Abstract
Objective
This study sought to confirm if thoracic endovascular aortic repair (TEVAR) was an appropriate therapeutic strategy for blunt thoracic aortic injury (BTAI).
Methods
Between 3/2005 and 12/2020, 104 patients with BTAI were brought to our hospital. The severity of each trauma case was evaluated using the Injury Severity Score (ISS); aortic injuries were classified as type I to IV according to Society for Vascular Surgery guidelines. Initial treatment was categorized into four groups: nonoperative management (NOM), open aortic repair (OAR), TEVAR, or emergency room thoracotomy/cardiopulmonary resuscitation (ERT/CPR).
Results
The patients’ mean age and ISS were 56.7 ± 20.9 years and 48.3 ± 20.4, respectively. Type III or IV aortic injury were diagnosed in 82 patients. The breakdown of initial treatments was as follows: NOM for 28 patients, OAR for four, TEVAR for 47, and ERT/CPR for 25. The overall early mortality rate was 32.7%. Logistic regression analysis confirmed ISS > 50 and shock on admission as risk factors for early mortality. The cumulative survival rate of all patients was 61.2% at 5 years after treatment. After initial treatment, eight patients receiving TEVAR required OAR. The cumulative rate of freedom from reintervention using TEVAR at 5 years was higher in approved devices than in custom-made devices (96.0 vs. 56.3%, p = 0.011).
Conclusions
Using TEVAR as an initial treatment for patients with BTAI is a reasonable approach. Patients with severe multiple traumas and shock on admission had poor early outcomes, and those treated with custom-made devices required significant rates of reintervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blunt thoracic aortic injury (BTAI) is the second most common cause of death in patients with blunt trauma [1, 2]. Thoracic endovascular aortic repair (TEVAR) has been reported as a first-line treatment of BTAI because of its favorable outcomes [3,4,5,6]. However, the overall mortality of patients damaged by BTAI is still extremely high. And the timing and choice of treatment, such as TEVAR, open aortic repair (OAR), and nonoperative management (NOM), still generate controversy in the management of BTAI [7, 8]. It has been agreed that anatomic suitability is important in deciding whether to use TEVAR [9], although age should not be a factor in deciding the type of repair [5]. The risk of death and spinal cord ischemia is significantly lower in all age groups after endovascular repair compared with open surgery [3, 4, 10], and these early benefits outweigh the concerns of potential late complications [5]. However, conventional open repair should be considered among patients with poor anatomic suitability for endovascular repair [5, 8].
In this study, we focus on the management of BTAI and evaluate both the short-term and long-term outcomes in patients with multiple organ injuries who underwent TEVAR.
Materials and methods
The Committee for the Protection of Human Subjects of the Japanese Red Cross Kobe Hospital and Hyogo Emergency Medical Center approved the collection and analysis of data. Informed consent was provided to all patients. This study included all patients diagnosed with BTAI who were transported to our institution between March 2005 and December 2020. TEVAR was introduced at our institution as a treatment for patients diagnosed with BTAI in March 2005. We performed a chart review on all patients. Clinical follow-up data were obtained via telephone interviews with the patient, a family member, or his/her primary physician.
One hundred and four patients diagnosed with BTAI were transported to our center. The initial evaluation was made by an emergency team that followed advanced trauma life support (ATLS) guidelines. Following hemodynamic stabilization, computed tomography (CT) angiography was performed according to the institutional protocol. The severity of the trauma was evaluated using the abbreviated injury scale (AIS) and Injury Severity Score (ISS) [11,12,13]. Scores on the AIS are determined by assigning a numerical score from 1 (minor) to 6 (maximum) for each injury to the head and neck; abdomen and pelvic contents; bony pelvis and limbs; face; chest; and body surface as follows: (1) minor; (2) moderate; (3) serious; (4)severe; (5) critical; and (6) maximum. The ISS is the sum of the squares of the highest AIS scoring injuries in the three highest-scoring body regions. In addition, aortic injury was evaluated using the Society for Vascular Surgery (SVS) classification as follows: type I, intimal tear; type II, intramural hematoma; type III, pseudoaneurysm; and type IV, rupture [5]. Shock at admission was defined as systolic blood pressure lower than 90 mmHg on hospital arrival.
