Abstract
Background
We have reported “sandwich technique,” via a right ventricular incision, to treat a post-infarction ventricular septal defect (VSD). This technique involves the placement of patches on both the left and right sides of the septum, pinching the VSD sealed with surgical adhesive between the two patches. In this study, we analyzed factors influencing 1-year mortality to determine the pitfalls in our procedure.
Methods
We evaluated 24 consecutive patients with post-infarction VSD who underwent the “sandwich technique” via a right ventricular incision. One-year survival and major residual leak were used as the criteria for the analysis of survival and technical success, respectively. In protocol 1, clinical variables were evaluated as predictors of one-year mortality. In protocol 2, surgical techniques were evaluated as predictors of major residual leak, which was found to be related to one-year mortality in protocol 1.
Results
In protocol 1, the one-year mortality was higher in patients with major residual leak (75 %, 3/4) than in those without (15 %, 3/20) (p = 0.035). In protocol 2, the patients with major residual leak had smaller patches than those without (41.9 ± 3.8 vs. 47.8 ± 4.8 mm, p = 0.031) and a smaller size difference between the patches and the VSD (22.5 ± 6.5 vs. 30.0 ± 5.7 mm, p = 0.028).
Conclusion
For the “sandwich technique” via a right ventricular approach to treat post-infarction VSD, the choice of patch size according to VSD size is an important variable for reducing major residual leak.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-infarction ventricular septal defect (VSD) is a life-threatening complication of transmural acute myocardial infarction, with a poor survival rate despite medical therapy [1, 2]. The 30-day mortality of post-infarction VSD was reported to be 10.5 % in an Annual Report by the Japanese Association for Thoracic Surgery in 2013 [3]. Problems with the previous surgical techniques include residual leak, uncontrolled bleeding, and technical difficulty [4–7]. To resolve these problems, we have developed a sandwich technique via a right ventricular (RV) approach [8–10]. This technique involves the placement of patches on both the left and right sides of the septum, pinching the VSD sealed with surgical adhesive between the two patches via a right ventricle (RV) approach (Fig. 1). Although there was no mortality after 30 days in our study, we believe that our technique may still have some pitfalls, with room to improve the surgical result, since there were several cases with major residual leak and several patients who did not survive for one year [10]. To investigate the possibility of improving treatment quality, we analyzed the factors influencing mortality.
Schematic drawing of the left (LV) and right (RV) ventricular patches with originally planned size (a). Ten millimeters of surgical bite from the trimmed edge of the ventricular septal defect (VSD) and an additional 10 mm from the suture line to the outer edge of the patch result in a patch size 40 mm larger than the size of the trimmed VSD (b)
Materials and methods
Between June 2001 and March 2013, 24 surgical cases of post-infarction VSD were treated with the sandwich technique via an RV approach, as first surgical treatment in all patients, at both the Yokohama City University Medical Center (20 cases) and the National Defense Medical College (4 cases). One other patient underwent the procedure as a reoperation to close a major residual leak after surgery with the infarct exclusion technique; the case was not included in this study. Approval for the use of these data was obtained from the institutional review board of Yokohama City University Medical Center on October 23, 2014.
Data are expressed as mean ± standard deviation. Statistical analysis was performed by SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA) using the Kaplan–Meier method, Chi-squared test, and Student’s t test. The follow-up rate for one year postprocedure was 100 %. Since there was no mortality after 30 days, 1-year mortality was used as survival criterion.
In protocol 1, correlations between one-year mortality and non-parametric perioperative clinical data were analyzed using the Chi-squared test. The perioperative parameters included major residual leak, redo, location of VSD (anterior or posterior), ventricle rupture, valve failure, and use of postoperative percutaneous cardiopulmonary support (PCPS).
In protocol 2, given the finding in protocol 1 that major residual leak was correlated with one-year mortality, the association between major residual leak and surgical variables was analyzed. The surgical non-parametric data included patch materials, blood contacting surface, size of sutures, number of sutures, use of pledgets, use of surgical adhesive, and use of extended sandwich repair technique [7, 11, 12], were analyzed using the Chi-squared test. The non-paired Student’s t test was used to analyze correlations between parameters related to patch size and major residual leak. The former parameters included patch size, VSD size after surgical trimming, and the difference between patch size and VSD size. Sizes of both VSDs and patches were expressed as the mean values of the longitudinal and transverse axes. Major residual leak was defined to include: (1) significant leak recognized during surgery that necessitated a second pump run; (2) significant leak recognized postoperatively that necessitated reoperation; and (3) significant leak recognized by postoperative echocardiography with Qp/Qs >1.5.
