = n2 = 4, p ≤ 0.05) higher in the district of Budgam and lower in the Srinagar district and it is directly proportional to the nest density at these sites. The soil type, preferred by L. marginatum for nest building was clay loam type in all the three locations under study; a maximum coarse gravel content of 2.75 in Budgam district contributed to the highest nest density. However, Sakagami and Michener (1962) observed that the nest cell construction gets terminated shortly in course gravel-to-sandy types of soils. The OM and the OC values, recorded in the nest soils, were comparatively lower; however, the reports infer that the ground-nesting bees generally prefer nesting in the areas with well-drained soils containing little organic matter. The pH value of the soil was weakly alkaline and lay in the range of 6.78 to 7.2. The EC, OC, OM, and nitrogen values were significantly higher in the district of Srinagar. The highest soil porosity i.e., 48.82% was recorded in Budgam district whereas the minimum value i.e., 52.0% was recorded in Pulwama. Furthermore, this finding also aligns with the outcomes of a study which confirmed that the moisture content percent, pH, and electrical conductivity of the alkali bee nest soil at 6-inch depth from the surface were 27.46%, 8.7 H+,* and 4.0 Ohms/cm, respectively (Bohart et al. 1960).
It was observed that various nest soil characteristics affect the abundance of L. marginatum in the habitats of all the experimental locations. These characteristics were found to be correlated with nest density, nest length, OM, clay content, soil nitrogen, soil alkalinity, slope, porosity, and moisture content. However, the bee abundance was found to be weakly correlated with the maximum number of physical characteristics of the soil. The abundance of the species was minimum in sandy areas due to which a weak correlation was found between the sand content and species abundance. In other terms, the species does not prefer dunes or sandy slopes. Likewise, the field observation also established the presence of a non-linear relationship between the bee abundance and the number of nests/m2. The nest characteristics are not the sole deciding factors for bee abundance in these habitats. Most of the nests were found to be filled with fresh dirt by late morning and opened again in the early hours of the next day. It is usually obvious when an open nest hole is active (with a bee inside) because the hole is roughly circular. The fresh holes are generally moist with round bits or pellets of soil mounted around the hole. In addition to various landscape-level variables, various soil characteristics were found to have a positive correlation with L. marginatum abundance. The moisture content affects the bee abundance by making the soil, easier to excavate. The soils at various nesting sites were found to be clay-rich type and yet quite hard, when dry. Many other nest soil features were also found to correlate with L. marginatum abundance. The species L. marginatum is known to build nest cells at a considerable depth, which is in partial agreement with the results by Hurd et al. (1964). They found that P. pruinosa builds nests at a depth of 16 to 30 cm. The moist soils were found to stimulate the digging activity in the genus Lasioglossum, which is also reported by Greenberg (1982). He mentioned that the densiest nesting areas are those with moist soils. In the current investigation, the soils at all three sites were clay-rich and quite hard, when dry. The possible reason for the higher abundance of the species in moist soils is due to the high specific heat of the water. Since soil moisture regulates the soil temperature, this phenomenon gets translated into a less variable and possibly more hospitable environment for the development of bee larvae.
