Abstract
Flavor volatiles profiles and some functional properties of soy protein products produced by five different extraction methods were studied. A method, consisting of Ethanol Washing and a Hydrothermal cooking Treatment, was proposed to produce a soy protein product (EWHT). Flavor volatiles profiles, emulsifying ability and whiteness of EWHT were improved in comparison with those of Acid Precipitated soy protein product (AP), soy protein product with Oil-Body Associated proteins and Polar Lipids removed (OBA/PL-less), soy protein product obtained from Countercurrent Extraction (CE) and Oil-Body Associated protein product with Polar Lipids (OBA/PL). The GC-MS and sensory analysis showed that the flavor volatiles content of EWHT was lower than that of the other soy protein products. The results from high performance size exclusion chromatography and dynamic light scattering showed that proteins with larger molecular weights and larger hydrodynamic radii were contained in EWHT, suggesting that EWHT formed soluble larger protein aggregates with a more flexible structure, thus the solubility of EWHT was similar to that of AP, OBA/PL-less and CE. The surface hydrophobicity of EWHT (994.8) was higher than that of the other soy protein products. Therefore the process for preparing EWHT can be an ideal processing method to produce soy protein with good quality relative to flavor and functionality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soy proteins are important in terms of high nutritional quality, good functionality and low cost [1]. The solubility and emulsifying properties make them an important commercial food ingredient [2]. However, traditional extraction of soy protein products from hexane-defatted soy flakes often results in undesirable flavor volatiles in the protein, which diminishes their value to the food industry.
Lipids are a major source of compounds responsible for the objectionable flavors in soy protein products [3]. Although most lipids have been removed, the hexane-defatted soy flakes still contain residual polar lipids that release flavor volatiles upon lipoxygenase catalysis [4]. The autoxidative decomposition products of unsaturated phospholipid fatty acids are also a source of volatile flavor compounds [5]. In addition, Anderson et al. [6, 7] reported that the precipitate of a defatted soy extract at pH 4.5 in the presence of NaCl, bound the flavor volatiles and non-protein materials. The residual lipid contents of soy protein products differ with their extraction methods [8, 9]. Hence there are some methods that can decrease the flavor compounds in soy protein products. One method is to maximize removal of the residual lipids during the extraction process of soy protein products. Another is to avoid acid precipitation during the extraction process of soy protein products while another is to remove the volatile flavor compounds and lipids before acid precipitation.
The residual lipids also affect the functional properties of soy protein products. Boatright et al. [10] reported that the solubility of soy protein isolate was improved after the removal of most of the polar lipids. Since solubility has a significant influence on other functional properties [11], removing residual lipids from soy protein products may also improve other functional properties of the soy protein products.
Over the past several decades, many researchers have attempted to remove undesirable flavor compounds and improve functional properties of soy protein products. Samoto et al. [6] reported that the flavor of soy protein isolate could be improved by removing oil-body associated proteins and polar lipids, but the protein recovery rate was only 30%. Liu et al. [12] prepared a soy protein product by a countercurrent extraction method under alkaline conditions to avoid acid precipitation during the production of soy protein; however, the protein content was relatively lower. In addition, ethanol washing was used to remove polar lipids and improve the flavor of soy protein products. Among these methods, ethanol washing was demonstrated to have high potential for improving the quality of soy protein products [13, 14], but might result in some denaturation of soy proteins as well as reducing the nitrogen solubility index [15]. We previously found that soluble soy protein aggregates possessing high solubility could be efficiently prepared from ethanol-washed insoluble soy protein concentrate by the method of hydrothermal cooking [16].
The objectives of our study were to propose a method which is referred to Ethanol Washed and Hydrothermally Treated process (EWHT) to produce a soy protein product. We determined the yields, compositions, functional properties and flavor volatiles content of EWHT and compare them to soy protein products prepared from other methods (AP, CE, OBA/PL-less and OBA/PL). Meanwhile, we examined the effects of different extraction methods on solubility, color, emulsifying properties and flavor.
Materials and Methods
Materials
Low-denatured, defatted soy flakes were provided by Xuchang Bangdi Industrial & Commercial Co., Ltd (Henan, China), and the processing conditions were not disclosed by the provider. Dialysis tubing (Molecular weight cut-off: 6,000–8,000 Da) was purchased from Union Carbide Corporation of USA (Danbury, Connecticut). The dialysis tubing was cut into lengths of 15 cm, immersed in 1 L of 2% sodium bicarbonate/1 mM EDTA in a 2-L glass beaker and boiled for 10 min. The dialysis tubing was then rinsed and boiled in double distilled water for 10 min, submerged completely in 50% ethanol/1 mM EDTA, and stored at 4 °C. The dialysis tubing was rinsed thoroughly with double distilled water prior to use. Other reagents and chemicals (analytical grade) were from the Guangzhou Chemical Co. (Guangzhou, China).