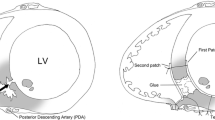
The surgical technique has been reported elsewhere [10] and is described briefly here. Under cardiopulmonary bypass, epicardial direct ultrasonography enabled the surgeon to visualize the lesion, to make an appropriate incision (3–7 cm in length) in the RV, and to perform a trabecular resection. Through the RV incision, the necrotic myocardium around the VSD was trimmed to obtain a firm enough tissue edge, first for stitching, and second for the introduction of the left ventricular (LV) side patch into the LV. The original plan was for the surgical bite to be 1.0 cm from the edge of the trimmed defect (Fig. 1a). With the addition of an outer margin extending 1.0 cm from the stitch, the length and width of the patches should be 4.0 cm larger than those of the trimmed defect (Fig. 1b). In practice, the size of the patch was not always as originally planned, since the size was determined by the surgeon. The patches were a combination of Dacron® or Teflon® felt patch (Bard Peripheral Vascular Inc., Tempe, AZ, USA) and an equine pericardial patch (Edwards Life Sciences Corp., Tokyo, Japan) or autologous pericardium for the surface in contact with blood. The felt patch and pericardial patches were attached using fibrin glue or gelatin–resorcinol–formaldehyde (GRF) glue. Gore-Tex® patches (W.L. Gore & Associates. Inc., Newark, DE, USA) 0.6 mm in thickness were used in two cases where no pericardial patch was attached. Eight or ten 4-0 or 3-0 polypropylene mattress sutures with an SH or MH needle (Ethicon, Inc., Somerville, NJ, USA), with or without felt pledgets, were first applied to the LV-side patch and then to the edge of the defect. After the LV-side patch was introduced into the ventricle, the sutures were lifted to make the LV-side patch fit the ventricular septum, so as to prevent the GRF glue leaking into the LV. GRF glue was used to close the VSD in all cases except one, and was applied to the defect three or four times. To prevent the presence of residual aldehyde, the ratio of adhesive to activator in the GRF glue was strictly controlled at 10:1 [13]. The amount of adhesive was measured using a syringe, and the amount of activator was controlled by counting the drops from a 27-gauge needle, where one drop was 4 µl. Two patches pinched the ventricular septum. The RV was usually closed with a running 4-0 or 5-0 polypropylene suture. When there was widespread necrosis at the edge of the free-wall side of the septal defect, we inserted the LV-side suture first through the LV free wall, on the proximal or distal side of the left anterior descending or posterior descending artery, and re-entered the RV using the felt strip to avoid cutting the necrotic ventricular septal wall (Fig. 2a). If the myocardial necrosis was widespread, we divided the sutures so that one suture was used to close the LV-side patch and the other to close the RV side (Fig. 2b), as was also reported by Asai et al. and Hosoba et al. [11, 12].
Schematic drawings of the extended sandwich technique in case of adequate firm tissue on the free-wall side. a If there is no viable myocardium in the ventricular septum near the ventricular free wall, the left ventricular (LV) patch is fixed to the intact ventricular free wall near the edge of the ventricular septal defect (VSD). Sutures are placed from inside the LV cavity through to the free wall just to the right of the left anterior descending artery (LAD), and after reinforcement with felt to avoid cutting the ventricular septal wall, the sutures are drawn back to the right ventricular (RV) cavity. b If there is no viable myocardium in the ventricular septum or the adjacent ventricular free wall, sutures are placed from inside the LV cavity to the outside of the LV free wall just to the left of the LAD. The sutures are then tied with reinforcement from a felt strip. Other sutures are placed from the outside of the RV free wall near the LAD to the inside of the RV cavity with reinforcement from another felt strip (extended “Sandwich” patch by Asai et al. 2013) [9]
Results
Protocol 1
Preoperative, operative, and postoperative data are shown in Tables 1 and 2. There were three cases with a significant intraoperative leak treated with a second pump run, and another single case with a major postoperative leak although no leak was detected during the first surgery. In all, major residual leak occurred in 17 % of patients (4/24) (Table 2). Thirty-day mortality was zero and one-year mortality was 25 % (6/24 patients). The causes of deaths were mediastinitis in two patients at 6 and 12 weeks after surgery, pneumonia in one patient at 8 weeks after surgery, arrhythmia in one patient at 12 weeks after surgery, heart failure in one patient at 23 weeks after surgery, and tracheostomy bleeding in one patient at 23 weeks after surgery. Correlations between 1-year mortality and clinical parameters are shown in Table 3. Three of the four patients (75 %) with major residual leak did not survive for one year, compared to 3 of the 20 patients (15 %) without major residual leak (p = 0.035). There were two redo VSD closure cases, neither of whom survived for one year (100 %), compared to 4 of 22 patients (18 %) without redo (p = 0.054). There were no statistically significant differences in second pump run, GRF glue use, location of VSD (anterior type or posterior type), postoperative PCPS use, or severe valve failure. There was only one patient who did not receive early repair (<7 days). There was one patient with high VSD who survived for a long term.
