Abstract
Nanoparticles are receiving many interests due to their broad efficiency in mitigating environmental stresses. The purpose of this research was to study the potential of selenium-nanoparticles (Se-NPs) to mitigate the adverse effects of salt stress on growth, physiology, and gene expression of Melissa officinalis. The pot experiment was conducted in a factorial based on completely randomized design (CRD) with three Se-NPs concentrations (0, 50, and 100 mg L−1) and four salinity levels (0, 50, 100, and 150 mM NaCl). The foliar spray of Se-NPs improved the growth of M. officinalis plants at different salinity concentrations, from which the most effective was 50 mg L−1. Plant growth decreased by progressing salinity concentration so that the minimum growth was observed at 150 mM NaCl. The Se-NPs improved salt tolerance in M. officinalis by decreasing lipid peroxidation through increased activity of antioxidant enzymes viz. superoxide dismutase (SOD), catalase (CAT), and peroxidase (POX). Furthermore, exposure to Se-NPs enhanced the transcript levels of phenylalanine ammonia-lyase and rosmarinic acid (RA) synthase genes in lemon balm plants under salt conditions. Plants treated with 100 mg L−1 Se-NPs at non-saline conditions revealed the highest RA accumulation. According to the findings, we suggest both 100 mg L−1 Se-NPs to alleviate the adverse effects of salinity on in the M. officinalis, as well as the biosynthesis pathway of RA as a valuable secondary metabolite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Melissa officinalis L., a perennial herbaceous plant known as Lemon balm, belongs to the mint family or Lamiaceae. It is native to Iran, Central Asia, the Mediterranean Basin, and south-central Europe. The extract of M. officinalis is used in various food and beverage products (Sharopov and Setzer 2018). Research showed that M. officinalis has medicinal compounds which could be used to treat different illnesses. Activities like antioxidant, anti-mutagen, anti-inflammatory, anti-hyperglycemic, and memory impairment are some of the biologically important functions, which have been proven in several experimental methods (Hasanein and Riahi 2015; Noguchi-Shinohara et al. 2015; Eudes Filho et al. 2017; Jahanban-Esfahlan et al. 2017).
Rosmarinic acid (RA) is considered the main phenolic compound of M. officinalis (Hamaguchi et al. 2009) and an ester of 3, 4-dihydroxiphenyllactic acid with multiple biological activities (Noguchi-Shinohara et al. 2015). M. officinalis extracts containing RA are safe and tolerable in healthy individuals due to lack of adverse effects (Noguchi-Shinohara et al. 2015). Of eight enzymes included in the biosynthesis of RA, phenylalanine ammonia-lyase (PAL) and rosmarinic acid synthase (RAS) play an important role. PAL catalyzes the conversion of Phenylalanine to cinnamic acid as an initial reaction. As a precursor for the formation of some natural products, four-coumaroyl-CoA reaction with 4-hydroxyphenyllactic acid results in the formation of 4-coumaroyl-4′-hydroxyphenyllactic acid using RAS enzyme (Park et al. 2016). Some studies indicated that the expression of these genes enhanced under saline conditions (Döring et al. 2014; Sadat Ejtahed et al. 2015).
Salinity is a major abiotic stress with negative effects on plants and environment (Sytar et al. 2017). Chlorides and sodium are the main salts in water resources. Previous studies indicated that salinity could both improve soil structure and result in unsolicited effects on photosynthetic pigment formation, proline content, and RA biosynthesis (Kiarostami et al. 2010; Bui 2013; Akcin and Yalcin 2016.
Nanotechnology has emerged as a useful strategy for improving agricultural productivity. Rapid and complete absorption of nano-fertilizers by plants is the main reason of their wide application (Sajyan et al., 2020). The use of nanoparticles can increase plants’ resistance against salt stress (Siddiqui et al. 2014; Oliveira et al. 2016; Mohamed et al. 2017). Also, the study on Nanoscale selenium (Se-NPs) indicates that it can be used as a therapeutic agent with no major adverse effect (Hosnedlova et al. 2018). Additionally, Se in the bulk form was shown to enhances the enzymatic activities of antioxidants and maintains the functions of photosynthetic system and, this way, play an important role in mitigating the tolerance of salt in tomato (Solanum lycopersicum), strawberry (Fragaria vesca), garlic (Allium sativum), and maize (Zea mays) (Diao et al. 2014; Jiang et al. 2017; Ashraf et al. 2018; Astaneh et al. 2018; Zahedi et al. 2019). Se-NPs can induce the contents and activity of RA-associated genes. Recently, Babajani et al. (2019) found that dose-dependent exposure of M. officinalis to Se-NPs supplementations could significantly induce the RAS expression gene. Therefore, it is interesting to investigate the role of nano selenium in M. officinalis under salt stress, especially RA biosynthesis. The purpose of this study was to evaluate the effect of Se-NPs on growth, pigments content, antioxidant enzymes activity, RA content, and the expression of its biosynthetic genes in M. officinalis in salt stress conditions.