Preparation of Soy Protein Products
Preparation of AP
The acid precipitated soy protein product was prepared as a control. Defatted soy flakes were ground to pass through a 60 mesh sieve. The yielded flour was then dissolved in distilled water at a liquid: solid ratio of 15:1 (v/w). The suspension was adjusted to pH 8.0 with 2 N NaOH, stirred for 1 h and centrifuged at 3,000g for 15 min at room temperature. The resulting supernatant was adjusted to pH 4.5 with 2 N HCl and centrifuged at 3,000g for 15 min. The protein-rich precipitate was re-dispersed in distilled water. The resulting suspension was adjusted to pH 7.5 with 2 N NaOH, dialyzed against distilled water for 36 h at 4 °C, freeze-dried and kept at 4 °C prior to use.
Preparation of OBA/PL and OBA/PL-less
OBA/PL and OBA/PL-less were prepared according to the method of Samoto et al. [6]. Defatted soy flour was dissolved in distilled water at a liquid:solid ratio of 15:1 (v/w). The mixture was adjusted to pH 7.5 with 2 N NaOH, stirred for 1 h and centrifuged at 10,000g for 10 min. The supernatant was adjusted to pH 2.8 with 2 N HCl and centrifuged at 10,000g for 10 min to obtain the precipitate, which was defined as OBA/PL. The resulting supernatant was diluted 4-fold with distilled water and adjusted to pH 4.5 with 2 N NaOH. The mixture was centrifuged at 10,000g for 10 min to obtain another precipitate, which was defined as OBA/PL-less. Each precipitate was suspended in distilled water. The resulting suspensions were adjusted to pH 7.5 with 2 N NaOH, dialyzed against distilled water for 36 h at 4 °C and freeze-dried.
Preparation of CE
CE was prepared according to the method of Liu et al. [12] except that three cycles of fractional Multi-Stage Countercurrent Extraction (MSCE) was conducted instead of continuous cycles. After 0.1% monoacylglycerol (w/w) was added into distilled water at 45 °C, defatted soy flour was suspended in distilled water at a liquid: solid ratio of 6:1 (v/w). The mixture was adjusted to pH 4.2 with 2 N HCl, stirred for 10 min and centrifuged at 1,500g for 15 min at room temperature. The resulting precipitate was divided equally into four parts. One part was suspended in distilled water at 45 °C at a liquid: solid ratio of 2:1 (v/w), adjusted to pH 8.0 with 1 N NaOH, stirred for 10 min and centrifuged at 1,500g for 15 min. The resulting supernatant was then added to another part of precipitate for the next cycle of protein extraction at 45 °C. Two more cycles were conducted, and the final supernatant was dialyzed and freeze-dried as described in the previous paragraph.
Preparation of EWHT
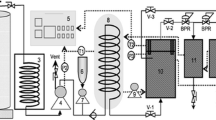
EWHT was prepared as follows: defatted soy flour was washed with 65% ethanol at a liquid: solid ratio of 4:1 (v/w) at 50 °C in MSCE system. After the removal of ethanol and moisture by vacuum, the soy flour was added to distilled water (1:100, w/v), adjusted to pH 9.0 with 2 N NaOH, stirred for 1 h, treated by hydrothermal cooking at 121 °C for 30 min and centrifuged at 3,000g for 10 min. The resulting supernatant was adjusted to pH 4.5 and centrifuged at 3,000g for 10 min. The precipitate was re-dispersed in distilled water. The resulting suspension was adjusted to pH 7.5, dialyzed and freeze-dried as described in previous sections (Fig. 1).
Thin Layer Chromatography (TLC) Analysis
Thin layer chromatography (TLC) analysis was performed to detect polar lipids by the method of Samoto et al. [17] with slight modifications. Lipids were extracted from each sample (0.5 g) with 10 mL of chloroform/methanol (2:1 v/v) for 2 h at 50 °C, dried and re-dissolved in 1.0 mL of chloroform/methanol (2:1 v/v). Ten microliters of the resulting solution were loaded on an H silica gel plate (Qingdao Haiyang Chemical Co., Ltd, China) and developed with a solvent system consisting of chloroform, methanol, acetic acid and water (85:15:10:3, by volume). The plate was sprayed with a 10% H2SO4 (w/w) solution and heated at 105 °C for 15 min to visualize the spots.
