Abstract
We investigated the population structure, reproduction, growth parameters and total mortality of Fragillianassa fragilis. Sampling was performed monthly from July 2017 to July 2018 at Casa Caiada Beach, Pernambuco, northeastern Brazil. The overall sex ratio was 1:1 but with a dominance of juvenile males and adult females. The carapace length (CL) ranged from 1.36 to 6.59 mm for males, 1.7 and 6.05 mm for females and 3.07 and 6.14 mm for ovigerous females. Reproduction was seasonal, with peaks during the warmest months of the year. The number of eggs ranged from 20 to 259. Growth parameters for males (L ∞ = 7 mm, K = 0.95 year–1, C = 0.4, and WP = 0.5) and females (L ∞ = 6.5 mm, K = 1.0 year–1, C = 0.9, and WP = 0.75) were quite dissimilar when compared to other ghost shrimps. Mortality was considered high for males and females (2.66 and 3.41, respectively), while the Growth Performance Index (Φ’) was similar for males (1.668) and females (1.626). This study provides new ecological information on ghost shrimp life-history strategies, as well as subsidies for conservation measures for this group, which is an important component of secondary production in coastal ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, a new classification of the family Callianassidae was published based on the results of a molecular phylogenetic analysis with morphological support. The family currently has 26 genera, 12 of which are recently proposed. Fragillianassa, one of the new genera included in this review, includes three species, F. fragilis (Biffar, 1970) and F. debilis (Hernández-Aguilera, 1998), previously belonging to the genus Biffarius; and the recently described F. joeli Pachelle and Tavares, 2020 (Poore et al. 2019; Pachelle and Tavares 2020). Of these three species, only two occur in Brazil, F. fragilis and F. joeli. The former extends from the USA (Florida), through the Gulf of Mexico, Puerto Rico, Antigua, and Venezuela to Brazil (Pernambuco and Ceará) (Botter-Carvalho et al. 2012; Pachelle et al. 2016); and the latter is known only from Trindade Island (Pachelle and Tavares 2020). Fragillianassa debilis occurs along the Pacific coasts of Mexico (Hernández-Aguilera 1998) and Costa Rica (Dworschak 2013; Vargas-Zamora et al. 2019).
These species are small-bodied, with the cephalothorax generally less than 5 mm long in adult F. debilis (Hernández-Aguilera 1998), not exceeding 7 mm. The main characteristics used to differentiate these species from other genera of the family Callianassidae are: the male and female major cheliped merus with a prominent truncate hook armed with serrations along the lower margin, excavated laterally at the base, with a deep notch at the base of the fingers. Pleopod 1 is present in males, while pleopod 2 is absent. The uropodal endopod is without facial spiniform setae (Poore et al. 2019).
The new genus is little studied, with only records of occurrence and information about morphological characteristics available, but no ecological information. Some studies have treated species of similar sizes, such as Arenallianassa arenosa (Poore, 1975) (formerly Biffarius arenosus), whose population structure (Butler et al. 2009), diet (Boon et al. 1997), and burrow morphology (Bird and Poore 1999) were studied in Australia; and Biffarius biformis (Biffar, 1971) whose larval stages were studied by Sandifer (1973) in the USA.
Fragillianassa fragilis preferentially inhabits the intertidal zone of sandy beaches, generally close to rivers and estuaries, such as the three beaches where they were found in Brazil (Forte Orange Beach, Itamaracá, Pernambuco; Casa Caiada Beach, Olinda, Pernambuco; and Pedra Rachada Beach, Paracuru, Ceará).
The members of the infraorder Axiidea have been studied extensively in recent decades, due to their importance to marine ecosystems. The bioturbation and bioirrigation that occur in the process of constructing and maintaining their burrows cause changes in sedimentary conditions, providing shelter to numerous organisms and consequently influencing the composition of benthic communities (Bird et al. 2000; Berkenbusch and Rowden 2003; Butler et al. 2009; Pillay and Branch 2011).
Analyses of population structure, reproduction, growth, longevity, and other length-frequency variables allow for comparison of this species with other species of ghost shrimps from different geographic areas. Here, we provide the first information on the population structure, reproduction, growth, longevity, mortality, and recruitment of F. fragilis, as well as information to aid in understanding the differences from other ghost shrimps.
