Abstract
We aimed to evaluate the effects of tropical crop systems and phosphorus (P) availability on arbuscular mycorrhizal fungi (AMF) spores’ community composition, and soil chemical properties in a Planosol at Tropical ecosystem from Brazilian Northeast. We collected rhizospheric samples containing soil and root fragments in a 5-year field experiment considering two groups of crop systems, i.e., no-till monocropping and agroforestry system, and testing two factors: the cropping system and the soil P availability. We identified the AMF community composition based on AMF spore’s morphology. We also characterized the soil chemical properties (e.g., soil pH, soil organic carbon, and available P) at samples level. Crop systems and soil P availability influenced the AMF community composition, and soil chemical properties. We found that: i) the abundance of Claroideoglomus claroideum, C. etunicatum, Rhizophagus intraradices, richness, Shannon’s index and Simpson’s index were positively correlated with no-till monocropping systems (Arachis hypogaea, Gossypium hirsutum, and Vigna unguiculata) and with all the studied agroforestry systems at low-P availability; and ii) soil pH, and soil organic carbon were positively correlated with no-till monocropping systems (Arachis hypogaea, Glicine hirsutum, Glicine max, and Sesamum indicum) at high-P availability, and Glicine max, Sesamum indicum, Zea mays, and agroforestry system at low-P availability. Our results highlight the positive effect of high P on AMF spores’ diversity, and the importance to consider both the crop system and soil P availability as key-factors promoting shifts into the AMF community composition and soil chemical properties in Tropical conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The interaction between tree species and arbuscular mycorrhizal fungi (AMF) under different P availabilities is important for plant fitness and its resistance to abiotic and biotic stresses (Tadersoo et al. 2020). The benefits of mycorrhizal symbiosis are closely related with the fine roots by helping the host plant to withstand abiotic and biotic stresses such as drought (Gao et al. 2020), soil anoxic conditions, root-feeding nematodes, and soil borne pathogens (Kariman et al. 2020; van’t Padje et al. 2020). This symbiosis also provides the host plants with water and essential plant-nutrients(Boutaj et al. 2020). In tropical environments, AMF can improve various plant physiological mechanisms by increasing growth, nutrient uptake, and yield (Passos et al. 2020). Therefore, understanding the effects of no-till monocropping and agroforestry system in low- and high-P availability on AMF community composition and soil chemical properties in a 5-year field experiment located at the Brazilian tropical seasonal dry forest is of interest in the field of soil ecology and can assist in the design of soil biology and management.
Plant roots support a wide diversity of AMF species by providing the fungi with C-rich exudates (Souza and Freitas 2017) and modifying rhizospheric conditions, i.e., soil pH by H+ extrusion (Ramos et al. 2008) and root exudation, i.e., allelopathy (Medeiros et al. 2021). Crop systems (e.g., no-till monocropping and agroforestry systems) can be classified as primary factors driving the succession of the AMF community composition of both annual plant species (e.g., A. hypogaea, G. max, G. hirsutum, S. indicum, V. unguiculata, and Z. mays), and perennial plant species (e.g., G. sepium, Mimosa caesalpiniaefolia, and Tabebuia alrea) as described by Guo et al. (2019), Hontoria et al. (2019), and McKenna et al. (2020). Given that the symbiotic phase among the AMF and plant species are genetically modulated (Souza 2015), plants can initiate the recruitment of specific AMF species by activating genes (e.g., myc+, CASTOR, DMI, SYMRK, etc), and to establish the morpho-physiological contact of the host plant (Souza 2015; Vergara et al. 2019).
In both no-till monocropping and agroforestry systems, plant-AMF interactions within the rhizosphere are generally modulated by soil P availability (Guo et al. 2019; Lei et al. 2020). The continuous use of P fertilization may disturb the AMF activity, i.e., sporulation and root colonization, thus promoting changes in the AMF community structure (Alvarado-Herrejón et al. 2019). The P fertilization practice can also indirectly affect rootability and rhizodeposition by changing soil pH and microbial activity such as the activity of P- and N-metabolism microorganisms (Na et al. 2019). According to Souza and Santos (2018), soil management practices that increase the plant-nutrient availability may weaken the interaction between plant species and AMF because the host does not need to sustain a symbiont inside its roots at high ATP investment. High P input could lead to a negative plant-soil feedback in the rhizosphere of annual plant species by weaking plant resistance induced by AMF against root-feeding nematode and fungal pathogens (Yang et al. 2020). The negative effects of conventional agriculture are well established for annual plant species, but information on the effects of no-till monocropping and agroforestry system into the Brazilian semi-arid is lacking (Sarto et al. 2020; Medeiros et al. 2021).
