Abstract
Triglyceride-based monomers represent a competitive alternative to petrochemical resources in the macromolecular compounds area. In the current study, several types of hydrophilic camelina oil (CO)-based monomers were synthesized using tunable experimental protocols that involve three different steps: first—conversion of the double bonds into epoxy rings, second—partial opening of the epoxy rings and methacrylic groups grafting and last—opening of the unreacted epoxy rings and hydrophilic units attaching. 1H-NMR, 13C-NMR and FTIR spectroscopy demonstrate the success of the CO functionalization with polymerizable and hydrophilic moieties—polyethylene glycol units—with different molecular weights, exhibiting self-emulsifiable properties. Several bulk and emulsion polymerization tests were performed for the synthesized monomers and their ability to build polymer networks using different photo-chemical procedures (using visible and UV radiations respectively) was demonstrated, without additional surfactants. FTIR spectroscopy indicates the polymerization success by the disappearance of the specific bands assigned to the double bonds from methacrylic groups and thermogravimetric analysis demonstrates that the emulsion polymerization leads to materials with an improved thermostability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environment is increasingly affected by petroleum-based chemical compounds and consequently many researchers have turned their attention to new raw materials which lead to substitutes for petrochemical compounds. To accomplish the requirements for sustainability, to solve environment problems and obtain novel materials with competitive properties, we are interested in some alternatives for products and materials derived from petroleum resources.
The use of plant oils as feedstocks in the macromolecular field and the polymer industry can be a successful solution to solve the environmental, financial and other problems such as depletion of non-renewable petroleum resources [1–4]. This study explores the chemical potential of camelina oil (CO), a novel and less-explored source of polyunsaturated fatty acids which makes it an excellent choice for the polymer industry [5].
Due to its high omega-3 and omega-6 fatty acid content, camelina oil has been exploited in the healthcare industry as an anti-age and emollient agent to improve skin elasticity and suppleness in toiletries, soaps, pharmaceuticals, sun care and nutraceuticals [6–8], but the most explored technical application of CO is as a feedstock for biofuels [9, 10].
The aim of this study was to design new monomers by grafting of polymerizable moieties and hydrophilic units onto the CO hydrophobic skeleton and also, to test the ability of the designed monomers by engaging them in some polymerization techniques under different conditions. Earlier reported work proved that CO-based systems obtained by co-polymerization of CO-derivatives with dimethacrylated polyethylene glycols exhibit promising and controllable properties in terms of surface hydrophilicity, water uptake, mechanical and thermal behavior [11].
Current research study demonstrated that designed CO-based monomers bearing both methacrylate and hydrophilic units exhibit suitable properties for polymerization techniques close to green chemistry due to their self-emulsifiable behavior. We present a tunable synthesis protocol, based on inexpensive reagents leading to monomers with potential industrial applications without elaborate purification methods. Different types of monomers were investigated in eco-friendly photo-polymerization procedures, bulk and emulsion, and thermal stability of the obtained polymers was investigated in order to establish the most appropriate polymerization procedure.
Materials and Methods
Materials
Camelina oil (CO) extracted in a cold-pressing process was kindly supplied by the University of Agricultural Sciences and Veterinary Medicine Bucharest. Polyethylene glycol methyl ether (PEG) with M ≈550 or 750 g/mol and methacrylic acid were purchased from Sigma–Aldrich. All other solvents and reagents were supplied by Sigma–Aldrich and used without any purification.
Characterization
1 H- and 13 C-NMR spectra were recorded on a Bruker Avance DRX 400 spectrometer, operating at 400.1 MHz for 1H and 100.6 for 13C nuclei equipped with a direct detection for nuclei probe-head and field gradients on the z axis. The NMR samples were prepared by dissolving 0.5 mL oil in 0.5 mL CDCl3. The chemical shifts are reported in ppm, using the TMS as internal standard.
Fourier transform infrared (FTIR) spectra were registered on a Vertex 70 Bruker FTIR spectrometer equipped with an attenuated total reflectance (ATR) accessory in order to determine the chemical structure and also the curing degree of all the studied specimens. All FTIR measurements were performed in the ATR–FTIR cell on Ge crystal, at room temperature. The FTIR spectra were recorded using 32 scans in 600–4000 cm−1 wave number region.
Critical micellar concentration (CMC) was estimated from the surface tension dependence on the synthesized monomers concentration in water in the range 0.025–35 mM.