Materials and methods
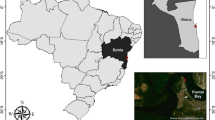
Samples of F. fragilis were collected monthly from July 2017 to July 2018, during low spring tides, in the municipality of Olinda on a sand-mud bank at Casa Caiada Beach (07°58’59.87” S and 34°50’6.08” W), Pernambuco, Brazil (Fig. 1). The bank, located in an area sheltered by breakwaters, is partly covered with macroalgae patches (Ulva, Halodule, Bryopsis, Caulerpa, and Cladophora) during part of the year and is completely covered from January to May (Fig. 2) (for more information about abiotic data see Botter-Carvalho et al. 2015; Costa et al. 2020a). About 80 shrimp were captured each month, using a manual suction pump. The specimens were individually packed in seawater and later preserved in 70% ethanol.
Species were identified, sexes determined based on the presence (female) or absence (male) of pleopod 2, and individuals were measured: carapace length (CL), tip of the rostrum to the end of the carapace; total length (TL), tip of the rostrum to the end of the telson; and propodus length of the major cheliped (PL), from the vertical projection of the proximal articulation to the vertical projection of the distal articulation, using a stereomicroscope with an ocular micrometer or digital caliper, to the nearest 0.01 mm.
Population structure
Size-frequency distributions were constructed using 0.5-mm CL size classes. The sex ratio (males to females) was compared to the total population, per rainy/dry season and size class, using chi-square tests. In this region the rainy season extends from April to September and the dry season from October to March (Pereira et al. 2016). Sex as a function of size was analyzed by Wenner’s curve, plotted with data for males and using the percentage of individuals by size class (see Wenner 1972).
To examine sexual dimorphism in terms of body size and chelipeds, we compared the CL and the PL between males (M) and females (F), using a Mann-Whitney test since the distribution was normal, but variances were not homogenous even with data transformation. The Mann-Whitney test was carried out in the program BioEstat 5.0 (Ayres et al. 2007).
The size at morphological maturation was calculated from the point of discontinuity of the regression line between the PL and CL. This point was defined by the smallest value obtained from the residual sum of squares (SQR) in the linear regression (Felder and Lovett 1989).
The breeding period was defined as those months with a maximum percentage of ovigerous females (OF), calculated based on the total number of adult females. Only females incubating embryos without eyes were used to estimate fecundity. The egg mass was mechanically removed from the pleopods and the eggs counted. Linear regressions were applied for the biometric relationships between fecundity and CL.
Growth, longevity, mortality, and recruitment
Growth was analyzed separately for males and females, using the von Bertalanffy seasonal growth function (VBGF) (Somers 1988; Soriano and Jarre 1988). Size–frequency distributions (0.5-mm intervals of CL) were recorded for each month. Growth parameters were calculated using the ELEFAN I tool contained in the program package FISAT (FAO-ICLARM Stock Assessment Tool) (Gayanilo et al. 2005). The values of t0 were calculated for both sexes from the equation proposed by López Veiga (1979), where hatching length was based on the 2.60-mm TL size of Biffarius biformis (Sandifer 1973). Maximum longevity (tmax), considered as the time necessary to reach 95% of L∞, was estimated using the formula proposed by Taylor (1958).
Graphical representations of von Bertalanffy growth curves with seasonal variations for males and females are according to de Graaf and Dekker (2006). To compare the growth performance between populations, the Growth Performance Index of Munro (Φ’) was applied (Munro and Pauly 1983; Pauly and Munro 1984).
Total mortality (Z) was estimated for males and females, using the length-converted catch curve method, considering that growth shows annual oscillations (C > 0) (Pauly 1984). As the population is not subject to exploitation, we assumed that the instantaneous mortality rate resulting from fishing (F) is zero and that the coefficients M and Z are equal, since Z = F + M (Paloheimo 1958; Pauly 1980).
The seasonal recruitment of F. fragilis was obtained using a time series of restructured length frequencies as input parameters for the best estimates of L∞, K, C, WP, and t0 (Pauly 1987; Moreau and Cuende 1991).
Results
Population structure
In total, 498 males (380 adults and 118 juveniles) and 489 females (386 adults, 67 juveniles, and 94 OF) were collected; 47 shrimp were damaged and therefore excluded from the morphometric analysis. Males ranged in size from 1.36 to 6.59 mm CL (3.79 ± 1.11), females (F) from 1.7 to 6.05 mm CL (3.88 ± 0.90), and ovigerous females from 3.07 to 6.14 mm CL (4.64 ± 0.64) (Fig. 3). Males and females showed a unimodal size distribution. When separated into size classes, the most dominant size classes for males (M) were 3–3.5 (N = 89), for females (F) 4–4.5 (N = 90), and for OF 4.5–5 (N = 30).