Analysis of Flavor Volatiles by Gas Chromatography-Mass Spectrometry (GC-MS)
Headspace solid-phase micro-extraction combined with GC-MS was utilized to quantify flavor volatiles in soy protein products according to the method of Hua et al. [8] with slight modifications. Protein products were dissolved in distilled water to a concentration of 8% (w/v). Twenty milliliters of each sample were then placed in a 50-mL triangular flask with a septum seal. The sampling was performed for 30 min at 50 °C using CAR/PDMS fibers (75 μm thickness; Supelco Company, USA). All analyses were performed on a GC-MS with an HP-1 column (30 m × 0.25 mm i.d., 0.25 μm film thickness; Agilent Company, USA). The temperature of the oven was kept at 50 °C for 3 min and then increased to 160 °C at a rate of 4 °C /min. The injector temperature was kept at 200 °C. The carrier gas was helium with a flow rate of 1.0 mL/min. The MS detector was operated in the scan mode with 70 eV electron impact. The peaks in GC-MS chromatograms, which represented the flavor compounds, were identified by NIST standard library searching. The flavor volatiles content was evaluated by total peak areas. Measurements for each sample were conducted in triplicate and the representative examples were selected for discussion.
Sensory Analysis
The sensory analysis method of the flavor profile tests (ISO standards, 1985) was used to quantify the beany flavor in soy protein products [18]. The soy protein products were dissolved in distilled water for 1 h at room temperature to a concentration of 8% (w/v). We chose room temperature for the sensory analysis. This is because some flavor volatiles might evaporate upon heating the soy protein solution to 50 °C (as was observed in the GC-MS analysis), rendering the panelists’ judgment imprecise. Ten trained panelists from the scientific staff of the institute, familiar with beany flavor, were asked to identify the beany notes in the protein products. Between evaluations, distilled water was used for palate clearance. The attribute scores of soy protein solutions were based on a 9-point scale (9–7, weak; 6–5, moderate; 3–4, strong; 1–2, very strong). Each value of flavor or odor reported is the mean of the ten panelists’ scores and the standard deviation for each sample (Table 3).
Color Determination of Soy Protein Products
CIE color system (CIELAB) coordinates were measured to characterize the color of soy protein products. The product was put into an empty and dry weighing bottle, in which the sample surface was flat and horizontal. Chroma Meter CR-400 was used to measure the CIELAB coordinates of each soy protein product according to the method of the International Illumination Commission in 1978 [19]. The three attributes of color in CIE color system were L*, a* and b*, where L* was the lightness variable proportional to value in the Munsell system [20], and a* and b* were chromatic coordinates designating positions on a red/green and yellow/blue axis, respectively (+a = red, −a = green; +b = yellow, −b = blue). The W value representing the whiteness of the protein samples was calculated as follows:
Measurements were performed in triplicate.
Molecular Weight (MW) Determined by High Performance Size Exclusion Chromatography (HPSEC)
Soy protein products were dispersed in 50 mM sodium phosphate buffer solution (50 mM NaCl, pH 7.2) for 1 h to a concentration of 0.5% (w/v). The solution was filtered through a cellulose acetate membrane (Satorious Company, Germany) with a pore size of 0.45 μm. Fifteen microliters of the filtrate were then loaded into a Waters 1525 liquid system equipped with a protein TSK G4000XL Column (7.8 mm × 30 cm; TOSOH Company, Japan) and a Waters 2487 ultraviolet detector. The column, which had an exclusion limit of 7,000 kDa and separation efficiency larger than 20 kDa, was operated at a flow rate of 0.7 mL/min with 50 mM sodium phosphate buffer solution (50 mM NaCl, pH 7.2). The absorbance of the eluent was monitored at 280 nm. The protein standards used for HPSEC analysis were ovalbumin (MW: 43 kDa), conalbumin (MW: 75 kDa), aldolase (MW: 158 kDa) and thyroglobulin (MW: 669 kDa). All standard samples were purchased from GE Healthcare UK Limited (Buckinghamshire, UK). All samples were measured in triplicate and the representative examples were selected for discussion.
Particle Size Distribution Determined by Dynamic Light Scattering
Soy protein products were diluted in 10 mM sodium phosphate buffer solution at pH 7.0 to a concentration of 0.5% (w/v). The solution was centrifuged at 8,000 rpm, and the supernatant was filtered through a cellulose acetate membrane (Satorious Company, Germany) with pore size of 0.45 μm. The particle size distribution of soy protein products was determined using a Malvern Nano-Zetasizer particle size analyzer at 25 °C. Measurements for each sample were performed at least in triplicate and the representative examples were selected as previously described.