Protocol 2
The size of the patches in the cases with major residual leak (41.9 ± 3.8 mm) was significantly smaller than in those with no leak (47.8 ± 3.8 mm) (p = 0.031) (Table 4). The size difference between the patch and the trimmed VSD in the cases with major residual leak (22.5 ± 6.5 mm) was significantly smaller than that in the cases without a leak (30.0 ± 5.7 mm) (p = 0.028). The size of the trimmed VSD did not show any significant relation to major residual leak. Correlations between major residual leak and surgical parameters are shown in Table 5. Major residual leak was observed in a case where we did not use GRF glue to close the VSD. However, the difference between the use of GRF glue and no use was not significant (p = 0.167). Other parameters, including blood-contacting surface, patch material, use of pledgets, size and number of suture, and the use of the extended sandwich approach, did not show significant correlation with major residual leak.
In addition, the relationship between one-year mortality and parameters related to patch size was studied, but no statistically significant differences were found. The patch size was 47.6 ± 5.2 mm in patients who survived to one year and 44.2 ± 3.8 mm in deceased patients (p = 0.249). The differences between the patch and VSD sizes were 29.8 ± 6.8 mm and 25.7 ± 3.8 mm, respectively (p = 0.198), while the trimmed VSD sizes were 18.3 ± 5.4 mm and 18.5 ± 2.3 mm, respectively (p = 0.924).
Discussion
Residual leak, uncontrolled bleeding, and technical difficulty are among the problems with the current surgical techniques for VSD repair [2, 4, 6, 7]. Using the Daggett infarct excision technique and the David–Komeda infarct exclusion technique, Deja et al. reported the results of 110 patients with a 30-day mortality of 35 % and a 5-year survival of 45 % [4]. A recent report by the Japanese Association for Thoracic Surgery described a somewhat better 30-day mortality of 10.5 % in 2013 [3]. The sandwich technique via the RV approach has provided good results, with a 30-day mortality of 0 % and a 5-year survival of 65 %, including hospital mortality [10]. We use one-year mortality to surgical technique since all the deaths in one year after the surgery were rather related to the surgical complication in our experience.
In this study, there was a correlation between major residual leak and one-year mortality. Other clinical parameters, including location of the VSD, postoperative PCPS use, which indicates postoperative right or bilateral ventricular failure, and severe valve failure involvement, did not show a significant correlation with one-year mortality. Although it might be generally thought that the high mortality of post-infarction VSD derives from the patient’s severe heart failure, our data did not fully support this, since the use of postoperative PCPS was not related to one-year mortality. Although the data in our study did not indicate a relationship between one-year mortality and patch size, our findings suggest that we can improve the surgical results if we reduce major residual leak.