The overall sex ratio was 0.98 M : 1 F and did not differ significantly from 1 : 1 (X² = 0.096, p > 0.05). The juvenile population was male-biased (2.81 M : 1 F; X² = 56.47, p < 0.001) but was skewed toward females for the adult population (0.72 M : 1 F; X² = 19.43, p < 0.001). Sex ratio as a function of size class indicated a predominance of males in the smallest (1.0 to 3.5 mm) and largest (5.5 to 7.0 mm) size classes, while females predominated in the intermediate classes (4.0 to 5.5 mm) (Fig. 4a). Significant differences were found between the sex ratio in the 2–2.5 and 3–3.5 mm size classes, with a predominance of males; and in the 4–4.5 and 4.5–5 mm classes, with a predominance of females (Fig. 4b). No seasonally significant differences were found between males (rainy season = 252 and dry season = 245; X2 = 0.787; p > 0.05) and females (rainy season = 254 and dry season = 244; X2 = 0.686; p > 0.05).
a Frequency distribution of carapace length (CL) size classes of Fragillianassa fragilis for males (black bars) and females (white bars). Arrows indicate significant differences in sex ratio by size class. b Percentage of males in total individuals of F. fragilis by size classes (CL). Open circles indicate significant differences
The CL and PL differed between males and females (CL: median = 3.66 mm for M and 4.13 mm for F; U = 105856.0; p < 0.0001; PL: median = 2.92 mm for M and 2.8 mm for F; U = 83736.5; p < 0.0001).
Size at maturity for males was approximately 3.3 mm CL, while females reached maturity at a slightly shorter length (2.9 mm CL).
Ovigerous females were found mostly during the dry season. The reproductive period was seasonal, with peaks on 2 January 2018, 30 January 2018, and 4 March 2018, when 79%, 81%, and 73% of adult females were ovigerous, respectively (Fig. 5). Females with uneyed embryos were observed over the months of the reproductive period, and females with eyed embryos were more abundant only on 18 November 2017.
Fecundity ranged from 20 to 259 embryos (mean = 91.86 ± 59.88) (N = 44) and was positively correlated with female size (R² = 0.33; F = 21.10; p < 0.001) (Fig. 6).
Growth, longevity, mortality, and recruitment
Regarding the growth curve parameters, males showed a larger asymptotic length (L∞) (7) than females (6.5). The growth coefficient (K) and amplitude of seasonal oscillations (C) were higher for females (K = 1.0 and C = 0.9) compared to males (K = 0.95 and C = 0.4). The period of slowest growth occurred in June (WP = 0.5) and September (WP = 0.75) for males and females, respectively. The goodness-of-fit index was Rn = 0.407 for males and Rn =0.511 for females. The estimated maximum longevity was around three years for both sexes (3.02 males and 2.95 females). The Growth Performance Index (Φ’) was similar for males (1.668) and females (1.626). Length converted to age, using a mathematical approach through the von Bertalanffy growth function with seasonal variations (from de Graaf and Dekker 2006), is shown in Fig. 7. Total mortality (Z) was estimated as 3.41 year–1 for females (r = − 0.999; IC = 3.03 ≅ 3.78) and 2.66 year–1 for males (r = − 0.979; IC = 1.65 ≅ 3.67). The recruitment patterns for males and females showed similar peaks, with higher percentages between April and June for both sexes. The highest percentages in these months ranged between 15 and 25% for males and 13–20% for females.
Discussion
The sex ratio for the overall population did not differ from the expected 1:1, but when juveniles and adults were separated, males predominated among juveniles and females among adults. For burrowing shrimps generally, a male-biased ratio is commonly reported among juveniles, while for adults, a female-biased ratio is the general rule (Dworschak 1988; Dumbauld et al. 1996; Rodrigues and Shimizu 1997; Shimizu 1997; Botter-Carvalho et al. 2007; Rosa et al. 2018; Costa et al. 2020b).
Wenner (1972) described four patterns of differences in the sex ratios of crustacean populations divided into size classes: (A) The “Pattern”, where the distribution does not differ from 1:1 in most classes, except the largest, which are dominated by males; (B) Reversal pattern (S-shaped curve), where the sex ratio changes inversely as the class size increases, which is common in populations with sex reversal; (C) Intermediate pattern, an intermediate state between the previous two, due to the inclusion of juvenile individuals; and (D) Anomalous pattern, where in the smallest classes the pattern is 1:1, and females are more abundant in the intermediate classes and males in the larger classes. More recently, Terossi and Mantellato (2010) suggested a fifth pattern, called the “Predominance Pattern”, which describes a population with a predominance of one sex in almost all size classes. The authors also suggest the possible existence of other patterns, especially in tropical and subtropical regions.