Gel fraction analysis (GF) was performed by Soxhlet extraction and the selected solvent to dissolve the sol fraction was THF. GF values were calculated according to previously reported work [11, 12].
Static contact angle (CA) values were determined at room temperature using a KSV CAM 200 apparatus. Ultrapure water droplets were used with a drop volume of 20 µL and the measurement was done within 10 s of the drop contact with the surface. The reported contact angles values represent the average of three determinations.
Thermogravimetric analysis (TGA) results were achieved on a Q500 TA instrument. A sample of about 2.5 mg was placed in a platinum crucible and heated from 20 to 500 °C at a heating rate of 10 °C/min under a constant N2 flow rate (balance flow 10 mL/min, oven flow 90 mL/min).
Methods
Synthesis of Hydrophilic CO-Based Compounds
Firstly CO was characterized by 1H-NMR and GC–MS in order to obtain the fatty acid profile and the reported results [11] are in good agreement with results reported in the literature [12].
1 H-NMR (CDCl 3 , TMS, δ in ppm): 5.25 (–HC=CH– from unsaturated acids), 5.34 (CH–O–CO, glycerol), 4.28, 4.15 (m, CH 2–O–CO, glycerol), 2.80 (–CH=CH–CH 2–CH=CH), 2.31 (CH 2–COO), 2.06 (–CH 2–CH=CH2), 1.62 (CH 2–CH2–COO), 1.25 (–(CH 2)n,), 0.98 (terminal CH 3, linolenic acid), 0.88 (terminal CH 3, all fatty acids except linolenic acid).
13 C-NMR (CDCl 3 , TMS, δ in ppm): 173.20 (–COO from 18:1, 1, 3 psn); 173.17 (–COO from 18:2 and 18:3, 1, 3 psn); 172.77 (–COO from 18:2 and 18:3, 2 psn); 131.89 (C16 from 18:3); 130.16, 130.06, 129.99 (C13 from 18:2); 129.96,129.93, 129.91 (C10 from 18:1); 129.88, 129.85 (C9 from 18:2); 129.81 (C13 from 18:3); 129.69, 129.67 (C9 from 18:1); 128.27,128.24 (C12 from 18:3); 128.17, 128.15 (C12 from 18:3); 128.10 (C10 from 18:2); 127.91 (C12 from 18:3); 127.79, 127.77 (C10 from 18:3); 127.13 (C10 from 18:1); 68.92 (glycerol, 2 psn.); 62.10 (glycerol, 1, 3 psn.); 34.18, 34.02, 33.81 (C2 all fatty acids); 31.95, 31.81 (C16 from 18:2); 31.56 (C16 from 18:3); 29.80–29.07 (internal aliphatic saturated C atoms); 27.23 (mono-allylic C atoms from all fatty acids); 25.64, 25.55, 24.89 (bis-allylic C11 and C14 from 18:2 and 18:3); 22.73, 22.62 (C17 from 18:1 and 18:2); 22.53 (C17 from 18:3); 14.30, 14.14, 14.10 (terminal C18 from all fatty acids).
FTIR (ATR, cm −1): 3010 (ν =CH–); 2923, 2856 (ν C–H asim, sim), 1747 (ν C=O); 1654 (ν C=C from unsaturated acids); 1457 (δ CH2); 1368 (δ CH3); 1232, 1158, 1102 (ν C–O); 718 (ρ CH2).
The functionalization pathway of the CO involves a three stage reaction. The epoxidation of the double bonds and methacrylic units attaching procedures was previously reported [11]. In addition, a selective reaction to open the epoxides with methacrylic acid (MA), using the same reaction conditions was performed in the current work resulting the methacrylated-epoxidized camelina oil (MECO) with different amounts of polymerizable units grafted on the CO structure and preserved unreacted epoxy groups without any side homopolymerization reaction detected (epoxy-methacrylate: 50–50 and 25–75). Appropriate epoxy: MA molar ratios were used, 1:0.8 or 1:0.65 to obtain MECO75 and MECO 50 % respectively.
Epoxidized CO. 1 H NMR (CDCl 3 , TMS, δ in ppm): 5.26 (CH–O–CO, glycerol), 4.28, 4.15 (CH 2–O–CO, glycerol), 3.12 (CH, internal protons of the epoxy ring), 2.89 (CH, marginal protons of the epoxy ring), 2.35 (CH 2–COO acyl group), 1.73 (CH 2 between two epoxy rings), 1.49 (CH 2–CH2–COO), 1.25 (CH 2, all alkyl chains), 1.05 (terminal CH 3 from linolenic acid), 0.88 (terminal CH 3 from all fatty acids except linolenic acid).