Various studies have investigated the effects of agriculture (Souza et al. 2015; Chave et al. 2019; Hontoria et al. 2019), and agroforestry system (Guo et al. 2019) on AMF community composition around the world. They have described a negative correlation between the AMF diversity and plant diversity. In such cases the monodominance lead of a plant-AMF simplification. We investigate for the first time in a Planosol at tropical ecosystem how both no-till monocropping and agroforestry systems impact AMF diversity, and soil chemical properties. We anticipated that the agroforestry system would promote positive changes in AMF community composition. To determine the impacts of crop systems (no-till monocropping vs. agroforestry systems), we asked (1) How does AMF community composition vary with crop system? (2) How does P availability influence AMF community structure in a tropical Planosol from Brazilian Northeast? In this context, we hypothesized that (i) the diversity of AMF species decreases with monocropping system at high-P availability; and ii) the variation in the AMF community composition is correlated with changes in soil chemical properties. Thus, we tested whether the AMF community, and soil properties might change at crop systems under low and high soil P availability.
Material and methods
Study sites
The experimental area was located at the experimental perimeter from EMEPA (Statal Research Company from Paraiba), Alagoinha, Paraiba, Brazil (6°57′00” S, 35°32′42” W, altitude of 317 m a.s.l). The climate is tropical wet and dry climate (As′ type according to the Köppen climate classification), with 995 mm of total annual precipitation and mean annual air temperature of +26.4 °C. Total monthly precipitation (mm), and mean air temperature (°C) from Alagoinha, Paraiba, Brazil from January to December 2018 (Fig. 1) were obtained online: https://portal.inmet.gov.br/, and http://www.aesa.pb.gov.br/aesa-website/meteorologia-chuvas/. The soil of the experimental area was classified as Planosol, with stagnant water profile and abrupt textural difference (e.g., presence of Bt horizon) between A horizon and B horizon (WRB 2006).
Total monthly precipitation (mm) and mean air temperature (°C) from January to December 2018 at the experimental area, Alagoinha, Paraiba, Brazil. Data obtained online: https://portal.inmet.gov.br/, and http://www.aesa.pb.gov.br/aesa-website/meteorologia-chuvas/
Experimental design
The field experiment has been conducted since 2013 using a randomized block design. In our study we considered a split-plot scheme with four blocks. In each block, we have nine treatments (e.g., divided into the two groups of crop systems) as plots (38 × 20 m), soil available P levels (e.g., low-P and high-P availability) as subplots (19 × 20 m), and 10 repetitions (e.g., sampling points located near the rows following 2 m of distance between each other) per subplot. Our treatments were divided into two main groups which simulated the follow systems: AF Group = Agroforestry systems (AF); and NTFS Group = No-till monocropping farming systems. Within AF group, we considered the following plant species combination into the plots: AF1 = [G. sepium (Jacq.) Steud. + Z. mays L. + B. decumbens Stapf.]; AF2 [M. caesalpiniaefolia Bentham + Z. mays L. + B. decumbens Stapf.]; and AF3 [T. alrea(Manso) Benth. & Hook. f. ex S. Moore + Z. mays L. + B. decumbens Stapf.]. Whereas for the NTFS group, we considered the following annual monocropping systems as plots: A. hypogaea L., Gossypium hirsutum L., Glycine max (L.) Merrill, S. indicum L., Vigna unguiculata (L.) Walp. and Z. mays L. Each plot had 38 × 20 m, and soil and crop’s management following recommendations according Cavalcanti (2008) and specifics recommendations according “iLPF net” (https://www.redeilpf.org.br/). All the plant combinations from AF groups are widely recommended into the Brazilian Northeast by the “iLPF net” as the best crop systems for semiarid environments because their resistance to abiotic stresses such as drought. For the no-till monocropping farming systems all annual plant species but G. max (L.) Merrill are widely used by the Brazilian smallholder farmers located into the tropical seasonal Brazilian dry forest (e.g., Caatinga ecoregion).
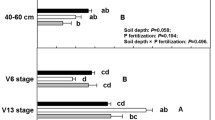
To better characterize the plant environment of our studied treatments, we described plant variables (e.g., shoot and fine root dry biomass, and plant yield), and some soil variables (e.g., litter deposition and Olsen’s available P) from the first year of the experiment. For plant variables, we selected 10 plants per subplot during flowering stage, that were harvested at the ground level. For the AF group, we estimated tree dry biomass using the allometric equations proposed by Laurindo et al. (2020). To estimate fine root (diameter: < 2 mm) dry biomass, we collected roots from the soil samples of each collected plant during flowering stages within soil monoliths (20 × 20 × 20 cm). Fine roots were washed using a 0.5-mm nylon mesh bag. We sorted fine roots into living and dead roots based on morphology and condition. Only living roots were considered to estimate dry biomass. Into the AF groups, fine roots included both tree and herbaceous species because it was difficult to distinguish between these precisely. Fine root dry biomass (g) was determined after drying the samples for 48 h at 70 °C. The plant yield of annual species was estimated by collecting 10 plants of each studied annual plant species during seed maturity stage (Fig. 2).