Fragillianassa fragilis appears to show an anomalous pattern (Fig. 4b). Anomalous patterns in the sexual proportions of crustaceans can be explained by factors such as longevity, differential migration, differential mortality, and differences in growth rates (Wenner 1972). In the present study, the variation of the 1:1 ratio in the smaller size classes may be due to the collection method, which is inefficient in capturing small organisms. The concentration of intermediate-sized females could indicate a differential growth rate, when females nearing sexual maturation (≅ 3 mm), would be concentrated in these classes. The decrease in the proportion of males in the 3.5-mm class (males reach sexual maturity at ≅ 3.3 mm) could also result from a differential mortality rate caused by combat, due to their agonistic and territorial behavior (Felder and Lovett 1989; Dumbauld et al. 1996; Rodrigues and Shimizu 1997; Shimoda et al. 2005).
Regarding the CL, the smallest individuals caught were smaller than those of A. arenosa (Butler et al. 2009), a callianassid species with lengths similar to F. fragilis, with the smallest individuals ranging between CL 2–3 mm for females and between 1–2 and 2–3 mm for males, while ovigerous females were similar in size, i.e., more than 3 mm. This value is also similar to that obtained through the SQR in the linear regression, which is considered the transition point between the juvenile and adult phases (males = 3.3 mm and females = 2.9 mm), very close to that defined by Butler et al. (2009) (CL > 3 mm).
Several studies have found continuous reproduction for burrowing shrimps in tropical regions, e.g. Callichirus major (Say, 1818) (Rodrigues and Shimizu 1997; Botter-Carvalho et al. 2007; Peiró et al. 2014), Lepidophthalmus sinuensis Lemaitre and de Almeida Rodrigues, 1991 (Nates and Felder 1999) and Upogebia omissa Gomes Corrêa, 1968 (Costa et al. 2020a), in contrast to populations in temperate regions, which tend to have short seasonal reproductive periods (Dumbauld et al. 1996; Kevrekidis et al. 1997; Tamaki et al. 1997). Fragillianassa fragilis did not follow the “tropical” pattern, indicating seasonal reproduction (October to May), corresponding to the dry season in northeastern Brazil, during the period with the highest temperatures of the year (maximum mean of 32 °C), low rainfall (mean of 83 mm) (Inmet 2018), and the highest salinities measured in the study period (39 and 38 in November and January).
Although temperature is a determining factor for the reproduction of many crustaceans, other variables may directly affect reproductive periods, such as salinity and nutrient variations (Rodrigues and Shimizu 1997; Nates and Felder 1999; Peiró et al. 2014). Casa Caiada Beach has a relatively constant high temperature throughout the year, indicating that temperature is not the determining factor stimulating the beginning of reproduction. From November 2017, we observed a gradual increase in the presence of macroalgae in the study area, with a peak in January 2018 when the bank was completely taken over, and a subsequent decrease in May 2018. This highly heterogeneous area has a mosaic of algae, with species of Ulva, Halodule, Bryopsis, Caulerpa, and a large Cladophora carpet. Thus, we suggest that the temporal increase in food availability, together with the plankton bloom, would likely trigger the beginning of the reproductive period for this species, when high concentrations of food would be available to females and to larvae after hatching.
Compared to other ghost shrimps, F. fragilis has relatively low fecundity, with a maximum of 259 eggs. For C. major, Costa et al. (2020b) found a maximum egg number of 11,460. Other studies have also found high egg numbers for C. major: 9931 (Peiró et al. 2014), 6600 (Souza et al. 1998), 6478 (Rosa et al. 2018), and 3530 (Botter-Carvalho et al. 2007). For Neotrypaea japonica (Ortmann, 1891; Tamaki et al. 1997) found 962 eggs. However, A. arenosa, a species of similar size, showed similar fecundity, with the number of eggs ranging from 31 to 216 (Butler et al. 2009). The difference in the number of eggs produced is most likely related to female size (Hill 1977; Thessalou-Legaki and Kiortsis 1997), since F. fragilis is much smaller than the other species. Other authors have related this low fecundity to more egg-laying in the same reproductive period (Dworschak 1988; Kevrekidis et al. 1997; Tamaki et al. 1997), but this cannot be proven from the present data for F. fragilis.
The relationship between egg number and female size was found to be significant, indicating that egg number is correlated with female size. Arenallianassa arenosa (Butler et al. 2009) produced a mean of 96 ± 54 eggs, with a significant relationship between egg number and size (R² = 0.51 and p < 0.001), similar to the present results. This pattern is common for burrowing shrimps and has been reported in several studies (Souza et al. 1998; Berkenbusch and Rowden 2000; Botter-Carvalho et al. 2007, 2015; Rotherham and West 2009; Peiró et al. 2014; Costa et al. 2020b).