Among the parameters we tested in protocol 2, a small patch size and a small difference between the sizes of the patch and the trimmed VSD correlated with major residual leak. Even after thorough resection of the necrotic myocardium during trimming of the edge of the VSD, the strength of the edge of the VSD seems insufficient to fully tolerate the tension of the suture. If the patch is not large enough, a tearing force could result from the sum of the tension vectors caused by the angled orientation of the sutures (Fig. 3a). This would entail the use of a part of the myocardium at high risk of ischemic damage as the anchoring site of the suture. We did not experience major residual leak with a patch size of more than 30 mm larger than the trimmed VSD size. To obtain a safety margin from the edge of the trimmed VSD after apparently necrotic myocardium was removed, we tried to allow a 10-mm-wide bite from the edge of the trimmed VSD (Fig. 3b). Since the margin outside the suture seems to have a role in dispersing the pressure of both the pinching force exerted by the sutures and the LV pressure on the patch over the wider area of the myocardium at the edge of the VSD, we also tried to leave a 10-mm margin of patch outside the stitch (Fig. 3c). Combining the bilateral surgical bite and the bilateral outer margin, it is recommended to use a patch 40 mm larger than the size of the trimmed VSD (Fig. 1b).
Schematic drawing of the patch and ventricular septal defect (VSD). a A small patch requires the sutures to be placed at an angle and the sum of the suture tension vectors (arrows, a, b) results in a force tearing the VSD (arrow, T). A small bite leads to a higher risk of a fragile suture-anchoring site, because of the ischemic damage to the myocardium at the suture. b Outer margins from the suture have a role in dispersing the pressure of both the pinching force from the sutures and the left ventricular pressure on the patch. c With a wider margin and GRF glue, both the pinching force from the suture and the LV pressure on the patch are dispersed over a wider area
Validity of our choice of the patches such as combination with Teflon or Dacron felt with pericardium or Gore-Tex patch with 0.6 mm thickness seems to be compatible to their patches.
The use of surgical adhesive plays an important role in treating post-infarction VSD [14, 15]. We did not use surgical adhesive in one patient and this resulted in a major residual leak that required a redo sandwich procedure. Although no statistical significance was demonstrated in this study, the lack of use of surgical adhesive might correlate with major residual leak. We hypothesized that surgical adhesive may have three major roles: the first is sealing the VSD and thus closing the defect, the second is reinforcement of the fragile myocardium by cross-linking the weak tissue to prevent suture cutting, and the third is the reduction of stress to the edge by increasing the stiffness of the edge and so preventing deformity of the patch on the VSD as a result of LV pressure. We must take care not to leave residual aldehyde at the repair site when we use GRF glue. Suzuki et al. [16, 17] reported aortic necrosis after surgical treatment using GRF glue in patients with acute type A aortic dissection. We have monitored the surviving patients by echocardiography every year at the outpatients clinic, and we did not observe any pseudoaneurysm formation at the VSD repair site. We believe that an appropriate ratio of adhesion and activator, in combination with felt reinforcement, might prevent pseudoaneurysm formation. Our in vitro test indicated that a 10:1 volume ratio of adhesive to activator provides the best force and minimal residual aldehyde [12]. Other surgical adhesives, such as bovine serum albumin–glutaraldehyde glue (Bioglue®, Cryolife, Inc., Kennesaw, GA), have also been used in the sandwich technique via the RV approach [10, 11]. Although the use of these surgical adhesives in the sandwich technique is currently not reimbursed by insurance in our country, we do recommend the use of surgical adhesives to avoid the risk of leak, with consent from the patient and institutional approval.
Extended approach
In six of the 24 patients, we recognized that there was ischemic myocardium at the free-wall side edge of the VSD from the RV view. Since suturing the patch to necrotic myocardium on the LV side may result in tearing of the tissue with major residual leak, we had to place sutures from the inside of the LV chamber to the RV or LV free wall that was free of ischemic changes (Fig. 2a, b). To prevent excessive stress on the ventricular free wall, reinforcement of the suture with Dacron or Teflon felt is required. The suture is then pulled back into the RV cavity (Fig. 2a). We can also separate sutures, one to fix the LV-side patch and the other to fix the RV-side patch (Fig. 2b); this is the so-called “extended sandwich patch” reported by Asai et al. [10]. Although we could not demonstrate the benefit of this technique in our small study, we believe that it might be useful in selected patients who have severe necrosis near the free-wall side edge or apical edge of the VSD. In addition, we reinforce LV free wall with bovine epicardium when oozing rupture or severe ischemic appearances are noticed, where we had not seen any marked LV remodeling in follow-up.
References
Lundberg S, Soderstrom J. Perforation of the interventricular septum in myocardial infarction. A study based upon an autopsy material. Acta Med Scand. 1962;172:413–8.