Materials and method
Chemicals and reagents
The spherical Se-NPs (average size: 10–45 nm, specific surface area: 30–50 m2 g−1, and true density: 3.89 g cm−3) were purchased from the NanoSany Corporation (Mashhad, Iran). Analytical grade chemicals were acquired from Merck (Darmstadt, Germany) and Sigma-Aldrich (Steinheim, Germany).
Plant material, culture condition, and treatment
In the present study, the experiments were considered under greenhouse conditions with 25/20 ± 2 °C maximum/minimum temperature, daily light integral of 25.5 ± 3.7 molm−2 d−1, and 75 ± 10% relative humidity. Seeds of M. officinalis (Mashhad Seedlings and Seeds Co. Iran) were cultivated into plastic pots (4–l volume pots, filled with a mixture of perlite, coco peat, and sand (5:7:23, w:w:w), 10 plants per pot). Germinated seedlings were irrigated daily with the standard Hoagland solution (EC 1.7 dSm_1, pH 6.0e6.5, 750 mL/pot/day) for 21 days. Thereafter, the plants with 4–5 fully expanded leaves (21-days-old) were exposed to non-saline (control) and different levels of salt (nutrient solution containing 0, 50, 100, and 150 mM NaCl, respectively, 200 mL/ pot/day) for 35 days until harvesting. Water (1000 mL/pot without NaCl) was applied once biweekly until harvesting to reduce the accumulation of salt in the mixture of perlite, coco peat, sand, and tape. Seven days after the salt treatment started, control and salt-treated plants were sprayed on upper surface leaves with 0, 50 and 100 mg L−1 Se-NPs-containing solution (50 mL per pot) once a week until harvesting (4 sprays in total). To prevent Se-NPs leaching to the soil during the spray treatment, soil surface was covered with aluminium foil which removed after spraying. Finally, plant samples (78-day-old plants) were harvested to evaluate their morphological, biochemical, and physiological responses.
Proline content
A slightly modified technique of Bates et al. (1973) was applied to analyze the proline content. Frozen leaf tissues (0.2 g) were homogenized in 3% sulfosalicylic acid solution (4 ml), and Wattman filter paper was used to filter the solution. Then, a solution of Ninhydrin, acetic acid and extract (with a proportion of 2 ml) were exposed to heat (100 °C) for one hour. The cooling process was carried out using an ice container to stop the reaction. The test tube to which added toluene solution (4 ml) was agitated quickly for 30 s. The supernatant absorbance was read at 520 nm after 20 min. Using the standard curve (range of 20–100 mg ml−1), the proline content was calculated (Bates et al. 1973).
Catalase, superoxide dismutase, and peroxidase activities
Pereira et al.’s (2002) method was used to evaluate the activity of catalase (CAT) enzyme. Potassium phosphate buffer at the pH of 7 (15 mM) and hydrogen peroxide (30 mM) was used. 10 μl enzyme extract was required in the final volume of 3 μl to start the assay. The absorbance was immediately recorded at 25 °C by 240 nm for 2 min and once every half minute with a spectrophotometer. The enzyme activity was analyzed in different treatments based on the breakdown of a mole of peroxide per gram of fresh weight, (Pereira et al. 2002). The enzyme extract, prepared according to Sairam method (Sairam et al. 2002), was used to determine the activity of SOD and CAT antioxidant enzymes.
Beauchamp and Fridovich’s (1971) method were utilized to determine SOD activity. The activity of the SOD enzyme in the wavelength of 560 nm was determined using optical reduction of Nitro Blue Tetrazolium (NBT). Firstly, 50 mM phosphate buffer solution (pH = 7.5) was prepared. Next, Riboflavin, EDTA, methionine, and NBT (4 mM, 0.1 M, 13 mM, and 75 mM, respectively) were added to prepare the reaction mixture. Potassium phosphate buffer was added to the container covered by aluminum foil to avoid light intensity. Forty μl of the enzyme extract was added to the final volume of 3 ml, and then tube was placed near a light bulb (40 W) (distance = 50 cm). The absorbance rate, recorded at 560 nm, declined to 50% in the presence of enzymes compared to tubes without any enzymes (Beauchamp and Fridovich 1971).