The growth parameters (L ∞ and K) were quite different from those reported for other ghost shrimps (Shimizu 1997; Pezzuto 1998; Souza et al. 1998; Botter-Carvalho et al. 2007; Simão and Soares-Gomes 2017; Rosa et al. 2018; Costa et al. 2020b). This discrepancy may be directly correlated to animal body size. The estimated L ∞ for males and females was very close to the longest CL found during the study period (6.59 and 6.14 mm), and the K values, which are inversely proportional to the L ∞ value, were relatively high.
The linear regression between the growth parameters found here and for C. major (Shimizu 1997; Souza et al. 1998; Botter-Carvalho et al. 2007; Simão and Soares-Gomes 2017; Rosa et al. 2018; Costa et al. 2020b) and Audacallichirus mirim (de Almeida Rodrigues, 1971) (Pezzuto 1998), indicated a strong and significant relationship (r² = 0.71, p < 0.001) (Fig. 8). Comparison of growth performance using the Growth Performance Index (Φ’) indicated a similarity between species. This comparative approach has the additional advantage of allowing inferences about the growth of these organisms, given these growth characteristics (Munro and Pauly 1983).
Linear regression between L ∞ and K of ghost shrimps. Species: Fragillianassa fragilis (1) Current study; Callichirus major (2) Shimizu (1997), (3) Souza et al. (1998), (4) Botter-Carvalho et al. (2007), (5) Simão and Soares-Gomes (2017), (6) Rosa et al. (2018), (7) Costa et al. (2020b) (exploited), (8) Costa et al. (2020b) (unexploited); and Audacallichirus mirim (9) Pezzuto (1998)
The estimated longevity for males and females was around 3 years, similar to previous estimations for other ghost shrimps: between 2 and 2.5 years for Lepidophthalmus louisianensis (Schmitt 1935) (Felder and Lovett 1989); from 2 to 3 years for Filhollianassa filholi (Milne-Edwards, 1879) (Berkenbusch and Rowden 1998); and for C. major, 3 years (Botter-Carvalho et al. 2007), 3.16 years (Simão and Soares-Gomes 2017), 3.45 years (Rosa et al. 2018), and 3.5 years (Costa et al. 2020b).
The period of least growth for females (WP) occurred in September, one month before the reproductive period which occurred from October to May, showing a mobilization of energy for vitellogenesis rather than body growth (Tamaki et al. 1997; Pezzuto 1998). For males, WP occurred in June, coinciding with low mean temperatures (21 °C) and lower salinity (30).
Total mortality (Z/year) was relatively high, with similar levels to exploited populations such as C. major by Botter-Carvalho et al. (2007) (Z = 3.92 and Z = 3.08) and Costa et al. (2020b) (Z = 2.42 and Z = 3.19) for males and females, respectively. There was a strong relationship between mortality and body size, where high mortality rates were generally correlated with small L ∞ sizes; however, this is due primarily, to the relationship between low L ∞ values and high K values (Pauly 1980). Another factor affecting mortality is the environmental quality of an area, which, when degraded, can also negatively affect the local population, boosting natural mortality (Conides et al. 2012). The mean temperature in the study area was 28.5 °C, relatively high, mainly because breakwaters in the area trap water and sediment. This urban beach is also subject to solid waste dumping, constituting a major problem of environmental degradation, which could directly influence species mortality.
Overall, F. fragilis, compared to other ghost shrimp, showed differences in growth parameters, reproductive strategy, and mortality. These differences were mainly due to the difference in size, since other species reach much larger sizes. The population characteristics of F. fragilis, such as its short life cycle, rapid growth and development, and high mortality rates indicate that the species is quite resilient and that its population stock continues steady, even in an extremely degraded environment. This study provides the first information on population structure and reproductive aspects of F. fragilis, which is the only species of the new genus studied. New ecological information on ghost shrimp life-history strategies is vitally important to develop conservation measures for this group, which is an important component of secondary production in coastal ecosystems.
Data availability
Data will be made available upon reasonable request.