13 C-NMR (CDCl 3 , TMS, δ in ppm): 173.07, 173.05 (–COO from 18:1, 1, 3 psn); 172.99 (–COO from 18:2 and 18:3, 1, 3 psn); 172.59 (–COO from 18:1, 2 psn); 128.85 (C12 from 18:3), 128.04 (C10 from 18:2 and 18:3), 68.75 (glycerol, 2 psn.); 61.91 (glycerol, 1,3 psn.); 57.95–53.61 (C atoms from epoxy rings); 33.95, 33.84, 33.79 (C2 from all fatty acids); 31.78, 31.71 (C16 from 18:2); 31.52 (C16 from 18:3); 29.56-28.79 (internal aliphatic saturated C atoms); 27.73, 27.67 (C8 and C11 from 18:1); 27.21–26.00 (C11 and C14 from diepoxy, 18:3); 24.63 (C17 from 18:1 and 18:2); 22.55, 22.52, 22.43 (C17 from 18:3); 13.97, 13.85, 13.56 (terminal CH3 groups from 18:2); 10.49, 10.35 (terminal CH3 from 18:3).
FTIR (ATR, cm −1): 2925, 2857 (ν C–H asim, sim.); 1743 (ν C=O); 1457, 1376 (δ CH from CH2 and CH3); 1236, 1160, 1104 (ν C–O); 826 (ν C–O–C from the epoxy ring); 728 (ρ CH2).
Methacrylated-Epoxidized CO
1H NMR (CDCl 3 , TMS, δ in ppm): 6.13, 5.58 (CH 2= from methacrylic group); 5.26 (CH–O–CO, glycerol); 4.29, 4.16 (CH 2–O–CO, glycerol); 3.11 (CH, internal protons of the epoxy ring); 2.89 (CH, marginal protons of the epoxy ring); 1.95 (CH 3–C=CH2, methacrylate); 1.72 (CH 2 between two epoxy rings); 1.49 (CH 2–CH2–COO); 1.25 (CH 2, all alkyl chains); 1.05 (terminal CH 3 from linolenic acid); 0.88 (terminal CH 3 from all fatty acids except linolenic acid).
13 C-NMR (CDCl 3 , TMS, δ in ppm): 173.16 (–COO from 18:1, 1, 3 psn); 173.22, 172.76 (–C=O from methacrylate moieties); 136.25 (=CH2 from methacrylate moieties); 125.94 (>C=CH2 from methacrylic groups); 68. 96 (glycerol, 2 psn.); 65.83 (C–O atoms between oil chain and methacrylic groups); 62.09 (glycerol, 1, 3 psn.); 58.30–54.11 (C atoms from epoxy rings); 34.13, 34.01, 33.97 (C2 from all fatty acids); 31.94, 31.87 (C16 from 18:2); 31.68 (C16 from 18:2); 29.71–27.38 (internal aliphatic saturated C atoms); 27.30, 27.26, 27.22 (C8 and C11 from 18:1); 26.93 26.15 (C11 and C14 from 18:3); 25.69, 25.45, 24.80 (internal aliphatic saturated C atoms); 22.67 (C17 from 18:1 and 18:2); 22.59 (C17 from 18:3).
FTIR (ATR, cm −1): 2926, 2857 (ν C–H asim, sim.); 1735, 1700 (ν C=O); 1633 (ν C=C from the methacrylic group); 1453, 1376 (δ CH from CH2 and CH3); 1301, 1009, 946, 820 (δ C–H and δ =C–H); 1165, 1107 (ν C–O); 729 (ρ CH2).
PEGylation of MECO was performed with polyethylene glycol monomethyl ether with different molecular weights, MW ≈550 or ≈750 g/mol. Epoxy group: PEG molar ratio was 1:1. PEG was dissolved in tetrahydrofuran (THF) and heated to 60 °C. Boron trifluoride (0.05 %v on PEG) was added. MECO in THF with a small amount of HQ was added dropwise over the PEG and the mixture temperature was raised to 65 °C for 30 min [13]. The product was filtered through a bed of NaHCO3 to remove catalyst residues. The solvent was evaporated under a vacuum. Thus methacrylated-PEGylated CO (MCO-PEG), self-emulsifiable compounds were obtained as light yellow products with yields over 96 %.