Membrane lipid peroxidation
Heath and picker’s (1969) method, based on the formation of a malondialdehyde (MDA) complex, was used to determine membrane lipid peroxidation. First, 0.5 g of fresh leaf tissue was rubbed with 5 mL of trichloroacetic acid (TCA) 0.1%. Then, the homogenized solution was centrifuged for 5 min at 15000 rpm. In addition, 1 ml of supernatant was mixed with 3 ml of TCA 20% containing thiobarbituric acid (TBT) of 0.5% and placed in a 90 °C water bath for 30 min. Then, it was placed into ice and centrifuged for 5 min at 15,000 rpm at 4 °C, and its absorbance was measured at 532 nm. The absorbance of compounds other than MDA was also recorded at 600 nm. The OD difference was calculated by subtracting absorbance at 600 nm and 532 nm (Heath and Packer 1968).
Chlorophyll and carotenoids assay
To measure chlorophyll and carotenoids, 0.5 g of leaf tissue was weighed and then rubbed out with 4 ml of 80% acetone. The supernatant was obtained with centrifuged at 4000 rpm for 5 min. Then, 14 ml of acetone 80% was added to a tube containing supernatant. To measure chlorophyll a, b and carotenoids, a spectrophotometer (T80 model) was calibrated and zeroed using acetone 80% in 664, 647 and 470 nm wavelengths. The absorbance of the solutions was read and the concentration of chlorophyll, and carotenoid was calculated using the following formulas (Lichtenthaler and Wellburn 1983).
Rosmarinic acid (RA) assay
First, 0.5 g of plant tissue was mixed with 20 ml of ethanol 50% and transferred to a 70 °C water bath. The solvent of ethanol was evaporated using rotary apparatus. The extract was dissolved in ethanol 70%. Then, the dissolved extract was transferred to minus 20 °C refrigerator for 24 h. Next, it was passed through the Wattman paper and stored in the refrigerator until the assay day. The absorbance of samples was gained using 327 nm wavelength (Tepe 2008).
RNA extraction and expression analysis
RNA was isolated from leaves using the RNXTM – Plus kit (Sinaclon, Iran) according to the manufacturer’s instructions. Ten μl of total RNA of leaves was mixed with 1 μl of OligodT as a primer (Table 1), 9 μl of RNase-free water, 1x RT buffer, 40 units of RNase, nucleoside triphosphates (NTPs), and 20 units ml−1 of reverse transcriptase (Invitrogen). The following instruction was used to perform the reaction: 37 °C for 1 min, 60 °C for 55 min and 5-min step at 95 °C.
Real-time SYBER Green PCR (Applied Biosystems step-one) was used to perform the semi-quantification comparison of PAL and RAS genes expression. The cDNA from each Se treated sample was used as a template for qRT-PCR reaction with PAL and RAS specified primers (Table 1). The actin gene was used for internal control. For this purpose, cDNA sample, primers for targeted and internal genes, nuclease-free water, Power SYBER Green PCR Master Mix (ABI), and 48-well plate were used. The 48-well plate was placed on ice and 2 μl of cDNA was added. Next, nuclease-free water and Syber Green Master mix (7 and 10 μl, respectively) were added. The plate was sealed by a nylon sheet to prevent from contamination and evaporation. The first step of 5 min at 95 °C was followed by 40 cycles of 30 s at 95 °C, 30 s at annealing temperature (58 °C, depending on the gene), and 45 s at 72 °C, with a final extension of 10 min at 72 °C. Positive and negative controls were treated like other samples. Distilled water was used as the sample for negative control.
Statistical analysis
Data were analyzed in a completely randomized design with three replications by SPSS (Version 16). ANOVA and Fisher’s Least Significant Difference (LSD) was used to compare different saline treatments after slicing. The P < 0.05 was used to determine significantly different means. Furthermore, the Excel software was used for drawing charts.