References
Angelella GM, McCullough CT, O’Rourke ME (2021) Honey bee hives decrease wild bee abundance, species richness, and fruit count on farms regardless of wildflower strips. Sci Rep 11(1):1–2. https://doi.org/10.1038/s41598-021-81967-1
Antoine CM, Forrest JR (2021) Nesting habitat of ground-nesting bees: a review. Ecol Entomol 46(2):143–159. https://doi.org/10.1111/een.12986
Barbosa FM, Alves RM, Souza BD, Carvalho CA (2013) Nest architecture of the stingless bee Geotrigona subterranea (Friese, 1901) (Hymenoptera: Apidae: Meliponini). Biota Neotrop 13:147–152. https://doi.org/10.1590/S1676-06032013000100017
Bohart GE, Stephen WP, Eppley RK (1960) The biology of Heterostylum robustum (Diptera: Bombyliidae), a parasite of the alkali bee. Ann Entomol Soc Am 53(3):425–435. https://doi.org/10.1590/S1676-06032013000100017
Brady SG, Litman JR, Danforth BN (2011) Rooting phylogenies using gene duplications: an empirical example from the bees (Apoidea). Mol Phylogen Evol 60(3):295–304. https://doi.org/10.1016/j.ympev.2011.05.002
Crowley LM, Sadler JP, Pritchard J, Hayward SA (2021) Elevated CO2 impacts on plant-pollinator interactions: A systematic review and free air carbon enrichment field study. Insects 12(6):512. https://doi.org/10.3390/insects12060512
Dar SA, Lone GM, Parey SH, Hassan GhI, Rather BA (2017) Insect pollinators and their conservation. J Entomol Zool Stud 5(3):1121–1131
Dar SA, Wani AR, Sofi MA (2018) Diversity and abundance of insect pollinators of sweet cherry Prunus avium in Kashmir valley. Indian J Entomol 80(3):725–736. https://doi.org/10.5958/0974-8172.2018.00231.6
Dar SA, Mir GM, Parry MA, Ahmad SB, Ganie MA, Raja TA, Yaqoob MY (2016) Diversity and richness indices and the Whittaker plot value of insect pollinators of peach Prunus persica in landscapes of temperate India. Acad J Entomol 9(4):62–73
Eltz T, Brühl CA, Imiyabir Z, Linsenmair KE (2003) Nesting and nest trees of stingless bees (Apidae: Meliponini) in lowland dipterocarp forests in Sabah, Malaysia, with implications for forest management. For Ecol Manage 172(2–3):301–313. https://doi.org/10.1016/S0378-1127(01)00792-7
Free JB (1993) Insect Pollination of Crops, 2nd edn. Academic Press, New York
Frison TH (1992) Notes on the life history, parasites and inquiline associates of Anthophora abrupta Say, with some comparisons with the habits of certain other Anthophorinae (Hymenoptera). Trans Am Entomol Soc 48(2):137–156
Gess S, Gess FW (2010) Pollen wasps and flowers in southern Africa. SANBI Biodiversity Series 18
Gibbs J, Packer L, Dumesh S, Danforth BN (2013) Revision and reclassification of Lasioglossum (Evylaeus), L. (Hemihalictus) and L. (Sphecodogastra) in eastern North America (Hymenoptera: Apoidea: Halictidae). Zootaxa 3672(1):1–17. https://doi.org/10.11646/zootaxa.3672.1.1
Greenberg L (1982) Year-round culturing and productivity of a sweat bee, Lasioglossum zephyrum (Hymenoptera: Halictidae). J Kansas Entomol Soc 1:13–22
Hurd PD, Linsley EG, Whitaker TW (1964) Squash and gourd bees (Peponapis, Xenoglossa) and the origin of the cultivated Cucurbita. Evolution 25(1):218–234
Huda AN, Salmah MR, Hassan AA, Hamdan A, Razak MN (2015) Pollination services of mango flower pollinators. J Insect Sci 15(1):113. https://doi.org/10.1093/jisesa/iev090