1 H NMR (CDCl 3 , TMS, δ in ppm): 6.18, 5.59 (CH 2= from methacrylic group); 5.25 (CH–O–CO, glycerol); 4.29, 4.17 (CH 2–O–CO, glycerol); 3.42–3.78 ((CH2–CH2–O)n, PEG chains); 3.38 (terminal CH3 groups from PEG chains); 2.31 (CH 2–COO); 1.94 (CH 3–C=CH2, methacrylate); 1.50 (CH 2–CH2–COO); 1.25 (CH 2, all alkyl chains); 1.06 (terminal CH 3 from linolenic acid); 0.88 (terminal CH 3 from all fatty acids except linolenic acid).
13 C-NMR (CDCl 3 , TMS, δ in ppm): 174.14, 173.98 (–C=O from methacrylate moieties); 173.18 (–COO from 18:1, 1, 3 psn); 173.10, 173.04, 172.99, 172.94 (–COO from 18:2 and 18:3, 1, 3 psn); 72.57, 71.91 (C–O between the PEG units and the oil chains); 70.54, 70.32 (CH2 groups of the PEG units); 68.93 (glycerol, 2 psn.); 67.82 (C–O between methacrylate moieties and oil chain); 62.05 (glycerol, 1,3 psn.); 61.55 (CH2 groups of the PEG units); 58.91 (terminal CH3 groups from the PEG units); 34.08, 33.98 (C2 from all fatty acids); 31.79–28.92 (internal aliphatic saturated C atoms); 27.78, 27.29 (C8 and C11 from 18:1); 26.87, 26.56, 26.35, 25.57 (C11 and C14 from 18:3); 22.60, (C17 from 18:3); 18.11 (CH3 from methacrylic groups); 14.06 (terminal CH3 groups from 18:2); 10.58, 10.40 (terminal CH3 groups from 18:3).
FTIR (ATR, cm −1): 2924, 2861 (ν C–H asim, sim.); 1737 (ν C=O); 1634 (ν C=C from methacrylic group); 1457, 1356 (δ CH from CH2 and CH3); 946, 848 (δ C–H and δ =C–H); 1107 (ν C–O); 736 (ρ CH2).
Curing Tests
Bulk photo-polymerization. Mixtures of MCO75%-PEG type compound and a specific photo-initiating system consisting of camphorquinone (CQ, 0.2 %wt) and a tertiary amine–ethyl (dimethylamino) benzoate (EDMAB, 0.8 %wt) [14] were placed in a clean Teflon mold (10 mm wide, 2 mm thick, 20 mm long), covered with a glass slide (1 mm thick) and irradiated in the visible range at a wavelength of 450–500 nm, 4 min [11].
Emulsion photo-polymerization. 4 %v MCO75 %-PEG550 or 6 %v MCO75 %-PEG750 were mixed with 5 mL distilled water and 2 %wt 1-hydroxy-cyclohexyl phenyl ketone (Irgacure 184) as photo-initiator. The homogeneous solution was placed into a micro photochemical reaction assembly with quartz well, equipped with a UV lamp (output 5.5 watts) which emits radiations at 254 nm and were magnetically stirred for 2 h at room temperature. The irradiated samples were filtered and dried at 60 °C, 3 h.
Results and Discussion
CO was modified through a three-step reaction which involves a total conversion of the fatty acids double bonds into epoxy rings [11] followed by a selective ring-opening of the epoxides in order to attach polymerizable moieties. Subsequently a reaction was performed to increase the hydrophilicity with PEG units by opening of the unreacted epoxy groups, which represent the novel part of this study in terms of chemical modification of the CO structure.
Synthesis and Characterization Of The Obtained MCO-PEG Compounds
The changes that occur during the functionalization steps were monitored by 1H- and 13C-NMR and FTIR spectroscopy, with focus on the NMR which provide generous information regarding the selectivity of the reactions. The 13C-NMR shifts of the CO were assigned according to literature reported data [15].
The success of the epoxidation reaction was demonstrated in a previously reported study by 1H-NMR, and FTIR spectroscopy [11]. Additionally, 13C-NMR spectra were recorded and interesting insights into the evolution of the epoxidation reaction were obtained due to an accurate identification of every unsaturated carbon atom from the double bonds (Fig. 1).