Results
Growth parameters
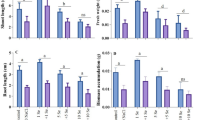
Figures 1 and 2 display the results of growth characterizations of shoot length, root length, shoot dry weight (SDW), and root dry weight (RDW). As shown, the SL, RL, RDW, and SDW of M. officinalis significantly reduced by salt stress. Among different saline levels, 150 mM NaCl showed a considerable reduction for both SL and RL of M. officinalis in comparison to non-saline control (Fig. 2a, b). In non-Se treated plants, severe salinity stress (150 mM NaCl) decreased shoot and root length by 38 and 33% as compared with non-saline conditions. However, the maximum shoot length (12.1 cm) and root length (16.6 cm) were obtained at 50 mg L−1 Se-NPs and non-saline conditions. A remarkable decrease occurred in SDW and RDW after severe salt stress, and the results were the same as those of SL and RL after using the Se-NPs (50 and 100 mg L−1) treatments. At non-saline conditions, 50 mg L−1 Se-NPs increased SDW and RDW by 16 and 30% compared with non-Se application (Fig. 1c, d).
Proline content and membrane lipid peroxidation
Both salinity and Se-NPs significantly changed the proline concentration in M. officinalis plants (P ≤ 0.05). The highest proline content was observed in plants exposed to150 mM NaCl and 100 mg L−1 Se-NPs to be 0.123 μmol g−1 FW. In addition, M. officinalis treated with 50 mg L−1 of Se-NPs can resist to proline content change under different saline conditions ranging from 0 to 150 mM (Fig. 3a). The MDA increased in the response of M. officinalis to salt stress levels. Salt stress at the concentration of 150 mM indicated a remarkable accumulation of MDA with or without Se treatment compared with non-saline conditions. In plants exposed to severe salinity stress, the application of 100 mg L−1 Se-NPs led to an enhancement of ~1.5-fold in MDA compared with non-Se-treated conditions (Fig. 3b).
SOD, catalase, and peroxidase activities
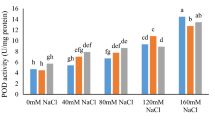
As shown in Fig. 4, all salt concentrations promoted a continuous increase in SOD, CAT, and POX in non-saline (control, 0 mM NaCl), 50 mM, 100 mM, and 150 mM NaCl. The SOD activity in non-Se-treated plants increased from 0.17 U mg−1 FW (control) to 0.64 U mg−1 FW (150 mM NaCl). Although 50 mg L−1 Se-NPs decreased SOD activity, their higher concentration (100 mg L−1) significantly increased the SOD activity, which led to the variation of SOD between 0.17 U mg−1 FW and 0.64 U mg−1 FW (Fig. 4a). The result was almost the same for CAT activity. In non-Se-treated plants, CAT activity increased over 3.5-fold (0.00028 U mg−1 FW) after adding 150 mM of salt compared with non-salt treated plants. These results for pots with 50 and 100 mg L−1 of Se-NPs were almost 2.5 (0.00022 U mg−1 FW) and 5-fold (0.00044 U mg−1 FW), respectively (Fig. 4b). Peroxidases play a major role in plant responses to stress conditions. The POX activity increased by progressing salt concentrations. The most significant increase was observed when M. officinalis plants were treated with 100 mg L−1 of Se-NPs and 150 mM NaCl as 17.04 U mg−1 FW (Fig. 4c).
Superoxide dismutase (SOD, a), catalase (CAT, b) and peroxidase (POX, c) activities of Melissa officinalis leaves under foliar application of selenium nanoparticles and salinity stress. The data are the mean of three independent replicates ± SD. Different letters indicate statistical significance at P < 0.05
Chlorophyll (Chl) and carotenoid content
The changes in pigment contents including Chl a and Chl b, as well as carotenoids were almost the same under salinity and Se-NPs application. The foliar spray of the Se-NPs significantly increased the pigment content in comparison to the none-Se-treated conditions. Chl a ranged from 0.6 mg g−1 FW at severe salinity and non-Se application to 2.1 mg g−1 FW at non-saline conditions and 100 mg L−1 Se-NPs (Fig. 5a). Chl b followed the same behavior (Fig. 5b). Although saline stress conditions decreased the content of carotenoids, Se nanoparticles compensated the deteriorate compacts of salinity. The carotenoid content varied from 0.23 mg g−1 FW at non-foliarly applied Se and severe salinity to 0.78 mg g−1 FW at 100 mg L−1 Se-NPs and non-saline treatment (Fig. 5c).
