Abstract
An 84-year-old man who underwent percutaneous coronary intervention for acute inferior myocardial infarction due to occlusion of the mid portion of the right coronary artery was transferred to our hospital because of post-infarction posterior ventricular septal rupture. We performed the extended sandwich technique via the right atrial approach as well as tricuspid and mitral valve replacement and permanent pacemaker implantation. Mild residual shunt was detected post-operatively, but the patient’s condition was controlled well with diuretics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventricular septal rupture (VSR) is a life-threatening complication of transmural acute myocardial infarction (MI). Surgical treatment remains the treatment of choice, but it is still a challenging operation associated with high mortality, especially the surgical repair of posterior VSRs secondary to inferior MI [1, 2]. We herein report a case of an elderly patient who underwent extended sandwich technique via the right atrial approach for post-infarction posterior VSR.
Case
An 84-year-old man with complaint of sudden-onset chest pain was referred to a nearby hospital. The patient was found to have acute inferior MI due to occlusion of the mid portion of the right coronary artery and subsequently underwent percutaneous coronary intervention (PCI). On the following day, a transthoracic echocardiogram revealed a VSR with left-to-right shunt flow; an intra-aortic balloon pump (IABP) was inserted, and he was transferred to our hospital.
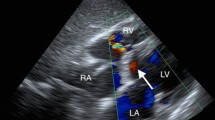
On admission, a pansystolic murmur was auscultated from the left sternal border. Biochemical tests revealed a serum creatinine level of 1.3 mg/dL and a creatine kinase level of 229 U/L. Chest X-ray showed a 54% cardiothoracic ratio with remarkable lung congestion, and electrocardiography showed normal sinus rhythm and ST-segment elevations in leads II, III, and aVF. Transthoracic echocardiography demonstrated a VSR in the middle level of the posterior ventricular septum (Fig. 1a and b), a D-shaped left ventricle (LV), right ventricular (RV) systolic dysfunction, and mild mitral regurgitation (MR). Post-infarction posterior VSR was diagnosed, and emergency surgery was performed.
Cardiopulmonary bypass was established with cannulation to the ascending aorta, superior vena cava, and inferior vena cava. An aortic cross-clamp was placed, and antegrade blood cardioplegic arrest was induced. We made a right atrial longitudinal incision and identified a VSR in the middle level of the posterior wall underneath the commissure of the septal and posterior leaflets of the tricuspid valve (TV) (Fig. 2). Exposure was secured after we excised the septal and posterior leaflets and the chordae of the TV and RV trabeculation hiding the defect. The necrotic myocardium around the VSR was resected, revealing a defect measuring 2 cm in diameter. The extended sandwich technique [3,4,5,6] was employed using a bovine pericardial patch placed on the LV side of the septum, combined with a Dacron patch placed on the RV side of the septum and Teflon felt pledgets placed on the LV and RV free wall (Fig. 3a and b). We blindly placed several LV side stitches because we could not view the defect from the front via the right atrial approach. BioGlue (CryoLife, Inc., Kennesaw, GA, USA) was applied to the defect between the two patches before final knotting. We replaced the TV with a 27-mm bioprosthesis. After the aortic cross-clamping was released, transesophageal echocardiography demonstrated massive MR. Hence, cardioplegic arrest was induced again, and the mitral valve (MV) procedure was performed via a transatrial approach through the interatrial septum. Although we could not observe the mitral subvalvular apparatus well because of the small left atrium and the status after TV replacement, the anterior mitral leaflet was almost tethered to the ipsilateral annulus. We performed MV replacement using a 25-mm bioprosthesis preserving the posterior mitral leaflet. We also performed permanent pacemaker implantation using an epicardial pacing lead because of the possibility of atrioventricular block after surgery.
Schema of the operation via the right atrial approach. Septal and posterior leaflets and the chordae of the tricuspid valve were excised to gain good exposure of the defect (a). The extended sandwich technique was employed using a bovine pericardial patch placed on the left ventricle (LV) side of the septum (a), combined with a Dacron patch placed on the right ventricle (RV) side of the septum (b) and Teflon felt pledgets placed on the LV and RV free wall (a, b). VSR ventricular septal rupture, SL septal leaflet, PL posterior leaflet, RA right atrium
The patient was weaned from the IABP on post-operative day 4 (POD 4) and was extubated on POD 5. He was dependent on ventricular pacing due to atrioventricular block. Post-operative transthoracic echocardiography revealed mild residual shunt flow, and the patient needed diuretics to prevent heart failure. However, he was stable and transferred to a nearby hospital for cardiac rehabilitation on POD 20.