The formation of the epoxy rings is evidenced by the appearance of the specific signals in the 58–53 ppm range (Fig. 1 b), and the subunitary epoxidation degree of 0.932, previously determined and reported [11] is reflected in residual signals assigned to the unsaturated carbons from the unreacted double bonds belonging to the linolenic (18:3) and linoleic (18:2) acids, more specifically C12=C13 from 18:3 and C9=C10 from 18:2 and 18:3. These internal double bonds are sterically crowded and less accessible and thus the epoxidation was more difficult and in fact, some of the double bonds remained unreacted. The disappearance of the signals assigned to the olefin bonds of 18:1 acids (129.67, 129.69 ppm –C9; 129.91, 129.93, 129.96 ppm –C10) as well as marginal double bonds from 18:2 and 18:3 acids (C12=C13 at 129.99, 130.06, 130.16 ppm and C15=C16 at 127.13, 131.89 ppm respectively) easily accessible during the synthesis reaction, accounts for a regioselectivity of epoxidation determined by steric hindrance. Thus, the high content of easily epoxidable oleic acid in the CO can be considered an advantage for this type of functionalization as compared to more unsaturated oils such as soybean and linseed [16].
The selective ring-opening reaction yielded camelina oil derived monomers with methacrylated and epoxy groups in different ratios. The 1H-NMR spectra of the MECO50% and MECO75% indicates the presence of methacrylate moieties by the characteristic signals around 6.1 and 5.5 ppm (=CH 2) and 1.9 (–CH 3). Also, the selectivity of the reaction, with preservation of some unreacted epoxy units was evidenced by the characteristic signals of oxirane groups around 3.1, 2.9 and 1.7 ppm.
The presence of methacrylate groups and unreacted oxiranes is evidenced in the 13C-NMR spectrum by the characteristic signals: around 125–136 ppm attributed to olefinic protons, 171 and 167 ppm attributed to C=O bonds, 65 ppm representing C–O linkage between MA and oil chains and around 18 ppm attributed to the carbon atoms from the terminal CH3 groups from methacrylates; signals from 58–54 ppm range evidenced the presence of the oxirane carbon atoms.
FTIR analysis confirms all that by the presence of the 1633 cm−1 and 945 cm−1 bands assigned to the stretching and deformation vibrations of the –HC=CH– and respectively =C–H bonds from methacrylic groups, which were used further to monitor the polymerization procedures.
The grafting of the hydrophilic moieties was performed by opening of the unreacted epoxy rings from the MECO50% and MECO75% structures with suitable PEG derivatives following previously reported procedure [13]. Tunable hydrophilicity was achieved both by using two polyethylene glycol monomethyl ethers with different molecular mass (PEG550 and PEG750 respectively) and by employing MECO with variable epoxy to methacrylate ratio. The final compounds were obtained in ~96 % yields as yellowish oily liquids. The attachment of PEG moieties (referred as PEGylation) was monitored by the same techniques as reported above.
The 1H-NMR spectrum for the MCO75%-PEG550 final compound (Fig. 2a) presents characteristic signals for the new entities grafted. Thus, the signal from 1.94 ppm corresponds to methyl protons and signals from 5.62 to 6.18 ppm corresponding to vinyl protons of the methacrylate moieties, clearly indicates the presence of these entities on the oil structure. The signals in the range between 3.38 and 3.76 ppm are specific for the protons from repetitive ethylene glycol units and the clear signal present at 3.38 ppm was assigned to the terminal CH 3 groups from the PEG chains, in this case the most easily detected signal. For all synthesized MCO-PEG type compounds, the NMR spectra displayed similar chemical shifts; they differ only by integral values and signal intensities of the 3.4–3.8 ppm range, characteristic for ethylene glycol repetitive units and specific signals of the methacrylated moieties.
We also examined the selectivity of the adopted procedure taking into account the possibility of another side reaction consisting in transesterification of PEG units with methacrylic acid. Reported 1H-NMR data of polyethylene glycol methyl ether acrylate synthesized by a more selective reaction using acryloyl chloride [17] evidenced a specific signal for the methylene group adjacent to carboxylate at 4.32–4.29 ppm. This signal was not present in 1H-NMR spectra of MCO-PEG compounds, thus the possibility of this side reaction was reduced to less than 3 % taking into account the precision of the NMR method.