Shoot dry biomass (g plant−1, a, b), root dry biomass (g monolith−1, c, d) and plant yield (e) from AF and NTFS groups as affected by soil P availability. The values are means (± SD, N = 40). Shoot dry biomass (SDB) from AF group was estimated by using the following equation: SDB (g plant−1) = [0.36 × DBH1.86]/1000. DBH = Diameter at breast height (cm). Root dry biomass was estimated from soil monoliths with 20 × 20 × 20 cm
Soil samples to characterize AMF community composition and soil chemical properties were collected in 2018 inside the field experiment considering two groups of crop systems, i.e., no-till monocropping and agroforestry systems. The soil environment was characterized by measuring litter deposition and estimating Olsen’s P at both low- and high-P availability. Before collecting the soil monoliths, an area of 20 × 20 cm on the soil surface was delimited for separately sampling the litter layer. Litter deposition (LP) was calculated by the following equation: LP (g cm−2) = DDPF (g)/ 400 cm−2. Where, DDPF is the dry dead plant fragments deposited on the monolith surface; and 400 cm−2 is the basal area of each soil monolith. Samples of each subplot from monoliths were air-dried and passed through a 2-mm sieve. Available P was determined using the Olsen’s P protocol (Olsen et al. 1954)(Table 1).
AF1 = G. sepium + Z. mays + B. decumbens; AF2 = M. caesalpiniaefolia + Z. mays + B. decumbens; AF3 = T. alrea + Z. mays + B. decumbens. a Independent sample t test comparing Low- × High-P groups. ns: not significative; * p < 0.05; and ** p < 0.01.
AMF community characterization
During each month (i.e., from January to December 2018), we collected three soil samples containing soil plus root fragments by each subplot (i.e., considering low- and high-P availability) within each experimental plot. All samples were collected at a soil depth of 0 to 20 cm. Each sample was divided in two portions. The first one to chemically analyse soil properties, and the second one to characterize AMF community composition by AMF species identification. We extracted AMF spores from each soil sample using the wet sieving protocol as described by Gerdemann and Nicolson (1963), followed by centrifugation using sucrose gradient (Jenkins 1964). First, the AMF spores were sorted under a dissecting microscope by their morphology (e.g., Acaulosporoid, Gigasporoid, Glomoid, and Radial-Glomoid) (Souza 2015). Next, the spores were identified in microscope using polyvinyl alcohol lacto-glycerol (PVLG), and Melzer’s reagent (Souza 2015). During taxonomical identification, we considered the morphological characteristics of the spore walls and presence of special structures (e.g., layer’s ornamentation, germination shield or orb, peridium, small bulbs on subtending hypha, and inner walls). The identification of AMF species was realized by consulting the International Culture collection of arbuscular mycorrhizal fungi database available on the INVAM (http://invam.caf.wvu.edu). We used the AMF classification adjusting new recently updated taxa (Oehl et al. 2011; Sieverding et al. 2011; Goto et al. 2012). As we did not aim to evaluate the seasonal variation of AMF community composition, in our results we showed the mean AMF’s abundance, and ecological indices (e.g., Chao, Shannon, Simpson and Pielou’s indices).
Soil sampling
We determined the following soil chemical properties: soil pH, total organic carbon, and available phosphorus. First, the soil pH was determined in a suspension of soil and distilled water (1 : 2.5, v: v, soil: water suspension). Next, the total organic carbon was determined through rapid dichromate oxidation method as described by Okalebo et al. (1993). Finally, the available P was determined as described by Olsen et al. (1954).
Data analysis
All analyses in our study were carried out using R software (R Core Team 2018). For all variables we tested normality by Shapiro-Wilk test. Also, we assessed homogeneity of variances using Bartletts test. The normality test was performed using the “shapiro.test” function, and the homogeneity test was performed using the “dplyr” package. To compare AMF community composition and soil chemical properties, we used two-way ANOVA followed by the Bonferroni test at 5% of probability. We performed non-metric multidimensional scaling (NMDS) using the “metaMDS” function of the “vegan” package to analyse differences between crop system and soil P availability in terms of AMF composition using Euclidean dissimilarities (Schmitz et al. 2020). Here, we performed a PERMANOVA, 9999 permutations) to determine differences in AMF composition using “adonis” function of the “vegan” package. To investigate a possible relationship between soil chemical properties and biotic (AMF species) variables, a CCA was used examining the similarity or dissimilarity in the AMF community composition of plots along the crop systems and soil P availability. The CCA was performed using the “ggord” and “ordiplot” of the “vegan” package.
Results
AMF community composition as influenced by crop system and soil P availability
The ecological indices varied between the crop system (p < 0.001), soil P availability (p < 0.05), and their interaction (p < 0.001). The highest values of Chao’s index were found on plots with A. hypogaea, V. unguiculata, and all the agroforestry systems (AF1, AF2, and AF3) at high soil P availability. For the AMF diversity and AMF dominance (Shannon and Simpson’s indices), we found the highest values on plots with A. hypogaea, G. hirsutum, V. unguiculata, and AF3 at high-P availability. Finally, for the evenness index (Pielou’s index), we found the highest values on plots with A. hypogaea, and AF1 plots at high-P availability. For G. hirsutum plots, we did not find significative differences on Chaos’s index, Shannon’s index (diversity), Simpson’s index (dominance), and Pielou’s index (evenness) between high- and low-P availability (Table 2).