Chlorophyll (Chl) a (a), Chl b (b), carotenoids (c), and rosmarinic acid (RA, d) contents in Melissa officinalis under foliar application of selenium nanoparticles and salinity stress. The data are the mean of three independent replicates ± SD. Different letters indicate statistical significance at P < 0.05
Rosmarinic acid (RA) content
Figure 5d shows the RA changes under salinity and foliar application of Se-NPs. The slight salinity stress (50 mM NaCl) significantly improved RA content, but 100 and 150 mM NaCl decreased its concentration. The highest RA content (3.03 mg g−1 DW) was observed in plants exposed to 50 mM NaCl and 100 mg L−1 Se-NPs. However, when salt concentration increased to 100 and 150 mM, a considerable decrease occurred in the RA accumulation. Under non-foliar application, the 2.7-fold reduction of RA content was observed at 150 mM NaCl compared with 50 mM NaCl (Fig. 5D).
Expression of PAL and RAS
Using the quantitative RT-PCR, the transcript levels of PAL and RAS genes were investigated in relation to the housekeeping genes of β-actin. The housekeeping gene of β-actin was used as the internal standard since it is the most common gene for normalization and can lower the probable errors. The PAL (Fig. 6s) and RAS (Fig. 6b) showed the strongest increase slightly more than 10-fold and well over 7-fold, respectively, in 50 mM of salt and 100 mg L−1 of Se-NPs while the β-actin was not affected by saline stress. The expression of these genes significantly decreased in pots with 100 and 150 mg L−1 salt compared to non-salt treated pots (Fig. 6).
The expression of phenylalanine ammonia-lyase (PAL, A) and rosmarinic acid synthase (RAS, b) genes in Melissa officinalis plants under foliar application of selenium nanoparticles and salinity stress. The mean values of three replicates followed by different letters indicate the significance of the difference between the treatments (P < 0.05, ANOVA, LSD)
Discussion
Increased salt concentration in plants could be problematic. However, the decrease in growth is less seen in plants sprayed with Se-NPs, especially at concentrations of 50 mg L−1 of Se-NPs. Although the mechanism is still unknown, our findings demonstrated that the Se application in nano form could have a positive impact on SL, RL, SDW, and RDW. In other words, both concentrations of Se-NPs (50 and 100 mg L−1) improved physiological characteristics of M. officinalis at different levels of salt in comparison with the condition without applying Se-NPs exogenous. Maneuvering on physiological and biochemical mechanisms demonstrated the useful role of Se-NPs in alleviating the negative impacts of salt on growth and fruit of strawberry plants studied very recently which supports the findings of the present study (Zahedi et al. 2019). Garlic is another plant whose physiological characteristics improved under NaCl stress using Se (Astaneh et al. 2018). Seeds of Cucurbita pepo treated with salt showed reduced length, and fresh and dry weights of the roots and shoots. However, nano-SiO2 was shown to improve growth properties by reducing hydrogen peroxide levels, as well as electrolyte leakage (Siddiqui et al. 2014). The salt-induced inhibitory effects were alleviated in tomato plants after Se-NPs application (10 mgl−1) that resulting in growth and development improvement (Morales-Espinoza et al. 2019).
Based on our results, the proline accumulation was not influenced by low concentrations of salt, but not by its higher concentrations. Also, this influence increased with the application of Se-NPs. Accumulation of proline, as an osmo-protectant amino acid, exhibits a relatively salt-alleviating effect of Se-NPs. The finding is in line with Hawrylak-Nowak’s (2009) study showing that Se treatments at 5 and 10 μM on cucumber leaves significantly increased the proline contents and photosynthetic pigments subjected to 50 mM of salt stress.