The 13C-NMR spectrum (Fig. 2b) evidenced the presence of PEG units by the characteristic signals identified in at 70.56 ppm assigned to C atoms from C–O bonds between PEG units and the oil chains, 67.90 and 63.89 ppm attributed to CH2 groups from the ethylene glycol repetitive units and 57.28 ppm assigned to terminal CH3 from the PEG structure. Also, we noticed the disappearance of the signals characteristic to the oxirane groups from 58–53 ppm range.
The success of the oil PEGylation was confirmed by FTIR spectroscopy too, through the strong vibration band at 1106 cm−1 characteristic for C–O bonds, belonging to (–CH2–CH2–O–)n repetitive units form PEG derivatives. Also, the appearance of the specific signals corresponding to –HC=CH– double bonds and =C–H bonds from the methacrylic moieties around 1635 and 944 cm−1 respectively, demonstrate the existence of these radicals covalently bound to the oil structure.
Establishment of the Percentages of Attached Entities
Based on 1H-NMR spectral data a system of equations was developed to determine the ratio between methacrylate and PEG groups. As a reference, the unmodified part of the molecule, the glycerol moiety (2H, 4.16 ppm; 2H, 4.29 ppm; 1H, 5.26 ppm) was used because it represents the part not affected by the performed chemical changes. The average number of the double bonds from the typical CO triglyceride molecule (=5.1) was taken into account.
The following notations were adopted: I vin—integral of vinyl protons; I met—integral of methyl protons; I PEG—integral of terminal CH3 protons from PEG chains; n—average number of double bonds; m—the methacrylation degree; p—the PEGylation degree; I +H —proton integral (from the reaction product, founded from the fixed part).
For example, to MCO75 %-PEG750 using multiple integrations for glycerol signals, the value of one H+ was established: I H+ = 0.5. Thus, for this compound: m = 0.72 and p = 0.24 meaning a methacrylation degree of almost 75 % and a PEGylation degree about 25 % which demonstrate a success of CO functionalization selective reactions achieved in desired proportions, according to molar ratio of the reagents involved. The ratio between polymerizable moieties/hydrophilic units was established for all synthesized compounds in the same manner.
Surfactant Behavior (CMC Measurement)
The reported literature data demonstrate that the ethoxylated oil derivatives are self-emulsifiable compounds [18], so the surfactant behavior of selected synthesized CO monomers (MCO75%-PEG550 and MCO75%-PEG750) has been tested, by surface tension measurements at different solution concentrations. By plotting surface tension versus concentration, the CMC values were estimated as follows:
-
CMC = 365 mg/L (0.184 mM) for MCO75%-PEG550.
-
CMC = 537 mg/L (0.24 mM) for MCO75%-PEG750.
These values are comparable with other common non-ionic surfactants [19] and other lipid-based surfactants [19–21] and proved that the synthesized CO compounds can be successfully exploited in emulsion polymerization procedures acting both as monomers and as surfactants without any involvement of a supplementary surfactant in the system.
Polymeric Materials
Bulk photo-polymerization. In order to test the polymerization ability of the obtained oil-based monomers a curing procedure was performed as a preliminary assay, using a green procedure based on photo-irradiation with VIS light, at 450–500 nm. Concerning the reaction conditions, suitable initiator amounts (0.2 % CQ and 0.8 % EDMAB) were used, in the absence of atmospheric oxygen and the reaction was performed for 4 min. This bulk-polymerization technique leads to the obtaining of soft samples, probably due to the very large size of the macromolecules involved in the system and also the steric hindrance of the polymerizable groups, due to the presence of long-chain PEG. After photo-polymerization all the samples were subjected to FTIR and TGA tests.
The FTIR analysis of the MCO-PEG irradiated materials indicates the complete disappearance of the peak around 1635 cm−1 assigned to –HC=CH– double bonds from methacrylic groups for all systems, with smaller or higher molecular weight of PEG.
Emulsion photo-polymerization. Preliminary UV-initiated polymerization attempts by an emulsion technique using water as the reaction medium were performed at concentrations superior to the determined CMC values preserving the homogeneity of the systems, in the presence of Irgacure 184 as initiator in 2 %wt from total amount of MCO-PEG.
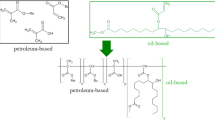
Figure 3 illustrates the experimental protocol involved to produce polymer networks starting from the camelina-oil based hydrophilic monomers, using the emulsion photo-irradiated polymerization.
Cured material obtained as a white precipitate was characterized by FTIR spectroscopy and thermogravimetric analysis (TGA).