Soil chemical properties as influenced by crop system and soil P availability
We observed significant differences on soil pH (p < 0.05), soil organic carbon (p < 0.001) and available P (p < 0.001) between the studied crop system and soil P availability. For soil pH, we found the highest values on plots at high-P availability with G. max and S. indicum, while at low-P availability the highest values were found on plots with all agroforestry systems (AF1, AfF2, and AF3). For soil organic carbon, we found the highest values at high-P availability with all no-till monocropping systems but V. unguiculata and AF3, while on low-P availability the highest values were found with A. hypogaea, and G. hirsutum plots. For the available P, the highest values at high-P availability were found with G. hirsutum plots, while on low-P availability, we found the highest values with A. hypogaea plots (Table 3).
The principal components analysis of the AMF community composition and soil chemical properties revealed that in different plots, these variables changed as a function of crop systems and soil available P conditions. According to the factor loadings of the principal components analysis, the abundance of C. claroideum, C. etunicatum, R. intraradices, richness, diversity (H′) and dominance (C) were positively correlated with no-till monocropping systems (A. hypogaea, G. hirsutum, and V. unguiculata) and agroforestry system (AF1, AF2, and AF3) at low-P subplots (Fig. 3a), while the soil pH, and soil organic carbon were positively correlated with no-till monocropping systems (A. hypogaea, G. hirsutum, G. max, and S. indicum) at high-P subplots, and G. max, S. indicum, Z. mays, and agroforestry system at low-P subplots (Fig. 3b).
Profile of arbuscular mycorrhizal fungi community composition (a), and soil chemical properties (b) plotted as Principal Component Analysis (PCA) of samples collected from different crop system and soil available P conditions. The two axes represent 66.75%, and 78.04% of data variance, respectively. Only high significant values are shown (p < 0.001). Agroforestry system are represented as follows: AF1 = G. sepium + Z. mays + B. decumbens; AF2 = M. caesalpiniaefolia + Z. mays + B. decumbens; AF3 = T. alrea + Z. mays + B. decumbens
Discussion
We demonstrated the influences of crop system and soil P availability on AMF community composition at AMF species level (Table 2), and on soil chemical properties (Table 3). Our results improved the understanding of the AMF responses to both no-till monocropping (e.g., A. hypogaea, G. hirsutum, G. max, S. indicum, V. unguiculata, and Z. mays) and agroforestry systems (Freschet et al. 2015; de Stefano and Jacobson 2018; Basirat et al. 2019; Melo et al. 2020). Our results detected dissimilar patterns of plant species (e.g., perennial plant species showing higher biomass than the annual plant species), root morphology (e.g., taproot vs. fibrous root), and crop systems (e.g., monocropping vs. agroforestry system) under field conditions (Fig. 2).
AMF community was characterized based on AMF spores’ morphology, which allowed us to understand the AMF investment for future symbiosis as an effect of the current plant-AMF symbioses that occurred in our field experiment. First, AMF species colonized the fine roots (Table S1) to establish the symbiotic process. Next, they improved plant performance (Fig. 2), and finally, they sporulate as influenced by the host plant (Table S2). According to many studies, the AMF sporulation is a highly carbon demanding process that starts during the plant senescence, i.e., when the AMF mycelium starts to be nutrient-limited(Souza and Freitas 2017; Liu et al. 2019; Medeiros et al. 2021). In our study, it is an important point because in the agroforestry system treatments, we have trees with a 5-years life cycle, whereas we have annual crops dominating the monocropping systems. It was expected to find in the agroforestry system less AMF spores than in the monocropping system (Deveautour et al. 2021). This hypothesis was supported for all studied agroforestry systems showing an average of 34.5% less AMF spores than the monocropping systems.
Our field study determined the significant effects of crop systems and soil P availability on the AMF spore abundance (Table S2), richness (Chao’s index), diversity (Shannon’s index), dominance (Simpson’s index) and evenness (Pielou’s index) (Table 2)(Liu et al. 2019; Soonvald et al. 2020). In tropical soils, the soil P availability is considered as the most important factor that drives AMF richness and diversity in agroforestry and monocropping systems (Belay et al. 2020). However, in both agroforestry and monocropping systems, the effect soil P availability on AMF community composition was positive in all studied treatments. Thus, our statement about the agroforestry system as the best treatment promoting positive changes on AMF community composition when compared to the no-till monocropping system was not supported. In general, we found the highest values of richness, diversity, dominance, and evenness (Table 2) on subplots with high-P availability. These phenomena may be related with the higher root dry biomass production (Fig. 2 and Table S2) at the high- P availability when compared to the low root dry biomass at the low- P availability sites (Impastato and Carrington 2020). The observed changes in the AMF community were primarily driven by the varying soil chemical properties (e.g., soil pH, soil organic carbon, and available P). Soil pH, soil organic carbon, and available P mainly explained the succession of the AMF community assemblage (Table 3, and Fig. 3).