The application of Se-NPs exhibited changes in the enzyme activity under saline conditions. The POX activity enhanced as the salt concentration increased in the present study. In saline condition, 50 mg L−1 of Se-NPs had a reverse effect on POX activity in comparison to non-saline condition while 100 mg L−1 of Se-NPs application showed a dramatic rise in POX activity, which led to an increase in POX just over 4-fold. The MDA and all enzymes including SOD and CAT followed the same behavior as POX. In addition, the results indicated that the pigment contents dropped significantly although the activity of these enzymes increased under saline condition. Kiarostami et al. (2010) reported that salt stress reduced Chlorophyll a and Chlorophyll b in rosemary, significantly. However, the Se-NPs application in the present study remarkably improved the negative consequences of salt. The results are in line with other reports. Bekhradi et al. (2015) indicated that the chlorophylls content decreased significantly while the lipid peroxidation increased in the Genovese genotype of basil with an increase in salt concentration. Like Se-NPs in the present study, the Nano-SiO2 reduced chlorophyll degradation in Cucurbita pepo leaves treated with salt. The increase in plant germination and growth properties through the application of nano-SiO2 may indicate oxidative damage reduction as a result of the expression of antioxidant enzymes such as CAT, POX, and SOD (Siddiqui et al. 2014). Evaluated under 50 mM of NaCl stress, the effects of Se-NPs on plant growth, and antioxidative metabolism in tomato, suggested that Se-NPs application increases the activity of SOD and POX enzymes. Results suggested that Se nanoparticles could mitigate the salt-induced oxidative stress by regulating the antioxidant defense mechanism in the tomato plant (Morales-Espinoza et al. 2019). Astaneh et al. (2018) showed that 8 mg L−1 of Se application in garlic leaves treated with 30 mM of NaCl and 4 mg L−1 under 60 mM of salt increased chlorophyll content, significantly. The findings of another study suggested that the photosynthesis and antioxidative capacity of maize under salt-induced stress were relieved by using Se (1 μM). In addition, Se application enhanced the net photosynthetic rate and relieved the damage to chloroplast induced by NaCl (Jiang et al. 2017). Ghazi (2018) reported that salinity produced adverse effects on different plant growth parameters of the Coriander plant, as well as the total chlorophyll content. Under different salinity levels of irrigation water, Se-NP at 50 ppm showed the best effects on physiological characteristics and total chlorophyll content of coriander plants. Carotenoids, with more than 750 members, are the second most abundant naturally-occurring pigments on earth (Kirti et al. 2014). Salt stress significantly decreased carotenes content in rosemary. Salinity affected leaf pigment contents of Rosmarinus officinalis at vegetative and generative growth stages (Kiarostami et al. 2010). Further, Se application in garlic leaves under saline condition significantly increased the carotenoid contents (Astaneh et al. 2018).
Regarding the RA in this study, 50 mM of salt stress did not change RA biosynthesis in M. officinalis while 50 and 100 mg L−1 of Se-NPs dramatically enhanced the amount of RA under 50 mM of salt, which suggests an optimum condition for RA biosynthesis. Transcript levels of PAL and RAS genes in M. officinalis under 100 and 150 mM NaCl noticeably decreased, similarly to RA biosynthesis representing the reduction of its biosynthesis at high saline levels. Döring et al. (2014) evaluated the formation of the main phenolic ingredient of the pharmaceutically important plant M. officinalis under O3 stress. There was a quick up-regulation of both RAS and PAL genes from the beginning of the exposure. Based on the result, a significant relation was observed between the specific activity of RAS and the decrease of RA concentration in M. officinalis leaves. Sadat Ejtehad et al. (2015) showed increased PAL gene expression and RA biosynthesis in Salvia officinalis and Salvia virgata under salicylic acid application.
The transcription-level determination of RA-involved genes encoding enzymes in the biosynthesis of RA using quantitative RT-PCR shows the order of processes happening during and after salt treatment of M. officinalis plants. In this study, Se-NPs was shown as an inducer for the expression of PAL and RAS genes with or without salt stress. In the none-saline condition, low levels of Se-NPs (50 mg L−1) induced the activity of genes and consequently the contents of RA. Low level of salt stress (50 mM) and high level of Se-NPs (100 mg L−1) showed the optimum condition for inducing RA associated genes in M. officinalis.
In general, Se-NPs could improve the negative effects of salt at 50 mg L−1 concentration regarding physiological features of RL, SL, SDW, and RDW. Further, the results demonstrated that 100 mg L−1 of Se-NPs could lead to the enhancement of proline, pigment content, and enzyme activity of POX, SOD, and CAT, especially in high levels of salt. Based on the findings, the optimal Se-NPs and salt supplementation for RA biosynthesis and expression of RAS and RAL genes were 50 mM of salt and 100 mg L−1 of Se-NPs. The interactive role of salt and Se-NPs could promote the salt-induced inhibitory mechanisms in M. officinalis.
Finally, the results of the current study indicated that Se-NPs foliar spray of M. officinalis is a valuable method for improving M. officinalis tolerance to salt. The beneficial effects of Se-NPs on M. officinalis growth performance under different salt levels could be attributed to the protection of photosynthetic pigments for enhancing photosynthetic capacity, accumulation of proline and oxidative enzyme activity for efficient reactive oxygen species (ROS) homeostasis, and enhancing RA and RAS and RAL levels for tackling the salinity problem.
Data availability
All data are subject to transparency.
Code availability
Not applicable.