Discussion
There has been a reduction in the incidence of post-infarction VSR because of aggressive primary PCI [1], but a few studies have also shown that the number of VSR operations has remained constant during the past 2 decades [7, 8]. Anterior MI is more likely to cause apical or anterior septal defects, and inferior or lateral MI is more likely to cause basal or posterior septal defects at the junction of the septum and the posterior wall [1]. Although posterior VSRs occur less frequently than anterior VSRs, they are commonly associated with RV dysfunction [9]; they are also associated with technical difficulties in exposure and suturing because of their basal anatomic location and proximity to the mitral subvalvular apparatus. Thus, surgical repair of posterior VSRs is very challenging [1, 2].
Post-infarction VSRs are almost always hidden by RV trabeculations coupled with the chordae and papillary muscles of the TV. Thus, VSRs can be difficult to expose and repair via the right atrial approach, and many surgeons prefer left ventriculotomy through the infarction to access the VSR, as it allows the most direct view of the defects, and infarctectomy or aneurysmectomy can be more easily performed if needed. Furthermore, the placement of the patch on the right side of the septum via the right atrial approach is not considered optimal because of the left-to-right pressure gradient, which may lead to residual shunt [10, 11].
Posterior VSRs are usually near the atrioventricular grove, close to the septal portion of the TV [12]. In the present case, the defect was located at the mid-muscular portion rather than at the basal portion. However, we detected the defect easily through the right atrial approach and the exposure was enhanced by tricuspid valvectomy [12, 13]. These are in contrast to the posterior transventricular approach, which requires the heart to be elevated for adequate exposure and may be superior in terms of preservation of ventricular function and avoidance of hemorrhagic complications associated with long ventriculotomy [10,11,12,13]. The transatrial approach may also be useful in redo surgery for VSR because it avoids the problems associated with dissection and difficulty with access to the ventricular chamber, often complicated by the presence of patent grafts and deterioration of cardiac function due to further ventriculotomy in an already compromised ventricle [14].
In this case, we repaired the VSR using the sandwich technique, in which two patches pinch the ventricular septum, which can ensure secure sutures with less tension [3,4,5,6]. Furthermore, BioGlue (CryoLife, Inc.) is expected to not only prevent leaks at the VSR and suture holes but to also strengthen the edge of the VSR by cross-linking the fragile necrotic myocardium [3,4,5,6]. In the present case, we performed extended repair because of widespread necrosis at the apical rim of the septal defect [4,5,6]. Although mild residual shunt was detected post-operatively, the patient’s condition was controlled well with diuretics. The reported incidence of major residual shunt using the sandwich technique varies from 0 to 17% [5, 6].
As TV replacement was needed, the transatrial approach trades possible TV replacement for ease of VSR repair even if the VSRs are near the atrioventricular grove [11,12,13]. Besides, we also had no choice but to replace the MV because the MR worsened after the VSR repair. Judging from intra-operative findings, the reason MR worsened after the VSR repair was likely due to having trapped the mitral subvalvular apparatus, resulting in its distortion when we placed the LV side stitches, rather than due to the progression of papillary muscle dysfunction. As we mentioned above, we blindly placed several LV side stitches because we could not view the defect from the front via the right atrial approach. Of course, this could be related to our limited surgical experience with VSR repair via the right atrial approach. In fact, the advocates of transatrial approach did not report such a complication [11,12,13]. However, with wider bites to ensure solid anchoring of the patch, this may happen more due to their proximity to the mitral subvalvular apparatus. Thus, if the posterior VSRs are located somewhat away from the basal portion or close to the apical septum, where the exposure is constrained and the suture line is difficult to place, it may be appropriate to select the LV or RV incision approach. Asai et al. claimed that the RV damage from the RV incision approach can be minimized when the incision is made close to the left descending artery or the posterior descending artery because of the direction of the coronary blood supply to the RV [4, 5].
Currently, no specific surgical method has been proven to improve the result of post-infarction VSR. Furthermore, experience in VSR repair via the right atrial approach is limited to a small series [10,11,12,13,14]. However, we believe that the sandwich technique via the right atrial approach could be an alternative for post-infarction posterior VSR, if the anatomy of the defect is favorable.