Acid soils may reduce the abundance of AMF species with large spores (e.g., Gigaspora species), and select AMF species with less dense mycelial networks (e.g., Glomeraceae species) (Wang et al. 2021). On the other hand, soil organic carbon may improve AMF community composition by creating favourable condition to glomalin-related soil protein production and root activity (Wei et al. 2019). Finally, high P availability usually decreases the abundance of Gigaspora and Scutellospora species (Zhang et al. 2020). In our study, we found that crop systems which promoted (i) soil acidification (e.g., A. hypogaea, G. hirsutum, and AF2 at high P sites, while G. hirsutum, V. unguiculata, and Z. mays at low P sites); ii) soil rhizodeposition (e.g., A. hypogaea, G. hirsutum, G. max, and V. unguiculata at high P sites); and iii) soil fertility (e.g., G. hirsutum at high P sites) have promoted the AMF-plant interaction by inducing Claroideoglomeraceae and Glomeraceae spore germination (e.g., Claroideoglomus, and Rhizophagus) and hyphal growth rate into these soil conditions during our field study. Like the results found in our study for agroforestry and monocropping systems (Wang et al. 2017; Liu et al. 2020), the reducing of Gigaspora and Scutellospora species might make the agroforestry system much more prone to the negative plant-soil feedback than the monocropping systems.
The shifts in the AMF community composition in high-P availability affected the richness, diversity, dominance, and evenness (Table 2) of the AMF species for specific crop systems (e.g., A. hypogea, G. max, G. hirsutum, V. unguiculata, and Z. mays). Previous studies on the variation in the AMF community detected substantial changes into the AMF community structure as affected by host traits (Šmilauer et al. 2019; Higo et al. 2020; Pires et al. 2020). Consistently, we found that crop systems determined the AMF community structure and the abundance of R. intraradices, C. claroideum, and C. etunicatum. In the present study, the ecological indices of AMF community were positively correlated with high-P availability. This finding suggests a P-soil-driven succession pattern of the AMF assemblages in accordance with host species (Higo et al. 2020). In addition to AMF-plant feedback, plants can establish and alter their relationship with AMF species initiatively trough rootability (e.g., root exudation), which vary in diversity, intensity, and quantity over plant developmental stages (e.g., growth stage vs. reproductive stage) and plant nutritional status modulated by soil fertility (Tsiknia et al. 2020; Deveautour et al. 2021). This condition indicated that both the plant species and soil nutrient availability can drive the succession of AMF community in semi-arid conditions in the rhizosphere of annual and perennial plants via the modification of rootability and rhizodeposition patterns. By contrast, the soil P availability cannot directly explain the shift in the AMF community by its own. Considering that the nutritional requirements of both monocropping and agroforestry systems were satisfied with fertilization during seedling (unpublished data), our results indicate that (i) into the monocropping systems (e.g., A. hypogaea, G. hirsutum, G. max, and V. unguiculata) and the agroforestry system (e.g., AF1 and AF2) the host plants increased their ability to recruit and structure their rhizosphere to AMF from Glomeraceae and Claroideoglomeraceae; (ii) the plots with high soil carbon content showed the highest values of biomass production, rootability which was promoted by the studied crop systems (e.g., A. hypogea, G. max, G. hirsutum, V. unguiculata, and Z. mays). In the first one, soil properties were influenced by rootability and rhizodeposition processes (Fig. 3) at high P availability, while in the second one is related to the effects of crop systems that promoted changes on soil chemical properties, such as improving plant nutrient availability (Gebremikael et al. 2016). In semi-arid ecosystems the high-quality organic residues deposition (e.g., litter) promote both rhizodeposition and biological activity next to the root zone (Deveautour et al. 2021). These results agreed with our previous hypothesis that soil ecosystem with high contents of H+ (e.g., acid soils) and organic compounds (e.g., root exudates) can promote soil chemical properties, soil organic carbon pools, and root system as described by Delgado-Baquerizo et al. (2018). Also, based on the importance of the relationship between host plants and AMF species in semi-arid conditions for plant growth and health (Souza and Freitas 2017; Souza and Santos 2018), we hypothesized that the shifts patters of AMF community structure, which is mainly driven by crop systems, will endanger the plant-soil feedback over the year. Further experimentations with plants inoculated with R. intraradices, C. claroideum, and C. etunicatum, which are based on AMF isolated and aggregated effect with annual and perennial plants under different P-fertilization rates (Fig. 3) can be useful in testing our main hypothesis.
Conclusion
The AMF community, and soil chemical properties responded differently to the changes in soil conditions promoted by the studied crop systems (e.g., no-till vs. agroforestry system) and soil P availability. The AMF community was directly affected by soil properties at both low- and high- P availability, which may suggest a P-soil-driven succession pattern of the AMF assemblages in accordance with host species. The significative abundance of C. claroideum, C. etunicatum, and R. intraradices in our study may suggest shifts into both rootability and rhizodeposition patterns. Hence, the monocropping with A. hypogea, G. max, G. hirsutum, V. unguiculata, and Z. mays in a no-till farming promoted soil carbon content, thus creating favourable conditions to AMF sporulation; and the agroforestry system increased the host plants ability to recruit and structure their rhizosphere to AMF from Glomeraceae and Claroideoglomeraceae.