References
Akcin A, Yalcin E (2016) Effect of salinity stress on chlorophyll, carotenoid content, and proline in Salicornia prostrata pall. And Suaeda prostrata pall. Subsp. prostrata (Amaranthaceae). Braz J Bot 39:101–106. https://doi.org/10.1007/s40415-015-0218-y
Ashraf MA, Akbar A, Parveen A, Rasheed R, Hussain I, Iqbal M (2018) Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol Biochem 123:268–280. https://doi.org/10.1016/j.plaphy.2017.12.023
Astaneh RK, Bolandnazar S, Nahandi FZ, Oustan S (2018) The effects of selenium on some physiological traits and K, Na concentration of garlic (Allium sativum L.) under NaCl stress. Inf Process Agric 5:156–161. https://doi.org/10.1016/j.inpa.2017.09.003
Babajani A, Iranbakhsh A, Ardebili ZO, Eslami B (2019) Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ Sci Pollut Res 26:24430–24444. https://doi.org/10.1007/s11356-019-05676-z
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bekhradi F, Delshad M, Marín A, Luna MC, Garrido Y, Kashi A, Babalar M, Gil MI (2015) Effects of salt stress on physiological and postharvest quality characteristics of different Iranian genotypes of basil. Hortic Environ Biotechnol 56:777–785. https://doi.org/10.1007/s13580-015-1095-9
Bui E (2013) Soil salinity: a neglected factor in plant ecology and biogeography. J Arid Environ 92:14–25. https://doi.org/10.1016/j.jaridenv.2012.12.014
Diao M, Ma L, Wang J, Cui J, Fu A, Liu H-Y (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul 33:671–682. https://doi.org/10.1007/s00344-014-9416-2
Döring AS, Pellegrini E, Della Batola M, Nali C, Lorenzini G, Petersen M (2014) How do background ozone concentrations affect the biosynthesis of rosmarinic acid in Melissa officinalis? J Plant Physiol 171:35–41. https://doi.org/10.1016/j.jplph.2013.11.005
Eudes Filho J, Silveira D, Soares AIC, Carneiro FP, de Assis MS, Leite FB, Ferreira (2017) Effects of lemon balm (Melissa officinalis) on behavioral deficits and memory impairment of rats surviving sepsis. J Med Plant Res 11:153–160. https://doi.org/10.5897/JMPR2016.6266
Ghazi D (2018) The contribution of Nano-selenium in alleviation of salinity adverse effects on coriander plants. J Soil Sci Agric Eng 9(12):753–760
Hamaguchi T, Ono K, Murase A, Yamada M (2009) Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-β aggregation pathway. Am J Pathol 175:2557–2565. https://doi.org/10.2353/ajpath.2009.090417
Hasanein P, Riahi H (2015) Antinociceptive and antihyperglycemic effects of Melissa officinalis essential oil in an experimental model of diabetes. Med Princ Pract 24:47–52. https://doi.org/10.1159/000368755
Hawrylak-Nowak B (2009) Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol Trace Elem Res 132:259–269. https://doi.org/10.1007/s12011-009-8402-1
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: II. Role of electron transfer. Arch Biochem Biophys 125:850–857. https://doi.org/10.1016/0003-9861(68)90523-7
Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Peng Q, Barao M, Opatrilova R, Zidkova J, Bjørklund G, Sochor J, Kizek R (2018) Se-NPslenium and its nanomedicine applications: a critical review. Int J Nanomedicine 13:2107. https://doi.org/10.2147/IJN.S157541
Jahanban-Esfahlan R, Seidi K, Monfaredan A, Shafie-Irannejad V, Abbasi MM, Karimian A, Yousefi B (2017) The herbal medicine Melissa officinalis extract effects on gene expression of p53, Bcl-2, Her2, VEGF-A and hTERT in human lung, breast and prostate cancer cell lines. Gene 613:14–19. https://doi.org/10.1016/j.gene.2017.02.034
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039. https://doi.org/10.1038/srep42039
Kiarostami K, Mohseni R, Saboora A (2010) Biochemical changes of emopenRosmarinus officinalisemclose under salt stress. J Stress Physiol Biochem 6(3):114–122
Kirti K, Amita S, Priti S, Mukesh Kumar A, Jyoti S (2014) Colorful world of microbes: carotenoids and their applications. Adv Biol 2014:1–13. https://doi.org/10.1155/2014/837891
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11(5):591–592. https://doi.org/10.1042/bst0110591