Madsen JC, Daggett WM Jr. Post-infarction ventricular septal defect and free wall rupture. In: Edmunds Jr LH, editor. Cardiac surgery in the adult. New York: McGraw-Hill; 1977. p. 629–55.
Masuda M, Kuwano H, Okumura M, Arai H, Endo S, Doki Y, et al. Thoracic and cardiovascular surgery in Japan during. Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2013;2015(63):670–701.
Deja MA, Szostek J, Widenka K, Szafron B, Spyt TJ, Hickey MS, et al. Post infarction ventricular septal defect–can we do better? Eur J Cardiothorac Surg. 2000;18:194–201.
Komeda M, Fremes SE, David TE. Surgical repair of postinfarction ventricular septal defect. Circulation. 1990;82:IV243–7.
Isoda S, Osako M, Kimura T, Nishimura K, Yamanaka N, Nakamura S, et al. Surgical repair of postinfarction ventricular septal defects-2013 update. Ann Thorac Cardiovasc Surg. 2013;19:95–102.
Asai T. Postinfarction ventricular septal rupture: can we improve clinical outcome of surgical repair? Gen Thorac Cardiovasc Surg. 2016;64:121–30.
Isoda S, Imoto K, Uchida K, Hashiyama N, Yanagi H, Tamagawa H, et al. Sandwich technique via right ventricle incision to repair post-infarction ventricular septal defect. J Card Surg. 2004;19:149–50.
Isoda S, Osako M, Kimura T, Mashiko Y, Yamanaka N, Nakamura S, et al. Midterm results of the “sandwich technique” via a right ventricle incision to repair post-infarction ventricular septal defect. Ann Thorac Cardiovasc Surg. 2012;18:318–21.
Isoda S, Imoto K, Uchida K, Nishimura K, Karube N, Suzuki S, et al. “Sandwich technique” via a right ventricle incision to repair postinfarction ventricular septal defects. J Card Surg. 2015;30:488–93.
Asai T, Hosoba S, Suzuki T, Kinoshita T. Postinfarction ventricular septal defect: right ventricular approach-the extended “sandwich” patch. Semin Thorac Surg. 2012;24:59–62.
Hosoba S, Asai T, Suzuki T, Nota H, Kuroyanagi S, Kinoshita T, et al. Mid-term results for the use of the extended sandwich patch technique through right ventriculotomy for postinfarction ventricular septal defects. Eur J Cardiothorac Surg. 2013;43:e116–20.
Isoda S, Kimura T, Mashiko Y, Nakamura S, Maehara T. In vitro study of the optimum volume ratio of activator to adhesive in gelatin-resorcin-formalin glue. Gen Thorac Cardiovasc Surg. 2011;59:326–8 (Deleted: Ito T, Hagiwara H, Maekawa A, Yamazaki T. Finite element analysis regarding patch size, stiffness, and contact condition to endocardium in surgery for postinfarction ventricular septal rupture. Gen Thorac Cardiovasc Surg 2013;61:632-639).
Musumeci F, Shukla V, Mignosa C, Casali G, Ikram S. Early repair of postinfarction ventricular septal defect with gelatin-resorcin-formol biological glue. Ann Thorac Surg. 1996;62:486–8.
Yamamoto N, Ohara K, Nie M, Torii S, Imai H, Yoshimura H. Double-patch closure using gelatin resorcine formol glue of a ventricular septal perforation following acute myocardial infarction. Jpn J Thorac Cardiovasc Surg. 2002;50:294–7.
Suzuki S, Imoto K, Uchida K, Takanashi Y. Aortic root necrosis after surgical treatment using gelatin-resorcinol-formaldehyde (GRF) glue in patients with acute type A aortic dissection. Ann Thorac Cardiovasc Surg. 2006;12:333–40.
Suzuki S, Masuda M. An update on surgery for acute type A aortic dissection: aortic root repair, endovascular stent graft, and genetic research. Surg Today. 2009;39:281–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Isoda, S., Imoto, K., Uchida, K. et al. Pitfalls for the “Sandwich technique” via a right ventricular incision to repair post-infarction ventricular septal defects. Gen Thorac Cardiovasc Surg 65, 187–193 (2017). https://doi.org/10.1007/s11748-016-0722-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-016-0722-4