References
Alvarado-Herrejón M, Larsen J, Gavito ME, Jaramillo-López PF, Vestberg M, Martínez-Trujillo M, Carreón-Abud Y (2019) Relation between arbuscular mycorrhizal fungi, root-lesion nematodes, and soil characteristics in maize agroecosystems. Appl Soil Ecol 135:1–8. https://doi.org/10.1016/j.apsoil.2018.10.019
Basirat M, Mousavi SM, Abbaszadeh S, Ebrahimi M, Zarebanadkouki M (2019) The rhizosheath: a potential root trait helping plants to tolerate drought stress. Plant Soil 445:565–575. https://doi.org/10.1007/s11104-019-04334-0
Belay Z, Negash M, Kaseva V, Kahiluoto H (2020) Native forests but not agroforestry systems preserve arbuscular mycorrhizal fungal species richness in southern Ethiopia. Mycorrhiza 30:749–759. https://doi.org/10.1007/s00572-020-00984-6
Boutaj H, Meddich A, Chakhchar A, Wahbi S, Alaoui-Talibi Z, Douira A, Filali-Maltouf A, Modafar C (2020) Arbuscular mycorrhizal fungi improve mineral nutrition and tolerance of olive tree to Verticillium wilt. Arch Phytopathol Plant Protect 53:673–689. https://doi.org/10.1080/03235408.2020.1792603
Cavalcanti JFA (2008) Recomendações de adubação para o Estado de Pernambuco. Instituto Agronômico de Pernambuco, Recife
Chave M, Angeon V, Pault R, Collombet R, Tchamitchian M (2019) Codesigning biodiversity-based agrosystems promotes alternatives to mycorrhizal inoculants. Agron Sustain Dev. https://doi.org/10.1007/s13593-019-0594-y
de Stefano A, Jacobson MG (2018) Soil carbon sequestration in agroforestry systems: a meta-analysis. Agrofor Syst 92:285–299. https://doi.org/10.1007/s10457-017-0147-9
Delgado-Baquerizo M, Oliverio AM, Brewer TE, Benavent-González A, Eldridge DJ, Maestre FT, Sinhg BK, Fierer N (2018) A global atlas of the dominant bacteria found in soil. Science 359(6373):320–325. https://doi.org/10.1126/science.aap9516
Deveautour C, Donn S, Bennett AE, Power S, Powell JR (2021) Variability of arbuscular mycorrhizal fungal communities within the root systems of individual plants is high and influenced by host species and root phosphorus. Pedobiologia. https://doi.org/10.1016/j.pedobi.2020.150691
Freschet GT, Kichenin E, Wardle DA (2015) Explaining within-community variation in plant biomass allocation: a balance between organ biomass and morphology above- vs belowground? J Veg Sci 26(3):431–440. https://doi.org/10.1111/jvs.12259
Gao D, Pan X, Zhou X, Wei Z, Li N, Wu F (2020) Phosphorus fertilization and intercropping interactively affect tomato and potato onion growth and rhizosphere arbuscular mycorrhizal fungal community. Arch Agron Soil Sci. https://doi.org/10.1080/03650340.2020.1768530
Gebremikael MT, Steel H, Buchan D, Bert W, Neve S (2016) Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci Rep. https://doi.org/10.1038/srep32862
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46(2):235–244. https://doi.org/10.1016/S0007-1536(63)80079-0
Goto BT, Silva GA, de Assis DMA, Silva DK, Souza RG, Ferreira ACA, Jobim K, Mello CMA, Vieira HEE, Maia LC, Oehl F (2012) Intraornatosporaceae (Gigasporales), a new family with two new genera and two new species. Mycotaxon 119:117–132. https://doi.org/10.5248/119.117
Guo J, Wang G, Wu Y, Shi Y, Feng Y, Cao F (2019) Ginkgo agroforestry practices alter the fungal community structures at different soil depths in eastern China. Environ Sci Pollut Res 26:21253–21263. https://doi.org/10.1007/s11356-019-05293-w
Higo M, Azuma M, Kamiyoshihara Y, Kanda A, Tatewaki Y, Isobe K (2020) Impact of phosphorus fertilization on tomato growth and arbuscular mycorrhizal fungal communities. Microorganisms. https://doi.org/10.3390/microorganisms8020178
Hontoria C, García-Gonzáles I, Quemada M, Roldán A, Alguacil MM (2019) The cover crop determines the AMF community composition in soil and in roots of maize after a ten-year continuous crop rotation. Sci Total Environ 660:913–922. https://doi.org/10.1016/j.scitotenv.2019.01.095
Impastato CJ, Carrington ME (2020) Effects of plant species and soil history on root morphology, arbuscular mycorrhizal colonization of roots, and biomass in four tallgrass prairie species. Plant Ecol 221:117–124. https://doi.org/10.1007/s11258-019-00997-y
Jenkins WR (1964) A rapid centrifugation technique for separating nematodes from soil. Plant Dis Rep 48:692