Surface Hydrophobicity
Surface hydrophobicity was determined with a fluorescence probe, 1-anilinonaphthalene-8-sulfonic acid (ANS), according to the method of Kato and Nakai [21] modified by Haskard and Li-Chan [22]. In brief, stock solutions of 8 × 10−3 M ANS− and 1.5% (w/v) protein were prepared in phosphate buffer solution (pH 7, I = 0.01 M). The buffer solution (4 mL) and ANS− stock solution (20 μL) were added to 10, 20, 30, 40 and 50 μL of protein solution. Samples were shaken in a vortex mixer for 5 s before measurement. Fluorescence Intensity (FI) was measured at wavelengths of 390 nm (excitation) and 489 nm (emission) using a Shimadzu RF-5301 PC fluorophotometer at 20 ± 0.5 °C with a constant excitation and emission slit of 5 nm. The FI for each sample with probe was computed by subtracting the FI attributed to protein in buffer solution. The initial slope of the FI versus protein concentration plot was calculated by linear regression analysis and used as an index of the sample’s surface hydrophobicity. For each sample, the mean and standard deviation were calculated from at least three measurements.
Solubility
Aqueous solutions (1%, w/v) of protein products were stirred for 1 h. The solutions were adjusted to the desired pH (pH 2–11) with 2.0 N HCl or 2.0 N NaOH and centrifuged at 10,000g for 20 min at 20 °C. After appropriate dilution, the protein content of the supernatants was determined by the Lowry method [23] using bovine serum albumin as the standard.
The solubility of soy protein was calculated as follows:
All determinations were conducted at least in duplicate.
Emulsifying Properties
The emulsifying activity indexes (EAI) and the emulsion stability indexes (ESI) of soy protein products were determined according to the method of Pearce and Kinsella [24]. The mixtures of 1.8 mL protein dispersion (0.1%, w/v, in 0.2 mol/L sodium phosphate buffer solution at pH 7.0) and 0.6 mL corn oil were homogenized for 1 min at 20,000 rpm to prepare the emulsion. An aliquot of the emulsion (100 μL) was immediately taken from the bottom of the homogenized emulsion and diluted (1:50, v/v) in SDS solution (0.1% w/v 0.2 mol/L phosphate buffer at pH 7.0). After being shaken in a vortex mixer for 5 s, the diluted emulsions were loaded into a spectrophotometer and the absorbance was measured at 500 nm. Another emulsion sample (100 μL) was taken ten minutes after the homogenization and measured in the same procedure. EAI and ESI values were calculated by the following equations:
where DF is the dilution factor (50), c is initial concentration of protein (g/mL), φ is optical path (0.01 m) and θ is fraction of oil used to form the emulsion (0.25). A 0 and A 10 are absorbance of the diluted emulsions at 0 and 10 min, respectively. Measurements were performed in triplicate.
Statistical Analysis
An analysis of variance (ANOVA) of the data was performed using the SAS system (version 9.0, SAS Institute Inc., Cary, NC, USA), and Duncan’s multiple-range test with a confidence interval of 95% was used to compare the means.
Results and Discussion
In order to better understand the effect of extraction methods on flavor volatiles and functional properties of soy protein products, we chose AP as the control, because the acid precipitation method is a widely used and traditional method to produce a soy protein product. Our study focuses on the improvement of flavor and functional properties of soy protein products extracted by other methods in comparison to the acid precipitation method.
Chemical Compositions
Protein, total lipids and moisture levels of defatted soy flakes and soy protein products are shown in Table 1. Protein contents (db, dry basis) of different soy protein products were significantly higher than that of defatted soy flakes (85.1–91.1% vs. 55.4%, respectively). The amounts of total lipids (db) in CE, EWHT and OBA/PL-less (2.84, 1.40 and 2.41%, respectively) were significantly lower than that of defatted soy flakes, AP and OBA/PL (5.96, 4.48 and 7.88%, respectively). EWHT exhibited the highest protein content and the least total lipids content among all the soy protein products, which indicated that most lipids in soy flakes were removed by multi-stage countercurrent ethanol washing. EWHT apparently contained less non-protein impurities and consequently, higher protein content. As the control, the method for preparing AP is a conventional and commercial method for producing a soy protein product, and its protein recovery rate is higher [25]. Protein recovery rate and product yield of EWHT were just below those of AP but higher than those of the other soy protein products.