Therapeutic strategies for BTAI
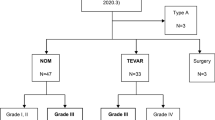
Therapeutic strategies for BTAI patients were based on our institutional flowchart (Fig. 1). Initial treatments were categorized as NOM, OAR, TEVAR, or emergency room thoracotomy/cardiopulmonary resuscitation (ERT/CPR). The patients’ characteristics are summarized in Table 1. In general, patients with type I or II aortic injury were treated with NOM. By contrast, patients with type III or IV aortic injury were treated with TEVAR, OAR, ERT/CPR or NOM, depending on their status on admission and anatomical factors. TEVAR was selected as the first-line treatment if the patient was responding to ATLS and there was no evidence of fatal organ damage, and of he/she was anatomically suitable. NOM was applied in 21 patients with type I or II aortic injury, and in seven patients with type III or IV. When NOM was selected, blood pressure was strictly controlled, and repeated CT scans were conducted.
OAR was selected in four cases, including one patient with type II and three with type III, after TEVAR was deemed anatomically unsuitable in three cases and one patient developed acute type-A aortic dissection (ATAAD). A cardiopulmonary bypass with full heparinization and left thoracotomy was used for graft replacement in all patients except the one with ATAAD, on whom a full sternotomy was conducted.
Forty-seven patients with type III or IV aortic injury underwent TEVAR as an initial treatment, and patient characteristics are summarized in Table 2. TEVAR was performed in the operating room under general anesthesia. Left subclavian artery revascularization was not performed before TEVAR in emergency cases. The dose of intraoperative heparin infusion was individualized depending on concomitant organ lesions. Femoral access was preferred for placing the device when possible. The size of the stent-graft was selected based on aortic diameter, the length of the lesion, and device availability at the time of TEVAR. Custom-made devices were applied in 14 patients, including two with a self-expanding Gianturco Z-stent (GZ; Cook Inc., Bjaeverskov, Denmark) covered with thin-wall Dacron graft (UBE woven graft, porosity 150 mL/min/cm2; Ube Inc., Yamaguchi, Japan) [14] and 12 with a curved nitinol stent-graft (MKSG; Matsui-Kitamura stent graft, Kanazawa, Japan) [15, 16], between March 2005 and June 2010. In addition, commercially available stent grafts (approved devices) were applied in 33 patients, including nine with GORE® TAG®, 23 with conformable GORE® TAG®, and one with GORE® EXCLUDER® cuff (W. L. Gore & Associates, Inc. Flagstaff, Arizona, USA) between July 2010 and December 2020.
All patients with cardiopulmonary arrest (CPA) who did not respond to ATLS underwent ERT/CPR and eventually died. A direct aortic cross-clamp was used in 10 patients, intra-aortic balloon occlusion was performed in three patients, and extracorporeal support was used in two patients.
Postoperative assessment
Postoperative intravenous contrast was performed to assess the aortic configuration and other organs—brain/chest/abdomen/pelvis CT scans—after 6 months, and annually thereafter. Early hospital mortality was defined by death within 30 days of being admitted to hospital, and long-term outcomes were evaluated according to the cumulative long-term survival rate. To evaluate the rate of reintervention, patients with initial TEVAR treatment were singled out and their freedom from reoperation rate calculated.
Statistical analyses
The analysis was retrospective. Data were collected from chart reviews by the authors (S. I., C. N., T. H.). Categorical variables are described as numbers and percentages, and continuous variables are described as mean ± standard deviation. Outcomes were compared among groups for the total study population, a chi-squared test was performed for proportions analysis, and a one-way analysis of variance with Bonferroni adjustment was performed for mean analysis, with p < 0.05 considered statistically significant. Survival analysis was performed according to the Kaplan–Meier method, and statistical differences were analyzed using the log-rank test. Multivariable logistic regression modeling yielding odds ratio (OR) and 95% confidence interval (CI) was used to determine variables that are independently associated with 30-day mortality. Shock on admission and ISS > 50 were selected as variables in multivariable logistic regression analysis [13, 17, 18]. The receiver operating characteristic (ROC) curve was used in this study for mortality. The ROC curve is derived by calculating the sensitivity and specificity of the test. Data analyses were performed with Bell Curve for Excel (version 3.20, Social Survey Research Information, Tokyo, Japan) and JMP Statistical Discovery software (version 13.0.0; SAS Institute Inc., Cary, NC).