Kariman K, Scanlan C, Boitt G, Rengel Z (2020) Feremycorrhizal symbiosis confers growth and nutritional benefits to mycorrhizal and non-mycorrhizal crops. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2020.108060
Laurindo LK, de Souza TAF, Silva LJR, Kormann S (2020) Produção primária líquida. In Laurindo LK, Souza T Indicadores da qualidade do solo em sistemas agroflorestais e ecossistemas associados, 1st edn. PPGEAN, Curitibanos, pp. 43–54
Lei H, Liu A, Hou Q, Zhao Q, Guo J, Wang Z (2020) Diversity patterns of soil microbial communities in the Sophora flavescens rhizosphere in response to continuous monocropping. BMC Microbiol. https://doi.org/10.1186/s12866-020-01956-8
Liu S, Xu J, Huang H, Zhu J, Tang J, Chen X (2020) Changes in the mycorrhizal fungal community in host roots over five host generations under low and high phosphorus conditions. Plant Soil 456:27–41. https://doi.org/10.1007/s11104-020-04694-y
Liu W, Zhang Y, Jiang S, Murray PJ, Liao L, Li X, Zhang J (2019) Spatiotemporal differences in the arbuscular mycorrhizal fungi communities in soil and roots in response to long-term organic compost inputs in an intensive agricultural cropping system on the North China plain. J Soils Sediments 19:2520–2533. https://doi.org/10.1007/s11368-019-02244-3
McKenna TP, Crews TE, Kemp L, Sikes BA (2020) Community structure of soil fungi in a novel perennial crop monoculture, annual agriculture, and native prairie reconstruction. PLoSONE 15:e0228202. https://doi.org/10.1371/journal.pone.0228202
Medeiros AS, Goto BT, Ganade G (2021) Ecological restoration methods influence the structure of arbuscular mycorrhizal fungal communities in degraded drylands. Pedobiologia. https://doi.org/10.1016/j.pedobi.2020.150690
Melo PL, Cherubin MR, Gomes TC, Lisboa IP, Satiro LS, Cerri CEP, Siqueira-Neto M (2020) Straw removal effects on sugarcane root system and stalk yield. Agronomy. https://doi.org/10.3390/agronomy10071048
Na X, Ma C, Ma S, Ma X, Zhu X, Xu P, Zhu H, Cao X, Liang W (2019) Monocropping decouples plant-bacteria interaction and strengthens phytopathogenic fungi colonization in the rhizosphere of a perennial plant species. Plant Soil. https://doi.org/10.1007/s11104-019-04311-7
Oehl F, Sieverding E, Palenzuela J, Ineichen K, da Silva GA (2011) Advances in Glomeromycota taxonomy and classification. IMA Fungus 2:191–199. https://doi.org/10.5598/imafungus.2011.02.02.10
Okalebo JR, Gathua KW, Woomer PL (1993) Laboratory methods of plant and soil analysis: a working manual. Technical bulletin n. 1. Nairobi: tropical soil biology and fertility Programme/soil science society East Africa/UNESCO/ROSTA. https://www.worldcat.org/title/laboratory-methods-of-soil-and-plant-analysis-a-working-manual/oclc/51374874
Olsen S, Cole C, Watanabe F, Dean L (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA circular Nr 939, US Gov. print. Office, Washington
Passos JH, Maia LC, Assis DMA, Silva JA, Oehl F, Silva IR (2020) Arbuscular mycorrhizal fungal community structure in the rhizosphere of three plant species of crystalline and sedimentary areas in the Brazilian dry Forest. Microb Ecol. https://doi.org/10.1007/s00248-020-01557-y
Pires GC, Lima ME, Zanchi CS, Freitas CM, Souza JMA, Camargo TA, Pacheco LP, Wruck FJ, Carneiro MAC, Kemmelmeier K, Moraes A, Souza ED (2020) Arbuscular mycorrhizal fungi in the rhizosphere of soybean in integrated crop livestock systems with intercropping in the pasture phase. Rhizosphere 17:100270. https://doi.org/10.1016/j.rhisph.2020.100270
R Core Team (2018) R version 3.5.0. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ramos AC, Façanha AR, Feijó JA (2008) Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol 178(1):177–188. https://doi.org/10.1111/j.1469-8137.2007.02344.x
Sarto MVM, Borges WLB, Sarto JRW, Pires CAB, Rice CW, Rosolem CA (2020) Soil microbial community and activity in a tropical integrated crop-livestock system. Appl Soil Ecol. https://doi.org/10.1016/j.apsoil.2019.08.012
Schmitz D, Schaefer CERG, Putzke J, Francelino MR, Ferrari FR, Corrêa GR, Villa PM (2020) How does the pedoenvironmental gradient shape non-vascular species assemblages and community structures in Maritime Antarctica? Ecol Indic 108:105726. https://doi.org/10.1016/j.ecolind.2019.105726.