Kim J, Williams N, Kremen C (2006) Effects of cultivation and proximity to natural habitat on ground-nesting native bees in California sunflower fields. J Kansas Entomol Soc 79(4):309–320. https://doi.org/10.2317/0507.11.1
Kjeldahl JG (1883) Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z Anal Chem 22(1):366–382. https://doi.org/10.1007/BF01338151
Kukuk PF, Sage GK (1994) Reproductivity and relatedness in a communal halictine bee Lasioglossum (Chilalictus) hemichalceum. Insect Soc 41(4):443–455. https://doi.org/10.1007/BF01240647
Lincoln C, Blair BD (1977) Extension entomology: a critique. Annu Rev Entomol 22(1):139–155. https://doi.org/10.1146/annurev.en.22.010177.001035
Michener CD (1964) Evolution of the nests of bees. Am Zool 1964:227–239. https://doi.org/10.1093/icb/4.2.227
Michener CD, Winston ML, Jander R (1978) Pollen manipulation and related activities and structures in bees of the family Apidae. Univ Kansas Sci Bull 51(19):575. https://doi.org/10.5962/bhl.part.17249
Michener CD (2000) The Bees of the World. The Johns Hopkins University Press, Baltimore, USA
Osgood EA Jr (1972) Soil characteristics of nesting sites of native bees associated with the low-bush blueberry in Maine. Maine Agric Exp Stn Tech Bull 59:1–8
Packer L (2006) Use of artificial arenas to predict the social organisation of halictine bees: data for fourteen species from Chile. Insect Soc 53(3):307–315. https://doi.org/10.1007/s00040-006-0873-x
Plateaux-Quénu C, Plateaux L, Packer L (2000) Population-typical behaviours are retained when eusocial and non-eusocial forms of Evylaeus albipes (F) (Hymenoptera, Halictidae) are reared simultaneously in the laboratory. Insect Soc 47(3):263–270. https://doi.org/10.1007/PL00001713
Packer L, Sampson B, Lockerbie C, Jessome V (1989) Nest architecture and brood mortality in four species of sweat bee (Hymenoptera; Halictidae) from Cape Breton Island. Can J Zool 67(12):2864–2870. https://doi.org/10.1139/z89-406
Rozen Jr, Jerome G (1989) Life history studies of the “primitive” panurgine bees (Hymenoptera, Andrenidae, Panurginae). American Museum Novitates 2962:1–27
Rodrigo Gómez S, Ornosa C, Selfa J, Guara M, Polidori C (2016) Small sweat bees (Hymenoptera: Halictidae) as potential major pollinators of melon (Cucumis melo) in the Mediterranean. Entomol Sci 19(1):55–66. https://doi.org/10.1111/ens.12168
Rehan SM, Leys R, Schwarz MP (2013) First evidence for a massive extinction event affecting bees close to the KT boundary. PLoS One 8(10):e76683. https://doi.org/10.1371/journal.pone.0076683
Sakagami SF, Michener CD (1962) The nest architecture of the sweat bees. Univ Kansas Press, Lawrence, Kansas
Stockhammer KA (1966) Nesting habits and life cycle of a sweat bee, Augochlora pura (Hymenoptera: Halictidae). J Kansas Entomol Soc 1:157–192
Soberón J, Peterson T (2004) Biodiversity informatics: managing and applying primary biodiversity data. Philos Trans R Soc Lond B Biol Sci 359(1444):689–698. https://doi.org/10.1098/rstb.2003.1439
Sakagami SF (1982) Stingless bees. In: Hermann HR (ed), Social insects. Academic press, New York, pp 361–423. https://doi.org/10.1016/B978-0-12-342203-3.50011-4
Tuell JK, Fiedler AK, Landis D, Isaacs R (2014) Visitation by wild and managed bees (Hymenoptera: Apoidea) to eastern US native plants for use in conservation programs. Environ Entomol 37(3):707–718. https://doi.org/10.1603/0046-225X(2008)37[707:VBWAMB]2.0.CO;2