Results
Patient characteristics and management data (Table 1)
Of the 104 patients, the mean age was 56.7 ± 20.9 years, and the prevalence of shock on admission, including CPA, was 58%. The causes of BTAI included traffic accidents in 72% of cases and falls in 19%. The aortic injury site was observed in the isthmus in 93% of cases, and type III aortic injury was identified in 55% of cases, making it the most common type of injury. The mean ISS was 48.6 ± 20.6, and 49% of all patients had extra-thoracic injury with an AIS score > 4. The study population was divided into four groups according to treatment intentions on admission. The number of shocks on admission, the type of injury and mean ISS were significantly different among the four groups.
Therapeutic management of all patients was summarized in Fig. 1. Regarding 22 patients with type I or II aortic injury, emergency OAR was performed on one patient due to progression to ATAAD, while the remaining 21 patients received NOM at the onset. After initial treatment, 16 patients recovered, three underwent elective OAR, one underwent TEVAR, and one died of brain infarction. In 82 patients with type III or IV aortic injury, 25 patients did not recover after ATLS, and subsequent ERT/CPR was performed. Of the remaining 57 patients, seven did not receive immediate aortic repair, 47 underwent TEVAR, and three were given immediate OAR. However, eight patients who underwent immediate TEVAR required aortic reintervention and conversion to OAR in later periods (Table 2). Therefore, in BTAI patients with type III or IV aortic injury, 11 patients underwent OAR and all patients survived without any neurological morbidities.
Short-term outcomes
Thirty-four patients (33%) died within 30 days of admission, including 33 with type III or IV aortic injury and one with type II. The 30 day mortality of each initial treatment was 100% for ERT/CPR, 13% for TEVAR, 0% for OAR, and 11% for NOM (Table 1). All patients who received ERT/CPR died of hemorrhagic shock. Six TEVAR patients died from the following causes: hemorrhagic shock in one patient; uncontrolled coagulopathy in two; brain damage in three, and sepsis in one. There was no mortality in three patients who underwent elective TEVAR after initial NOM. Regarding the 18 patients treated with OAR; four who received immediate OAR and 14 who underwent elective OAR as a secondary treatment all survived. The best cutoff point for predicting mortality using ISS was a score of 50 (sensitivity 94.1%, specificity of 80.0%, ROC area 0.914). Logistic regression analysis confirmed ISS > 50 and shock on arrival as risk factors for 30 day mortality (Table 3). The mean follow-up period was 37.0 ± 45.8 months (range 0–170 months). Overall survival estimated by the Kaplan–Meier method was 62.6% at 1 year and 61.2% at 5 years (Fig. 2).
TEVAR outcomes
Of the 47 patients, the mean age was 58.0 ± 19.7 years. After initial TEVAR, eight patients (17%) required OAR, with six in the custom-made device group and two in the approved device group (two with GORE® TAG®), due to type Ia endoleak in six patients in the custom-made device group, and device infolding in one. These seven patients underwent the removal of the stent and graft replacement in a median of 23 (ranging from 3 to 136) days. In addition, one patient who developed deformation of the aorta 5 years after undergoing TEVAR using an approved device (GORE® TAG®) underwent open repair. There was no reintervention (undergoing OAR) in 23 patients with conformable GORE® TAG®. There was a significant difference in the prevalence of reintervention between the two groups (Table 2). The cumulative freedom from reintervention was better in approved devices than in custom-made devices over time (96.0 vs. 56.3%, p = 0.01) (Fig. 3).
Discussion
Historical estimates have reported a 75–85% prehospital mortality attributable to BTAI patients, and 15–23% in those who were alive on arrival as a hospital but died from associated injuries during the initial 24 h [19,20,21]. Sandu et al. [22] reported 21.6% of their cohort died before confirmation in 273 of BTAI cases. That was similar to the findings of this study, in which the ERT/CPR group comprised 22.1% of BTAI patients. While the early mortality rate among our study cohort seems higher than that in other reports [23, 24], one possible explanation is that the mean value of ISS was 48.6 in this study. Bolorunduro et al. [17] reported that the mortality rate in patients with ISS > 25 was 55.8 times higher than those with ISS < 9. In addition, older patients and those with decompensated shock or CPA on arrival were included in this study, resulting in the possibility that our patient cohort may have included more critical patients.