Residual Lipids in Soy Protein Products Prepared by Different Extraction Methods
In the soybean oil extraction process with n-hexane, a considerable amount of oil-body membrane protein is retained in soy flakes [6, 26]. Therefore, membrane-originating proteins may be recovered as lipophilic proteins associated with phospholipids in soy protein products. The results of the lipid components in soy protein products indicated the presence of phospholipids such as phosphatidylethanolamine, phospatidylcholine and phosphatidylinositol (Fig. 2), but the amount of lipids in soy protein products varied with the extraction methods (Table 1). The lipid content of OBA/PL was the highest among all the soy protein products (Table 1), as indicated by an obvious TLC lane (Fig. 2, Lane e), whereas the lipids content of AP was lower than that of OBA/PL, as indicated by a less visible TLC lane (Fig. 2, Lane a). The phospholipids in CE, EWHT and OBA/PL-less were not detected, because the lipids content of these products might be lower than the detection limit of TLC.
TLC analysis of lipids in different soy protein products. Chloroform/methanol (2:1, v/v) was added to each soy protein products. Ten microliters of the extracted sample were then loaded on a silica gel plate. PE phosphatidylethanolamine, PC phosphatidylcholine, PI phosphatidylinositol. Lane S commercial soy lecithin, a AP, b CE, c EWHT, d OBA/PL-less, e OBA/PL
Flavor Volatiles of Soy Protein Products
A total of 15 different volatile flavor components were detected in soy protein products (Table 2), with n-hexanal, 2-heptanone, 1-octen-3-ol, 2-pentyl furan, 2-octenal, nonanal and decanal being the main volatiles. These compounds have been reported as the products of lipoxygenase catalyzed oxidation of polyunsaturated fatty acids [27, 28]. Benzaldehyde, n-hexanol, and other minor components were also reported to be present in soy extract [8, 9, 29, 30]. Flavor volatiles content, which was represented by total peak areas, of EWHT, OBA/PL-less and CE was less than that of AP and OBA/PL. Although it was effective for OBA/PL-less and CE to produce soy protein products with less flavor compounds, the flavor volatiles content of EWHT was the lowest among these products. Furthermore, each peak area of these volatiles from EWHT was much smaller than that from AP and OBA/PL when their content in EWHT, AP and OBA/PL was compared. The whole peak area from EWHT was less than one-third and about one-half of that from OBA/PL and AP, respectively. This decrease in volatiles content in EWHT corresponded with a substantial improvement in the flavor of EWHT in comparison with that of OBA/PL and AP, indicating that ethanol washing and the hydrothermal cooking treatment were very efficient at reducing the odor of soy protein products. The content of 1-octen-3-ol in EWHT (3.14%) was much lower than that in CE, OBA/PL-less, AP and OBA/PL (12.81, 21.07, 26.59 and 75.64%, respectively) (Table 2). The results suggested that 1-octen-3-ol was one of the main flavor compounds, which was also reported by Samoto et al. [6]. The improvement in the flavor of EWHT might be attributed to the removal of the precursor of 1-octen-3-ol and other flavor compounds.
As we pointed out previously, residual lipids and other impurities in soy flakes, which could serve as substrates for lipoxygenase oxidation, were removed during the extraction process of CE, EWHT and OBA/PL-less; thus, these products were lower in flavor volatiles content than AP or OBA/PL. The flavor volatiles content was also consistent with the results of TLC analysis as discussed earlier. The removal of residual lipids may also prolong the shelf life of soy protein products because of the reduction of lipid oxidation and resulting flavor compounds.
Table 3 shows the results of the sensory analysis among AP, CE, EWHT, OBA/PL-less and OBA/PL. Samples were evaluated as 8% dispersions in water and scored on a scale of 1–9 where 9 was bland and 1 was a strong odor or flavor. EWHT received a flavor score of 6.24 and an odor score of 5.88, and was described as beany and astringent. EWHT received the highest score of flavor and odor, which meant that the flavor of EWHT was better than that of the other soy protein products. Meanwhile, the flavor or odor score of OBA/PL was the lowest among these soy protein products, whereas EWHT, CE and OBA/PL-less had better odor than did OBA/PL and AP. This might be related to the lipids content of soy protein products as previously described. The method for production of EWHT reported here would make it easy to produce a soy protein product with less flavor volatiles, which might expand the application to many food items.
Color of Soy Protein Products
There were significant differences in L*, a* and b* data among the soy protein products prepared by five different extraction methods (Table 4). EWHT exhibited the highest degree of whiteness, followed by OBA/PL-less, CE and AP in decreasing order. OBA/PL exhibited the least degree of whiteness. We attributed this to the association of soy protein products with lipids and other impurities (e.g. pigment). The lipids and other colored impurities were removed to some extent in the extraction process of EWHT, OBA/PL-less and CE, resulting in a higher degree of whiteness among these soy protein products.