FTIR spectroscopy proved the success of the photo-polymerization reaction by disappearance of the specific signal assigned to –HC=CH– bonds (Fig. 4) around 1635 and 945 cm−1, respectively, for both specimens involved in this preliminary study.
Gel Fraction Analysis
The insoluble fraction of the photo-polymerized materials was established through GF measurement in THF. GF values were determined by gravimetric measurements of the polymers based on MCO-PEG, obtained through bulk and emulsion techniques.
The GF values of CO-based systems were calculated and the results are as follow: 31 ± 1 % for MCO-PEG550 and 44 ± 2 % for MCO75%-PEG750 obtained through bulk photo-polymerization procedure and 53 ± 2 % for MCO-PEG550 and 62 ± 3 % for MCO75 %-PEG750 obtained using emulsion photo-polymerization technique.
Taking into account the obtained GF values we can assume that emulsion photo-polymerization technique is more efficient for the synthesized CO-based monomers and leads to a great polymerization degree in comparison with bulk photo-polymerization.
Contact Angle (CA) Measurement
CA values represent proof of the dependence of hydrophilicity on the PEG molecular weight. The two materials synthesized by employing bulk photo-polymerization method were analyzed by measuring the CA.
Emulsion polymerization technique leads to some materials inappropriate for CA tests. An increase of the material hydrophilicity meaning a decrease of the CA value was registered due to the different PEG molecular weight grafted on the oil chains as follow: 42 ± 1.7° for MCO75%-PEG550 and 28 ± 1.9° for MCO75 %-PEG750, both obtained through bulk photo-polymerization.
Thermo-Gravimetric Analysis for the Synthesized Polymeric Materials
Bulk vs Emulsion Photo-Polymerization
A critical evaluation of the general features for the polymeric materials obtained by photo-polymerization was further performed by TGA/DTG in order to establish the influence of the PEG molecular weight on the thermal properties. A more important aspect that was investigated through this study is the efficiency of the type of polymerization process (bulk/emulsion). TGA experimental results are summarized in Table 1 and the registered curves are shown in Fig. 5. All the studied samples exhibit similar thermal behavior, a single step of decomposition was observed as the temperature increases.
As expected the influence of the molecular weight for the PEG grafted on the oil-based monomer is clearly observed. The thermostabilities (T d5%) of the systems are enhanced when a PEG with a high molecular weight was used no matter what type of polymerization was chosen. Besides that, a more relevant aspect that could be emphasized from the TGA results concerns the polymerization efficiency. It can be noticed that the 5 % weight loss temperatures measured by TGA are considerably higher for the samples obtained using photo-induced emulsion polymerization in comparison with the samples obtained by bulk photo-polymerization. This behavior could be assigned to a greater efficiency in term of conversion of the emulsion polymerization toward the bulk-polymerization. Thus we can assume that through emulsion polymerization process a higher conversion could be attained for the oil macromonomer due an increased mobility of the methacrylated oil chains with attached PEG moieties favoring crosslinking. This is sustained by the increased thermostability of the polymer samples obtained with longer PEG chains favoring the freedom degree of the system. Additionally, the higher energies of the UV radiations against the VIS radiations could probably contribute to the obtaining of a high conversion degree. No significant differences were observed between all the polymerized samples concerning the temperatures of the maximum weight loss rate (T max) as well as the residual mass recorded at the end of the experiment (500 °C). The small amount of char suggests that all the samples undergo an almost complete thermal degradation at high temperatures independent of the polymerization process used.
In conclusion, camelina oil is proving to be a promising renewable raw material for polymer industry.
The adopted inexpensive and adjustable functionalization strategy leads to some monomers with polymerizable groups and hydrophilic groups on the oil structure whose ratio between grafted entities can be tuned by synthesis methodology and readily determined by using the 1H-NMR spectra. Preliminary photo-chemical polymerization tests proved that the synthesized monomers can be successfully polymerized using two different procedures. Moreover the major advantage of the designed monomers with increased hydrophilicity is the ability to exhibit a double role: monomer and surfactant favoring a green polymerization technique in water which leads to a superior polymerization degree demonstrated by gel fraction analysis.