References
Ayres M, Ayres M Jr, Ayres DL, Santos AAS (2007) Bioestat 5.0: aplicações estatísticas nas áreas das ciências biológicas e médicas. IDSM, Belém
Berkenbusch K, Rowden AA (1998) Population dynamics of the burrowing ghost shrimp Callianassa filholi on an intertidal sand flat in New Zealand. Ophelia 49:55–69. https://doi.org/10.1080/00785326.1998.10409373
Berkenbusch K, Rowden AA (2000) Intraspecific burrow plasticity of an intertidal population of Callianassa filholi (Crustacea: Decapoda: Thalassinidea) in relation to environmental conditions. N Z J Mar Freshw Res 34:397–408. https://doi.org/10.1080/00288330.2000.9516943
Berkenbusch K, Rowden AA (2003) Ecosystem engineering — moving away from ‘just-so’ stories. N Z J Ecol 27:67–73
Bird FL, Poore GCB (1999) Functional burrow morphology of Biffarius arenosus (Decapoda: Callianassidae) from southern Australia. Mar Biol 134:77–87
Bird FL, Boon PI, Nichols PD (2000) Physicochemical and microbial properties of burrows of the deposit-feeding thalassinidean ghost shrimp Biffarius arenosus (Decapoda: Callianassidae). Estuar Coast Shelf Sci 51:279–291. https://doi.org/10.1006/ecss.2000.0676
Boon PI, Bird FL, Bunn SE (1997) Diet of the intertidal callianassid shrimps Biffarius arenosus and Trypea australiensis (Decapoda: Thalassinidea) in western Port (Southern Australia), determined with multiple stable-isotope analyses. Mar Freshw Res 48:503–511. https://doi.org/10.1071/MF97013
Botter-Carvalho ML, Santos PJP, Carvalho PVVC (2007) Population dynamics of Callichirus major (Say, 1818) (Crustacea, Thalassinidea) on a beach in northeastern Brazil. Estuar Coast Shelf Sci 71:508–516. https://doi.org/10.1016/j.ecss.2006.09.001
Botter-Carvalho ML, Carvalho PVVC, Santos PJP (2012) New records confirming the occurrence of the ghost shrimps Biffarius biformis (Biffar, 1970) and B. fragilis (Biffar, 1971) (Decapoda, Callianassidae) in Brazil and the southwestern Atlantic. Biota Neotrop 12:88–93. https://doi.org/10.1590/S1676-06032012000300009
Botter-Carvalho ML, Costa LB, Gomes LL, Clemente CCC, Carvalho PVVC (2015) Reproductive biology and population structure of Axianassa australis (Crustacea, Axianassidae) on a sand-mud flat in north-eastern Brazil. J Mar Biol Assoc U K 95:735–745. https://doi.org/10.1017/S002531541400174X
Butler SN, Reid M, Bird FL (2009) Population biology of the ghost shrimps, Trypaea australiensis and Biffarius arenosus (Decapoda: Thalassinidea), in western Port, Victoria. Mem Mus Vict 6:43–59
Conides AJ, Nicolaidou A, Apostolopoulou M, Thessalou-Legaki M (2012) Growth, mortality and yield of the mudprawn Upogebia pusilla (Petagna, 1792) (Crustacea: Decapoda: Gebiidea) from western Greece. Acta Adriat 53:87–102
Costa LB, Marinho NCM, Carvalho PV, Botter-Carvalho ML (2020a) Population dynamics of the mud shrimp Upogebia omissa (Crustacea: Gebiidea: Upogebiidae) from the southwestern Atlantic coast of Brazil. Reg Stud Mar Sci 36:101281. https://doi.org/10.1016/j.rsma.2020.101281
Costa LB, Marinho NCM, Gomes PB, Santos PJP, Carvalho PV, Botter-Carvalho ML (2020b) Interpopulation differences of the burrowing shrimp Callichirus major on urban beaches under different levels of fishing pressure. Ocean Coast Manage 197:105310. https://doi.org/10.1016/j.ocecoaman.2020.105310
de Graaf GJ, Dekker P (2006) A simple spreadsheet model to incorporate seasonal growth into length-based stock assessment methods. Naga Worldfish Center Q 29:46–54
Dumbauld BR, Armstrong D, Feldman KL (1996) Life-history characteristics of two sympatric thalassinidean shrimps, Neotrypaea californiensis and Upogebia pugettensis, with implications for oyster culture. J Crust Biol 16:689–708. https://doi.org/10.1163/193724096X00784
Dworschak PC (1988) The biology of Upogebia pusilla (Petagna) (Decapoda, Thalassinidea). Mar Ecol 9:51–77. https://doi.org/10.1111/j.1439-0485.1988.tb00198.x
Dworschak PC (2013) Axiidea and Gebiidea (Crustacea: Decapoda) of Costa Rica. Ann Nat Hist Mus Wien B115:37–55