Molecular Weight Distribution
In order to investigate the molecular weight profile of soy protein products and to gain a clearer understanding of the molecular basis of the improvement in functional properties, we determined the molecular weights and particle size distributions of soy protein products. As shown in Fig. 3, four standard proteins for the column were used to estimate the molecular weights of soy protein products. The molecular weight profiles were similar for the soy protein products of AP, CE, OBA/PL-less and OBA/PL. One peak with molecular weight more than 669 kDa was observed, and the peaks with molecular weights between 669 and 158 kDa were not observed for EWHT. Since the molecular weights for soybean 7 and 11S were about 180 and 350 kDa, respectively, the observed peak was most probably those of protein aggregates. EWHT had a higher content of the protein with molecular weight less than 43 kDa than did the other soy protein products. This may be because 7S is glycoprotein, which is easier to dissociate than 11S with 65% ethanol washing and the hydrothermal cooking treatment [16]. The treatment conditions during the extraction processes of soy protein products such as temperature and organic solvent may result in some denaturation of soy proteins, therefore, soy proteins may aggregate to larger molecules and dissociate to smaller molecules, which can be observed through HPSEC (Fig. 3).
High performance size exclusion chromatogram of soy protein products determined on a protein TSK G4000XL Column. The column was operated at a flow rate of 0.7 mL/min with 50 mM sodium phosphate buffer solution (50 mM NaCl, pH 7.2). The absorbance of the eluent was monitored at 280 nm. Standard proteins: 1 Thyroglobulin (MW: 669 kDa); 2 Aldolase (MW: 158 kDa); 3 Conalbumin (MW: 75 kDa); 4 Ovalbumin (MW: 43 kDa)
Particle size distributions of soy protein products from dynamic light scattering analysis are shown in Fig. 4. The particle sizes of AP, CE, OBA/PL-less and OBA/PL were all around 10 nm as determined by quantitative distribution, but the particle size of EWHT was about 30 nm. This meant that soluble protein aggregates might be formed for EWHT solution, which corresponded to the results from size exclusion chromatography. Gosal et al. [31] reported that two types of aggregates, “string of beads” and “particles” could be formed depending on heating conditions when globular protein solutions were heated. The concentration of the soy protein solution was only 1% (w/v), and the EWHT solution was treated at 121 °C for 30 min; accordingly, the “string of beads” type of aggregate might be formed for the EWHT solution, thus the structure of EWHT became less compact and more flexible, and the particle size of EWHT was larger than that of the other soy protein products.
Surface Hydrophobicity
At a constant pH (e.g. pH 7.0), ANS binds to the exposed hydrophobic surfaces in partially unfolded proteins with much higher affinity than to native or completely unfolded proteins, resulting in an increase in fluorescence emission compared with the emission of free ANS in aqueous solution [32]. The highest surface hydrophobicity was obtained for EWHT, followed by AP, CE, OBA/PL-less and OBA/PL in decreasing order (Fig. 5). Petruccelli et al. [33] reported that treatment conditions, including temperature, time and protein concentration, had an influence on the surface hydrophobicity of protein. The hydrothermal cooking treatment for 30 min at low concentration (1%, w/v) might result in an increased exposure of the hydrophobic group of soy protein to the solvent. The results from HPSEC and dynamic light scattering analysis were consistent with the data of surface hydrophobicity. As we pointed out previously, the EWHT solution contained particles of larger hydrodynamic radii than did the other soy protein products, suggesting that EWHT formed larger protein aggregates with a more flexible structure. The incompact and flexible structure of EWHT might be propitious to the exposure of the hydrophobic group of soy protein in aqueous solution, thus the surface hydrophobicity of EWHT was the highest among these products.
Solubility of Soy Protein Products
The solubility of soy protein products was determined at pH values ranging from 2 to 11 (Fig. 6). The isoelectric points of soy protein products were between pH 4 and pH 5, the usual isoelectric region for most soy protein products. No obvious difference in solubility was observed among EWHT, AP, CE and OBA/PL-less at pH 2–4 and pH 5–11. OBA/PL also showed similar behavior with the other soy protein products at pH 4–5, but it exhibited the lowest solubility at all other pH levels compared to the other soy protein products. The treatment conditions of AP, CE and OBA/PL-less in the extraction processes were mild and resulted in low denatured soy protein products. The soluble aggregates might have formed for EWHT. Therefore, the solubility of EWHT was similar to that of AP, CE and OBA/PL-less. The relatively low solubility of OBA/PL, which might be due to high lipids content in OBA/PL, was similar to the findings reported by Boatright et al. [34]. The transfer of direct free radicals from oxidized lipids to amino acids and proteins resulted in protein denaturation and the formation of insoluble aggregates [35] and consequently, poor solubility.