Mohamed AKS, Qayyum MF, Abdel-Hadi AM, Rehman RA, Ali S, Rizwan M (2017) Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch Agron Soil Sci 63:1736–1747. https://doi.org/10.1080/03650340.2017.1300256
Morales-Espinoza MC, Cadenas-Pliego G, Pérez-Alvarez M, Hernández-Fuentes AD, Cabrera dela Fuente M, Benavides-Mendoza A, Valdés-Reyna J, Juárez-Maldonado A (2019) Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed under NaCl Stress. Molecules (Basel, Switzerland) 24:3030. https://doi.org/10.3390/molecules24173030
Noguchi-Shinohara M, Ono K, Hamaguchi T, Iwasa K, Nagai T, Kobayashi S, Yamada M (2015) Pharmacokinetics, safety, and tolerability of Melissa officinalis extract which contained rosmarinic acid in healthy individuals. Alzheimers Dement 11:P902. https://doi.org/10.1371/journal.pone.0126422
Oliveira HC, Gomes BC, Pelegrino MT, Seabra AB (2016) Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide 61:10–19. https://doi.org/10.1016/j.niox.2016.09.010
Park WT, Arasu MV, Al-Dhabi NA, Yeo SK, Jeon J, Park JS, Lee SY, Park SU (2016) Yeast extract and silver nitrate induce the expression of phenylpropanoid biosynthetic genes and induce the accumulation of rosmarinic acid in Agastache rugosa cell culture. Molecules 21:426. https://doi.org/10.3390/molecules21040426
Pereira G, Molina SMG, Lea P, Azevedo RAD (2002) Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea. Plant Soil 239:123–132. https://doi.org/10.1023/a:1014951524286
Sadat Ejtahed R, Radjabian T, Tafreshi SAH (2015) Expression analysis of phenylalanine ammonia lyase gene and rosmarinic acid production in Salvia officinalis and Salvia virgata shoots under salicylic acid elicitation. Appl Biochem Biotechnol 176:1846–1858. https://doi.org/10.1007/s12010-015-1682-3
Sairam RK, Rao KV, Srivastava G (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046. https://doi.org/10.1016/S0168-9452(02)00278-9
Sajyan TK, Alturki SM, Sassine YN (2020) Nano-fertilizers and their impact on vegetables: contribution of Nano-chelate super plus ZFM and Lithovit®-standard to improve salt-tolerance of pepper. Annal Agr Sci. https://doi.org/10.1016/j.aoas.2020.11.001
Sharopov F, Setzer WN (2018) Medicinal Plants of Tajikistan. In: Egamberdieva D, Öztürk M (eds) Vegetation of Central Asia and Environs Springer, Cham. https://doi.org/10.1007/978-3-319-99728-5_7
Siddiqui MH, Al-Whaibi MH, Faisal M, Al Sahli AA (2014) Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ Toxicol Chem 33:2429–2437. https://doi.org/10.1002/etc.2697
Sytar O, Brestic M, Zivcak M, Olsovska K, Kovar M, Shao H, He X (2017) Applying hyperspectral imaging to explore natural plant diversity towards improving salt stress tolerance. Sci Total Environ 578:90–99. https://doi.org/10.1016/j.scitotenv.2016.08.014
Tepe B (2008) Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia virgata (Jacq), Salvia staminea (Montbret & Aucher ex Bentham) and Salvia verbenaca (L.) from Turkey. Bioresour Technol 99:1584–1588. https://doi.org/10.1016/j.biortech.2007.04.008
Zahedi SM, Abdelrahman M, Hosseini MS, Hoveizeh NF, Tran L-SP (2019) Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ Pollut 253:246–258. https://doi.org/10.1016/j.envpol.2019.04.078
Acknowledgments
The authors would like to thank the staff of the Islamic Azad University (IAU) laboratory in Neyshabur (Iran) for their effective cooperation.
Funding
This study was financially supported by Islamic Azad University, Damghan Branch (Iran).
Author information
Authors and Affiliations
Contributions
SGH performed the experiments, analyzed the data, and drafted the paper. NM and ASA designed and supervised the experiments, analyzed the data, and drafted the paper. FSN designed the experiments and analyzed the data. All the authors read the final manuscript and approved the submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghasemian, S., Masoudian, N., Saeid Nematpour, F. et al. Selenium nanoparticles stimulate growth, physiology, and gene expression to alleviate salt stress in Melissa officinalis. Biologia 76, 2879–2888 (2021). https://doi.org/10.1007/s11756-021-00854-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00854-2