Felder DL, Lovett DL (1989) Relative growth and sexual maturation in the estuarine ghost shrimp Callianassa louisianensis Schmitt, 1935. J Crust Biol 9:540–553. https://doi.org/10.1163/193724089X00566
Gayanilo FC, Sparre P, Pauly D (2005) FAO-ICLARM stock assessment tools II: FiSAT II: user’s guide. FAO Computerized Information, Rome
Hernández-Aguilera JL (1998) On a collection of thalassinids (Crustacea: Decapoda) from the Pacific coast of Mexico, with description of a new species of the genus Biffarius. Cienc Mar 24:303–312. https://doi.org/10.7773/cm.v24i3.754
Hill BJ (1977) The effect of the effluent on egg production in the estuarine prawn Upogebia africana (Ortmann). J Exp Mar Biol Ecol 29:291–302. https://doi.org/10.1016/0022-0981(77)90072-7
INMET. Instituto Nacional de Meteorologia. Available at: http://www.inmet.gov.br/portal/. Accessed 2 Nov 2018
Kevrekidis T, Gouvis N, Koukouras A (1997) Population dynamics, reproduction and growth of Upogebia pusilla (Decapoda, Thalassinidea) in the Evros delta (North Aegean Sea). Crustaceana 70:799–812
Lopez Veiga EC (1979) Fitting von Bertalanffy growth curves in short-lived fish species. A new approach. Invest Pesq 43:179–186
Moreau J, Cuende FX (1991) On improving the resolution of the recruitment patterns of fishes. Fishbyte 9:45–46
Munro JL, Pauly D (1983) A simple method for comparing the growth of fishes and invertebrates. Fishbyte 1:5–6
Nates SF, Felder DL (1999) Growth and maturation of the ghost shrimp Lepidophthalmus sinuensis Lemaitre and Rodrigues, 1991 (Crustacea, Decapoda, Callianassidae), a burrowing pest in penaeid shrimp culture ponds. Fish Bull 97:526–541
Pachelle PP, Tavares M (2020) Axiidean ghost shrimps (Decapoda: Axiidae, Callianassidae, Callichiridae, Micheleidae) of the Trindade and Martin Vaz Archipelago, Vitória-Trindade Seamounts Chain and Abrolhos, off southeastern Brazil. Zootaxa 4758(1):103–126. https://doi.org/10.11646/zootaxa.4758.1.4
Pachelle PP, Anker A, Mendes CB, Bezerra LEA (2016) Decapod crustaceans from the state of Ceará, northeastern Brazil: an updated checklist of marine and estuarine species, with 23 new records. Zootaxa 4131:1–63. https://doi.org/10.11646/zootaxa.4131.1.1
Paloheimo JE (1958) A method of estimating natural and fishing mortalities. J Fish Res Board Can 15:749–758. https://doi.org/10.1139/f58-039
Pauly D (1980) On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J Mar Sci 39:175–192. https://doi.org/10.1093/icesjms/39.2.175
Pauly D (1984) Fish population dynamics in tropical waters: a manual for use with programmable calculators. ICLARM Stud Rev 8:1–325
Pauly D (1987) A review of the ELEFAN system for analysis of length-frequency data in fish and aquatic invertebrates. In: Pauly D, Morgan GR (eds) Length-based methods in fisheries research. ICLARM Conf Proc 13, pp 7–34. https://hdl.handle.net/20.500.12348/3366
Pauly D, Munro JL (1984) Once more on the comparison of growth in fish and invertebrates. Fishbyte 2:21–22
Peiró DF, Wehrtmann IS, Mantelatto FL (2014) Reproductive strategy of the ghost shrimp Callichirus major (Crustacea: Axiidea: Callianassidae) from the southwestern Atlantic: sexual maturity of females, fecundity, egg features, and reproductive output. Invertebr Reprod Dev 58:294–305. https://doi.org/10.1080/07924259.2014.944672
Pereira PS, Araújo TCM, Manso VDAV (2016) Tropical sandy beaches of Pernambuco State. In: Short AD, Klein AHF (eds) Brazilian Beach Systems. Coastal Research Library, vol 17. Springer, pp 251–279. https://doi.org/10.1007/978-3-319-30394-9_10
Pezzuto PR (1998) Population dynamics of Sergio mirim Rodrigues 1971 Decapoda: Thalassinidea: Callianassidae in Cassino Beach, Southern Brazil. Mar Ecol 19:89–109. https://doi.org/10.1111/j.1439-0485.1998.tb00456.x
Pillay D, Branch GM (2011) Bioengineering effects of burrowing thalassinidean shrimps on marine soft-bottom ecosystems. Oceanogr Mar Biol Annu Rev 49:137–192
Poore GC, Dworschak PC, Robles R, Mantelatto FL, Felder DL (2019) A new classification of Callianassidae and related families (Crustacea: Decapoda: Axiidea) derived from a molecular phylogeny with morphological support. Mem Mus Vict 78:73–146. https://doi.org/10.24199/j.mmv.2019.78.05