Emulsifying Properties of Soy Protein Products
The emulsifying ability is dependent on the structure and flexibility of the protein [36]. At neutral pH (pH 7.0), EWHT exhibited the highest EAI (25.20 m2/g), followed by AP (11.49 m2/g), OBA/PL-less (10.67 m2/g), CE (10.67 m2/g) and OBA/PL (8.26 m2/g) (Table 5). The EAI data for these soy protein products were generally consistent with the data of surface hydrophobicity (Table 5; Fig. 5), indicating that the surface hydrophobicity played an important role in the emulsifying ability at neutral pH. Similar results were also reported by Yuan et al. [37]. The emulsifying activities were affected by the solubility of proteins [24], but it could be the surface hydrophobicity that had a major effect on the EAI of soy protein products in our study. EWHT exhibited a significantly higher emulsifying ability compared to the other soy protein products, which might be due to the effect of the surface hydrophobicity.
The ESI data of soy protein products are listed in Table 5. No significant differences were observed in ESI among the products of EWHT, AP, CE and OBA/PL-less. The ESI data of OBA/PL was significantly higher than that of the other soy protein products (40.5 vs. 12.6–25.4 min, respectively), which could be attributed to a higher lipids content in OBA/PL. The lipids in soy protein products affected the emulsion stability of these products, but the effect of lipids on the EAI of soy protein products was apparently not significant. The possible reason was that the differences attributable to lipids content were canceled out by the differences of solubility among these soy protein products, thus lipids had little influence on EAI; however, insoluble protein could contribute to the ESI, therefore the effect of lipids content on ESI were not canceled out, and the emulsion stability of OBA/PL was influenced by its higher lipid content. In addition, higher lipids content of OBA/PL might result in a more stable combination of lipid and protein in itself, which might partly affect the emulsion stability. It indicates that soy protein extraction methods may be considered when targeting desired soy protein emulsifying properties.
Conclusions
The method using ethanol washing in an MSCE system and the hydrothermal cooking treatment produced a soy protein product (EWHT) with better flavor, emulsifying ability and whiteness in comparison with the other extraction methods for producing soy protein products (AP, CE, OBA/PL-less and OBA/PL). Additionally, EWHT had greater yields with lower lipids content, and contained more than 90% protein (db). Our study indicated that the extraction methods used can affect flavor and functional properties of soy protein products. The method for the production of EWHT can be used as an alternative and is capable of producing a soy protein product with better functional properties and a lower flavor volatiles content.
References
Maheshwari P, Murphy PA, Nikolov ZL (1997) Characterization and application of porcine liver aldehyde oxidase in the off-flavor reduction of soy proteins. J Agric Food Chem 45:2488–2494
Kinsella JE (1979) Functional properties of soy proteins. J Am Oil Chem Soc 56:242–258
Wolf WJ (1975) Lipoxygenase and flavor of soybean protein products. J Agric Food Chem 23:136–141
Nash AM, Eldridge AC, Wolf WJ (1967) Fractionation and characterization of alcohol extractables associated with soybean proteins. Nonprotein Components. J Agric Food Chem 15:102–108
Kim H, Ho CT, Chang SS (1984) Isolation and identification of volatile flavor compounds in commercial oil-free soybean lecithin. J Am Oil Chem Soc 61:1235–1238
Samoto M, Miyazaki C, Kanamori J, Akasaka T, Kawamura Y (1998) Improvement of the off-flavor of soy protein isolate by removing oil-body associated proteins and polar lipids. Biosci Biotechnol Biochem 62:935–940
Anderson RL (1974) Ultracentrifugation and binding studies of acid-sensitive soybean proteins. Cereal Chem 51:707–717
Hua YF, Huang YR, Qiu AY, Liu XY (2005) Properties of soy protein isolate prepared from aqueous alcohol washed soy flakes. Food Res Int 38:273–279
Anderson RL, Warner K (1976) Acid-sensitive soy proteins affect flavor. J Food Sci 41:293–296