Sieverding E, da Silva GA, Berndt R, Oehl F (2011) Rhizoglomus, a new genus of the Glomeraceae. Mycotaxon 129(2):373–386. https://doi.org/10.5248/129.373
Šmilauer P, Košnar J, Kotilínek M, Šmilauerová M (2019) Contrasting effects of host identity, plant community, and local species pool on the composition and colonisation levels of arbuscular mycorrhizal fungal community in a temperate grassland. New Phytol 225(1):461–473. https://doi.org/10.1111/nph.16112
Soonvald L, Loit K, Runno-Paurson E, Astover A, Tedersoo L (2020) Characterizing the effect of crop species and fertilization treatment on root fungal communities. Scientific Report 10(18741). https://doi.org/10.1038/s41598-020-74952-7
Souza T (2015) Handbook of arbuscular mycorrhizal fungi. Springer
Souza TAF, Freitas H (2017) Arbuscular mycorrhizal fungal community assembly in the Brazilian tropical seasonal dry forest. Ecol Process 6(2):2–10. https://doi.org/10.1186/s13717-017-0072-x
Souza TAF, Rodrígues AF, Marques LF (2015)Long-term effects of alternative and conventional fertilization I: effects on arbuscular mycorrhizal fungi community composition. Russ Agricult Sci 41(6):454–461. https://doi.org/10.3103/S1068367415060245
Souza TAF, Santos D (2018) Effects of using different host plants and long-term fertilization systems on population sizes of infective arbuscular mycorrhizal fungi. Symbiosis 76:139–149. https://doi.org/10.1007/s13199-018-0546-3
Tadersoo L, Bahram M, Zobel M (2020) How mycorrhizal associations drive plant population and community biology. Science 367(6480). https://doi.org/10.1126/science.aba1223
Tsiknia M, Tsikou D, Papadopoulou KK, Ehaliotis C (2020)Multi-species relationships in legume roots: from pairwise legume-symbiont interactions to the plant – microbiome - soil continuum. FEMS Microbiol Ecol 97(2). https://doi.org/10.1093/femsec/fiaa222
Van’t Padje A, Werner GD, Kiers ET (2020) Mycorrhizal fungi control value of phosphorus in trade symbiosis with host roots when exposed to abrupt ‘crashes’ and ‘booms’ of resource availability. New Phytol 229(5):2933–2944. https://doi.org/10.1111/nph.17055
Vergara C, Araujo KEC, Souza SR, Schultz N, Saggin Júnior OJ, Sperandio MVL, Zilli JÉ (2019)Plant-mycorrhizal fungi interaction and response to inoculation with different growth-promoting fungi. Pesq Agrop Brasileira 58(0). https://doi.org/10.1590/s1678-3921.pab2019.v54.25140
Wang C, Zheng MM, Song WS, Chen RF, Zhao XQ, Wen SL, Zheng ZS, Shen RF (2021) Biogeographic patterns and co-occurrence networks of diazotrophic and arbuscular mycorrhizal fungal communities in the acidic soil ecosystem of southern China. Applied Soil Ecology:158. https://doi.org/10.1016/j.apsoil.2020.103798
Wang J, Ren C, Cheng H, Zou Y, Bughio MA, Li Q (2017) Conversion of rainforest into agroforestry and monoculture plantation in China: consequences for soil phosphorus forms and microbial community. Sci Total Environ 595(1):769–778. https://doi.org/10.1016/j.scitotenv.2017.04.012
Wei L, Vosátka M, Cai B, Ding J, Lu C, Xu J, Yan W, Li Y, Liu C (2019) The role of arbuscular mycorrhiza Fungi in the decomposition of fresh residue and soil organic carbon: a Mini-review. Soil Science Society pf America Journal 83(3):511–517. https://doi.org/10.2136/sssaj2018.05.0205
WRB (2006) World soil resources report, FAO, Rome
Yang Q, Biere A, Harvey JA, Ding J, Siemann (2020) Antagonistic interactions between above- and belowground biota reduce their negative effects on a tree species. Plant Soil 454:379–393. https://doi.org/10.1007/s11104-020-04642-w
Zhang J, Quan C, Ma L, Chu G, Liu Z, Tang X (2020) Plant community and soil properties drive arbuscular mycorrhizal fungal diversity: a case study in tropical forests. Soil Ecology Letters 3:52–62. https://doi.org/10.1007/s42832-020-0049-z
Acknowledgments
The authors wish to thank Valdemir Ribeiro Cavalcante (EMEPA), Andre Julio do Amaral (EMBRAPA), and João Henrique Zonta (EMBRAPA) for their assistance.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
We declare that all the authors made substantial contributions to the conception, design, acquisition, analysis, and interpretation of the data. All the authors participate in drafting the article, revising it critically for important intellectual content; and finally, the authors gave final approval of the version to be submitted to Biologia.
Corresponding author
Ethics declarations
Ethics approval
We confirm that this manuscript that not been published elsewhere and is not under consideration by another journal. All Authors have approved the manuscript and agree with submission to Biologia. We have read and have abided by the statement of ethical standards for manuscripts submitted to Biologia.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, S.I.A., de Souza, T.A.F., de Lucena, E.O. et al. High phosphorus availability promotes the diversity of arbuscular mycorrhizal spores’ community in different tropical crop systems. Biologia 76, 3211–3220 (2021). https://doi.org/10.1007/s11756-021-00874-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00874-y