Rodrigues S, Shimizu RM (1997) Autoecologia de Callichirus major (Say, 1818) (Crustacea: Decapoda: Thalassinidea). In: Absalão RS, Esteves AM (eds) Ecologia de Praias Arenosas do Litoral Brasileiro. Oecol Bras 3:155–170
Rosa LC, Freire KM, Souza MJ (2018) Spatial distribution and population dynamics of Callichirus major (Crustacea, Callianassidae) in a tropical sandy beach, northeastern Brazil. Invertebr Biol 137:308–318. https://doi.org/10.1111/ivb.12228
Rotherham D, West RJ (2009) Patterns in reproductive dynamics of burrowing ghost shrimp Trypaea australiensis from small to intermediate scales. Mar Biol 156:1277–1287. https://doi.org/10.1007/s00227-009-1169-2
Sandifer PA (1973) Mud shrimp (Callianassa) larvae (Crustacea, Decapoda, Callianassidae) from Virginia plankton. Chesapeake Sci 14:149–159. https://doi.org/10.2307/1350601
Shimizu RM (1997) Ecologia populacional de Scolelepis squamata (Muller, 1806) (Polychaeta: Spionidae) e Callichirus major (Say,1818) (Crustacea: Decapoda: Thalassinidea) da praia de Barequeçaba (São Sebastião, SP). PhD thesis, Universidade de São Paulo
Shimoda K, Wardiatno Y, Kubo K, Tamaki A (2005) Intraspecific behaviors and major cheliped sexual dimorphism in three congeneric callianassid shrimp. Mar Biol 146:543–557. https://doi.org/10.1007/s00227-004-1453-0
Simão DS, Soares-Gomes A (2017) Population dynamics and secondary production of the ghost shrimp Callichirus major (Thalassinidea): a keystone species of Western Atlantic dissipative beaches. Reg Stud Mar Sci 14:34–42. https://doi.org/10.1016/j.rsma.2017.05.002
Somers IF (1988) On a seasonally oscillating growth function. Fishbyte 6:8–11
Soriano ML, Jarre A (1988) On fitting Somer’s equation for seasonally oscillating growth, with emphasis on T-subzero. Fishbyte 6:13–14
Souza JRBD, Borzone CA, Brey T (1998) Population dynamics and secondary production of Callichirus major (Crustacea, Thalassinidea) on a southern brazilian sandy beach. Arch Fish Mar Res 46:151–164
Tamaki A, Ingole B, Ikebe K, Muramatsu K, Taka M, Tanaka M (1997) Life history of the ghost shrimp, Callianassa japonica Ortmann (Decapoda: Thalassinidea), on an intertidal sandflat in western Kyushu, Japan. J Exp Mar Biol Ecol 210:223–250. https://doi.org/10.1016/S0022-0981(96)02709-8
Taylor CC (1958) Cod growth and temperature. ICES J Mar Sci 23:366–370. https://doi.org/10.1093/icesjms/23.3.366
Terossi M, Mantelatto FL (2010) Sexual ratio, reproductive period and seasonal variation of the gonochoric shrimp Hippolyte obliquimanus (Caridea: Hippolytidae). Mar Biol Res 6:213–219. https://doi.org/10.1080/17451000903078630
Thessalou-Legaki M, Kiortsis V (1997) Estimation of the reproductive output of the burrowing shrimp Callianassa tyrrhena: a comparison of three different biometrical approaches. Mar Biol 127:435–442. https://doi.org/10.1007/s002270050030
Vargas-Zamora J, Vargas-Castillo R, Sibaja-Cordero JA Crustáceos (Decapoda y Stomatopoda) del R.V., Skimmer RV (2019) Victor Hensen en el Golfo de Nicoya, Pacífico, Costa Rica. Rev Biol Trop 67:286–305. https://doi.org/10.15517/rbt.v67i1.34729
Wenner AM (1972) Sex ratio as a function of size in marine Crustacea. Am Nat 106:321–350. https://doi.org/10.1086/282774
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, Finance Code 001. The authors also thank Dr. Janet W. Reid (JWR Associates) for the English language translation.
Author information
Authors and Affiliations
Contributions
NCMM and PVVCC: Methods, formal analysis, field sampling, writing the original draft of the manuscript. MLBC: Supervision, revising and editing the draft. All authors discussed the results and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marinho, N.C.M., Carvalho, P.V.V.C. & Botter-Carvalho, M.L. Population dynamics and reproduction of the ghost shrimp Fragillianassa fragilis (Biffar, 1970) (Axiidea: Callianassidae) in a sand-mud bank on the northeastern coast of Brazil. Biologia 78, 2463–2472 (2023). https://doi.org/10.1007/s11756-023-01379-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01379-6