In this study, ISS > 50 and a state of shock on admission were independent risk factors for early mortality. Starnes et al. [18] reported that 75% of patients who died of BTAI were hypotensive before or at hospital presentation, and hypotension was a strong predictor of early death. In addition, Konstantinidis et al. [25] reported that geriatric patients demonstrated a four-fold increase in mortality following vascular injuries. However, age was not a statistically significant risk factor for early mortality in this study.
The SVS guidelines for BTAI [5] recommended that patients diagnosed with type II, III and IV aortic injury should be repaired immediately. However, the management of type II aortic injuries remains controversial [7, 22, 26]. In this study, we selected a conservative management approach for type II aortic injuries as an initial treatment, except in the case of one patient who developed acute type A aortic dissection and underwent OAR. In addition, 20% of the patients underwent OAR electively. No aorta-related mortality occurred in patients with type I or II aortic injury; therefore, NOM could be recommended as an initial treatment for these patients with BTAI.
For patients with type III or type IV aortic injuries, TEVAR was selected as the first-line treatment [5, 6, 8, 24]. The use of custom-made devices resulted in a higher incidence of graft failure [27]. On the other hand, the use of commercially available stent grafts improved both early and long-term outcomes [6, 8, 24]. In this study, there was no reintervention required in the patients with conformable GORE® TAG® at the present time. The recent availability of devices with a more conformable and smaller diameter, of up to 21 mm, meant the number of cases requiring reintervention has reduced [28]. However, as patients in this cohort are younger than those who underwent TEVAR for atherosclerotic thoracic aneurysms, morphologic changes in the thoracic aorta may remain a concern during long-term follow-up examinations [8, 27, 28]. In addition, given the highly successful surgical results from OAR in this study, OAR must remain one of the therapeutic options for BTAI 5, 6, 8].
Our study had two limitations. First, it was a retrospective, single-center study. A multicenter study is needed in the future because of differences in the accuracy of diagnosis and therapeutic management between facilities. Second, the failure to follow up is a common limitation in retrospective cohort studies involving the trauma population. For this study, we were unable to follow up with CT imaging for 16 patients (15%).
Conclusions
It is reasonable to consider TEVAR as an initial treatment of patients with BTAI and multiple injuries. Patients with severe multiple traumas and those with shock on arrival had poor early outcomes, and those treated with custom-made devices required significant rates of reintervention.
References
Clancy TV, Gary Maxwell J, Covington DL, Brinker CC, Blackman D. A statewide analysis of level I and II trauma centers for patients with major injuries. J Trauma. 2001;51:346–51.
Richens D, Field M, Neale M, Oakley C. The mechanism of injury in blunt traumatic rupture of the aorta. Eur J Cardio Thorac Surg. 2002;21:288–93.
Tang GL, Tehrani HY, Usman A, Katariya K, Otero C, Perez E, et al. Reduced mortality, paraplegia, and stroke with stent graft repair of blunt aortic transections: a modern meta-analysis. J Vasc Surg. 2008;47:671–5.
Xenos ES, Abedi NN, Davenport DL, Minion DJ, Hamdallah O, et al. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48:1343–51.
Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187–92.
Akhmerov A, DuBose J, Azizzadeh A. Blunt thoracic aortic injury: current therapies, outcomes, and challenges. Ann Vasc Dis. 2019;12:1–5.
Jacob-Brassard J, Salata K, Kayssi A, Hussain MA, Forbes TL, Al-Omran M, et al. A systematic review of nonoperative management in blunt thoracic aortic injury. J Vasc Surg. 2019;70:1675–81.
McCurdy CM, Faiza Z, Namburi N, Hartman TJ, Corvera JS, Jenkins P, et al. Eleven-year experience treating blunt thoracic aortic injury at a tertiary referral center. Ann Thorac Surg. 2020;110:524–30.
Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JAMC Cardiovasc Imaging. 2008;1:739–48.
Neschis DG, Scalea TM, Flinn WR, Griffith BP. Blunt aortic injury. N Engl J Med. 2008;359:1708–16.
Palmer CS, Gabbe BJ, Cameron PA. Defining major trauma using the 2008 abbreviated injury scale. Injury. 2016;47:109–15.
Loftis KL, Price J, Gillich PJ. Evolution of the abbreviated injury scale: 1990–2015. Traffic Inj Prev. 2018;19:S109–13.
Baker SP, O’Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96.