Vercelli M, Novelli S, Ferrazzi P, Lentini G, Ferracini C (2012) A qualitative analysis of beekeepers’ perceptions and farm management adaptations to the impact of climate change on honey bees. Insects 12(3):228. https://doi.org/10.3390/insects12030228
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38. https://doi.org/10.1097/00010694-193401000-00003
Wcislo WT (1992) Attraction and learning in mate-finding by solitary bees, Lasioglossum (Dialictus) figueresi Wcislo and Nomia triangulifera Vachal (Hymenoptera: Halictidae). Behav Ecol Sociobiol 31(2):139–148. https://doi.org/10.1007/BF00166347
Wcislo WT (1993) Communal nesting in a North American pearly-banded bee, Nomia tetrazonata, with notes on nesting behavior of Dieunomia heteropoda (Hymenoptera: Halictidae: Nomiinae). Ann Entomol Soc Am 86(6):813–821. https://doi.org/10.1093/aesa/86.6.813
Wcislo-Luraniec E (1985) New details of leaf structure in Bilsdalea dura Harris (Coniferae) from the Jurassic of Kraków. Poland Acta Palaeobot 25:1–2
Woodcock BA, Ridding L, Freeman SN, Pereira MG, Sleep D, Redhead J, Aston D, Carreck NL, Shore RF, Bullock JM, Heard MS (2018) Neonicotinoid residues in UK honey despite European Union moratorium. PLoS One 13(1):e0189681. https://doi.org/10.1371/journal.pone.0189681
Wyman LM, Richards MH (2003) Colony social organization of Lasioglossum malachurum Kirby (Hymenoptera, Halictidae) in southern Greece. Insect Soc 50(3):201–211. https://doi.org/10.1007/s00040-003-0647-7
Acknowledgements
We highly acknowledge SKUAST-K Kashmir for its research help. This research was supported by Princess Nourah bint Abdulrahman; University Researchers Supporting Project number (PNURSP2023R365), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
One-way ANOVA and post hoc analysis of the nest characteristics of L. marginatum
S.No | Nest parameter | TSS (Type III) | d. f. | MSS | MD | F-value | Standard error (SE) | Significance |
|---|---|---|---|---|---|---|---|---|
1 | Tumulus length (cm) | 0.637 | 4 | 0.159 | 0.10 | 12.88 | 0.0645 | 0.010 |
2 | Turret height (cm) | 15.19 | 4 | 3.799 | 1.23 | 217.9 | 0.0761 | 0.012 |
3 | Nest depth (cm) | 300.44 | 4 | 75.11 | 32.41 | 4.522 | 2.353 | 0.041 |
4 | Nest density | 14.49 | 4 | 0.036 | 0.011 | 19.25 | 0.025 | 0.030 |
5 | Nest length (cm) | 78.66 | 4 | 19.66 | 4.32 | 1.475 | 2.108 | 0.020 |
6 | Nest slop height (m) | 2.406 | 4 | 0.601 | 0.21 | 29.175 | 0.083 | 0.030 |
7 | Nest diameter (mm) | 109.77 | 4 | 27.44 | 8.54 | 61.75 | 0.385 | 0.010 |
8 | No. individuals/nest | 122.9 | 8 | 12.57 | 3.34 | 35.76 | 2.34 | 0.002 |
9 | No. cell/nest tunnel | 29.35 | 8 | 7.44 | 2.34 | 15.76 | 1.50 | 0.001 |
10 | Cell length (cm) | 31.62 | 8 | 3.51 | 1.21 | 13.54 | 0.081 | 0.004 |
11 | Cell depth (cm) | 148 | 8 | 16.33 | 2.56 | 15.76 | 4.50 | 0.021 |
12 | Cell size (mm) | 64 | 8 | 7.2 | 1.54 | 0.46 | 0.15 | 0.001 |
13 | Cell diameter (mm) | 20.3 | 8 | 2.29 | 1.09 | 0.065 | 0.021 | 0.010 |
14 | Sex ratio (F/M) | 3.34 | 8 | 0.377 | 0.12 | 0.122 | 0.041 | 0.031 |
15 | Pollen mass (g) | 0.099 | 8 | 0.011 | 0.0 | 0.0005 | 0.0015 | 0.017 |
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dar, S.A., Javeed, K., Al-Shuraym, L.A. et al. Assessment of nest architecture and pollination efficiency of Lasioglossum (Evylaeus) marginatum (Helictidae: Hymenoptera). Biologia 78, 2835–2847 (2023). https://doi.org/10.1007/s11756-023-01419-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01419-1