References
Miao S, Wang P, Su Z, Zhang S (2014) Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater 10:1692–1704
Xia Y, Larock RC (2010) Vegetable oil-based polymeric materials: synthesis, properties, and applications. Green Chem 12:1893–1909
Ronda JC, Lligadas G, Galia M, Cadiz V (2011) Vegetable oils as platform chemicals for polymer synthesis. Eur J Lipid Sci Technol 11:346–358
Sharma V, Kundu PP (2006) Addition polymers from natural oils. Prog Polym Sci 31:983–1008
Reddy N, Jin E, Chen L, Jiang X, Yang Y (2012) Extraction, characterization of components, and potential thermoplastic applications of camelina meal grafted with vinyl monomers. J Agric Food Chem 60:4872–4879
Karvonen HM, Aro A, Tapola MS, Salminen I, Uusitupa MIJ, Sarkkinen ES (2002) Effect of alpha linolenic acid-rich Camelina sativa oil on serum fatty acid composition and serum lipids in hypercholestemic patients. Metabolism 51:1253–1260
Fröhlich A, O’Dea G, Hackett R, O’Beirne D, Eidhin DN, Burke J (2012) Stabilization of camelina oil with synthetic and natural antioxidants. J Am Oil Chem Soc 89:837–847
Vahvaselkä M, Laakso S (2010) Production of cis-9, trans-11-conjugated linoleic acid in camelina meal and okara by an oat-assisted microbial process. J Agric Food Chem 58:2479–2482
Ciubota-Rosie C, Ruiz JR, Ramos MJ, Perez A (2013) Biodiesel from Camelina sativa: a comprehensive characterisation. Fuel 105:572–577
Soriano NU, Narani A (2012) Evaluation of biodiesel derived from Camelina sativa. J Am Oil Chem Soc 89:917–923
Balanuca B, Lungu A, Hanganu A, Stan LR, Vasile E, Iovu H (2014) Hybrid nanocomposites based on POSS and networks of methacrylated camelina oil and various PEG derivatives. Eur J Lipid Sci Technol 116:458–469
Balanuca B, Lungu A, Conicov I, Stan R, Vasile E, Vuluga DM, Iovu H (2015) Novel bio-based IPNs obtained by simultaneous thermal polymerization of flexible methacrylate network based on a vegetable oil and a rigid epoxy. Polym Adv Technol 26:19–25
Budin JT, Breene WM, Putnam DH (1995) Some compositional properties of camelina (Camelina sativa L. Crantz) seeds and oils. J Am Oil Chem Soc 72:309–315
Hedman B, Piispanen P, Alami E, Norin T (2003) Synthesis and characterization of surfactants via epoxidation of tall oil fatty acid. J Surfact Deterg 6:47–53
Sulca NM, Munteanu AV, Popescu RG, Lungu A, Stan R, Iovu H (2010) Dibenzylidene sorbitol derivatives for improving dental materials properties. UPB Sci Bull Series B 72:25–36
Vlahov G (2009) 13C nuclear magnetic resonance spectroscopy to determine fatty acid distribution in triacylglycerols of vegetable oils with high–low oleic acid and high linolenic acid. Open Magn Reson J 2:8–19
Winkler M, Montero de Espinosa L, Barner-Kowollik C, Meier MAR (2012) A new approach for modular polymer–polymer conjugations via Heck coupling. Chem Sci 3:2607–2615
Trouvé G (2000) Utilization of ethoxylated fatty acid esters as self-emulsifiable compounds. US Patent 6,103,770
Joshi-Navare K, Khanvilkar P, Prabhune A (2013) Jatropha oil derived sophorolipids: production and characterization as laundry detergent additive. Biochem Res Int 2013:1–11
Vanavil B, Perumalsamy M, Seshagiri Rao A (2014) Studies on the effects of bioprocess parameters and kinetics of rhamnolipid production by P. aeruginosa NITT 6L. Chem Biochem Eng Q 28:383–390
Nitschke M, Costa SG, Contiero J (2010) structure and applications of a rhamnolipid surfactant produced in soybean oil waste. J Appl Biochem Biotechnol 160:2066–2074
Acknowledgments
The work was funded by the Sectoral Operational Programme Human Resources Development of the Romanian Ministry of Labour, Family and Social Protection through the Financial Agreement POSDRU/159/1.5/S/132395.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Balanuca, B., Stan, R., Hanganu, A. et al. Design of New Camelina Oil-Based Hydrophilic Monomers for Novel Polymeric Materials. J Am Oil Chem Soc 92, 881–891 (2015). https://doi.org/10.1007/s11746-015-2654-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2654-z