Tsuji Y, Tanaka Y, Kitagawa A, Hino Y, Taniguchi T, Sugimoto K, et al. Endovascular stent-graft repair for penetrating atherosclerotic ulcer in the infrarenal abdominal aorta. J Vasc Surg. 2003;38:383–8.
Sanada J, Matsui O, Terayama N, Kobayashi S, Minami T, Kurozumi M, et al. Clinical application of a curved nitinol stent-graft for thoracic aortic aneurysms. J Endovasc Ther. 2003;10:20–8.
Yamaguchi M, Sugimoto K, Tsukube T, Mori T, Kawahira T, Hayashi T, et al. Curved nitinol stent-graft placement for treating blunt thoracic aortic injury: an early experience. Ann Thorac Surg. 2008;86:780–6.
Bolorunduro OB, Villegas C, Oyetunji TA, Haut ER, Stevens KA, Chang DC, et al. Validating the injury severity score (ISS) in different populations: ISS predicts mortality better among Hispanics and females. J Surg Res. 2011;166:40–4.
Starnes BW, Lundgren RS, Gunn M, Quade S, Hatsukami TS, Tran NT, et al. A new classification scheme for treating blunt aortic injury. J Vasc Surg. 2012;55:47–54.
Parmley LF, Mattingly TW, Manion WC, Jahnke EJ. Nonpenetrating traumatic injury of the aorta. Circulation. 1958;17:1086–101.
Fabian TC, Richardson JD, Croce MA, Smith JS Jr, Rodman G Jr, Kearney PA, et al. Prospective study of blunt aortic injury: multicenter trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42:374–80.
Arthurs ZM, Starnes BW, Sohn VY, Singh N, Martin MJ, Andersen CA, et al. Functional and survival outcomes in traumatic blunt thoracic aortic injury: an analysis of the National Trauma Databank. J Vasc Surg. 2009;49:988–94.
Sandhu HK, Leonard SD, Perlick A, Saqib NU, Miller CC 3rd, Charlton-Ouw KM, et al. Determinants and outcomes of nonoperative management for blunt traumatic aortic injuries. J Vasc Surg. 2018;67:389–98.
Mosquera VX, Marini M, Lopez-Perez JM, Muniz-Garcia J, Herrera JM, Cao I, et al. Role of conservative management in traumatic aortic injury: comparison of long-term results of conservative, surgical, and endovascular treatment. J Thorac Cardiovasc Surg. 2011;142:614–21.
Steuer J, Björck M, Sonesson B, Resch T, Dias N, Hultgren R, et al. Durability of endovascular repair in blunt traumatic thoracic aortic injury: long-term outcome from four tertiary referral centers. Eur J Vasc Endovasc Surg. 2015;50:460–5.
Konstantinidis A, Inaba K, Dubose J, Barmparas G, Lam L, Plurad D, et al. Vascular trauma in geriatric patients: a national trauma databank review. J Trauma. 2011;71:909–16.
Spencer SM, Safcsak K, Smith CP, Cheatham ML, Bhullar IS. Nonoperative management rather than endovascular repair may be safe for grade II blunt traumatic aortic injuries: an 11 year retrospective analysis. J Trauma Acute Care Surg. 2018;84:133–8.
Tinelli G, Minelli F, Sica S, De Nigris F, Massetti M, Tshomba Y. TEVAR for traumatic thoracic injury with the first-generation stent graft. J Vasc Surg Cases Innov Tech. 2020;7:16–20.
García Reyes ME, Goncalves Martins G, Fernandez Valenzuela V, Domínguez González JM, Maeso Lebrun J, Bellmunt MS. Long-term outcomes of thoracic endovascular aortic repair focused on bird beak and oversizing in blunt traumatic thoracic aortic injury. Ann Vasc Surg. 2018;50:140–7.
Acknowledgements
We gratefully acknowledge the support of the Department of Emergency and Critical Care Medicine at the Japanese Red Cross Kobe Hospital and the Hyogo Emergency Medical Center. We thank Dr. Takahisa Mikami for his statistical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This study was approved by the ethics committee of our hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izumi, S., Nakai, C., Haraguchi, T. et al. Retrospective study of thoracic endovascular aortic repair as a first-line treatment for traumatic blunt thoracic aortic injury. Gen Thorac Cardiovasc Surg 70, 16–23 (2022). https://doi.org/10.1007/s11748-021-01661-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-021-01661-x