Recently, transcatheter repair of the VSR has been reported to be a definitive strategy or bridge therapy in clinically debilitated patients. However, outcomes are influenced by the timing of treatment, similar to surgical treatment. Moreover, posterior or basal defects are especially challenging as they frequently lack an adequate tissue rim to secure the device, and the location of the adjoining TV apparatus (especially the septal leaflet) makes the closure of basal defects more difficult [1]. The use of LV mechanical support devices has the potential to impart hemodynamic stability and to delay surgical treatment until scar tissue forms around the defect, sufficient to hold a suture patch [15].
Conclusion
We have reported a case of an elderly patient who underwent the extended sandwich technique via the right atrial approach for post-infarction posterior VSR. This method allows secure VSR repair without requiring the elevation of the heart or ventriculotomy, despite the possible need for TV replacement.
References
Jones BM, Kapadia SR, Smedira NG, Robich M, Tuzcu EM, Menon V, et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J. 2014;35:2060–8.
Cinq-Mars A, Voisine P, Dagenais F, Charbonneau É, Jacques F, Kalavrouziotis D, et al. Risk factors of mortality after surgical correction of ventricular septal defect following myocardial infarction: retrospective analysis and review of the literature. Int J Cardiol. 2016;206:27–36.
Isoda S, Osako M, Kimura T, Mashiko Y, Yamanaka N, Nakamura S, et al. Midterm results of the “sandwich technique” via a right ventricle incision to repair post-infarction ventricular septal defect. Ann Thorac Cardiovasc Surg. 2012;18:318–21.
Asai T, Hosoba S, Suzuki T, Kinoshita T. Postinfarction ventricular septal defect: right ventricular approach-the extended “sandwich” patch. Semin Thorac Cardiovasc Surg. 2012;24:59–62.
Hosoba S, Asai T, Suzuki T, Nota H, Kuroyanagi S, Kinoshita T, et al. Mid-term results for the use of the extended sandwich patch technique through right ventriculotomy for postinfarction ventricular septal defects. Eur J Cardiothorac Surg. 2013;43:e116–20.
Isoda S, Imoto K, Uchida K, Karube N, Kasama K, Yamazaki I, et al. Pitfalls for the “sandwich technique” via a right ventricular incision to repair post-infarction ventricular septal defects. Gen Thorac Cardiovasc Surg. 2017;65:187–93.
Moreyra AE, Huang MS, Wilson AC, Deng Y, Cosgrove NM, Kostis JB, MIDAS Study Group (MIDAS 13). Trends in incidence and mortality rates of ventricular septal rupture during acute myocardial infarction. Am J Cardiol. 2010;106:1095–100.
Arnaoutakis GJ, Zhao Y, George TJ, Sciortino CM, McCarthy PM, Conte JV. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2012;94(2):436–44.
Moore CA, Nygaard TW, Kaiser DL, Cooper AA, Gibson RS. Postinfarction ventricular septal rupture: the importance of location of infarction and right ventricular function in determining survival. Circulation. 1986;74:45–55.
Calderon M, Ott DA. Surgical treatment of postinfarction rupture of the interventricular septum. Tex Heart Inst J. 1991;18:282–5.
Massetti M, Babatasi G, Le Page O, Bhoyroo S, Saloux E, Khayat A. Postinfarction ventricular septal rupture: early repair through the right atrial approach. J Thorac Cardiovasc Surg. 2000;119:784–9.
Sharma V, Greason KL, Nkomo VT, Schaff HV, Burkhart HM, Park SJ, et al. Repair of acute inferior wall myocardial infarction-related basal ventricular septal defect: transatrial versus transventricular approach. J Card Surg. 2013;28:475–80.
Ashikhmina EA, Schaff HV. Tricuspid valvectomy to facilitate repair of postinfarction ventricular septal defect. J Card Surg. 2008;23:543–5.
Furukawa K, Iwasa S, Hayase T, Fukushima Y, Onitsuka T. Transtricuspid approach in redo surgery for post-infarction ventricular septal rupture. Gen Thorac Cardiovasc Surg. 2012;60:391–3.
La Torre MW, Centofanti P, Attisani M, Patanè F, Rinaldi M. Posterior ventricular septal defect in presence of cardiogenic shock: early implantation of the impella recover LP 5.0 as a bridge to surgery. Tex Heart Inst J. 2011;38:42–9.
Acknowledgements
We thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Furukawa, K., Shirasaki, Y., Ishii, H. et al. Extended sandwich technique via the right atrial approach for post-infarction posterior ventricular septal rupture. Gen Thorac Cardiovasc Surg 68, 629–632 (2020). https://doi.org/10.1007/s11748-019-01140-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-019-01140-4