Boatright WL, Hettiarachchy NS (1995) Effect of lipids on soy protein isolate solubility. J Am Oil Chem Soc 72:1439–1444
Bian YL, Myers DJ, Dias K, Lihono MA, Wu SW, Murphy PA (2003) Functional properties of soy protein fractions produced using a pilot plant-scale process. J Am Oil Chem Soc 80:545–549
Liu XQ, Tsumura K, Kugimiya W, Sato R, Saito Y, Samoto M (2005) Process for producing soy protein. C.N. Patent 1,675,239
Eldridge AC, Wolf WJ, Nash AM, Smith AK (1963) Protein purification, alcohol washing of soybean protein. J Agric Food Chem 11:323–328
Rackis JJ, Honig DJ, Sessa DJ, Steggerda FR (1970) Flavor and flatulence factors in soybean protein products. J Agric Food Chem 18:977–982
Baker EC, Mustakas GC, Warner KA (1979) Extraction of defatted soybean flours and flakes with aqueous alcohols: evaluation of flavor and selected properties. J Agric Food Chem 27:969–973
Zheng HG, Yang XQ, Tang CH, Li L, Ahmad I (2008) Preparation of soluble soybean protein aggregates (SSPA) from insoluble soybean protein concentrates (SPC) and its functional properties. Food Res Int 41:154–164
Samoto M, Maebuchi M, Miyazaki C, Kugitani H, Kohno M, Hirotsuka M, Kito M (2007) Abundant proteins associated with lecithin in soy protein isolate. Food Chem 102:317–322
ISO (1985) Sensory analysis method-flavor profile test, ISO 6564
Friele LFC (1978) Fine color metric (FCM). Color Res Appl 3:53–64
Kuehni RG (2002) The early development of the Munsell System. Color Res Appl 27:20–27
Kato A, Nakai S (1980) Hydrophobicity determined by a fluorescence probe method and its correlation with surface-properties of proteins. Biochim Biophys Acta 624:13–20
Haskard CA, Li-Chan ECY (1998) Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANS) fluorescent probes. J Agric Food Chem 46:2671–2677
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin-phenol reagent. J Biol Chem 193:265–275
Pearce KN, Kinsella JE (1978) Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem 26:716–723
Horan FE (1974) Soy protein products and their production. J Am Oil Chem Soc 51:67A–73A
Herman EM (1987) Immunogold-localization and synthesis of an oil-body membrane protein in developing soybean seeds. Planta 172:336–345
Sessa DJ (1979) Biochemical aspects of lipid-derived flavors in legumes. J Agric Food Chem 27:234–239
Rackis JJ, Sessa DJ, Honig DH (1979) Flavor problems of vegetable food proteins. J Am Oil Chem Soc 56:262–271
Kao JW, Hammond EG, White PJ (1998) Volatile compounds produced during deodorization of soybean oil and their flavor significance. J Am Oil Chem Soc 75:1103–1107
Sessa DJ, Rackis JJ (1977) Lipid-derived flavors of legume protein products. J Am Oil Chem Soc 54:468–473
Gosal WS, Murphy SBR (2000) Globular protein gelation. Curr Opin Colloid Interface Sci 5:188–194
Pallares I, Vendrell J, Aviles FX, Ventura S (2004) Amyloid fibril formation by a partially structured intermediate state of alpha-chymotrypsin. J Mol Biol 342:321–331
Petruccelli S, Añón MC (1995) Thermal aggregation of soy protein isolates. J Agric Food Chem 43:3035–3041
Boatright WL, Hettiarachchy NS (1995) Lipid components that reduce protein solubility of soy protein isolates. J Am Oil Chem Soc 72:1445–1451
Saeed S, Fawthrop SA, Howell NK (1999) Electron spin resonance (ESR) study on free radical transfer in fish lipid–protein interaction. J Sci Food Agric 79:1809–1816
Nazareth ZM, Deak NA, Johnson LA (2009) Functional properties of soy protein isolates prepared from gas-supported screw-pressed soybean meal. J Am Oil Chem Soc 86:315–321
Yuan DB, Yang XQ, Tang CH, Zheng ZX, Min W, Ahmad I, Yin SW (2009) Physicochemical and functional properties of acidic and basic polypeptides of soy glycinin. Food Res Int 42:700–706
AOAC (1995) Official methods of analysis of the Association of Official Analytical Chemists, 16th edn. AOAC, Arlington
Acknowledgments
This work was a part of the research project of the National Natural Science Foundation of China (NO. 20776050).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wu, N., Wang, L., Yang, X. et al. Comparison of Flavor Volatiles and Some Functional Properties of Different Soy Protein Products. J Am Oil Chem Soc 88, 1621–1631 (2011). https://doi.org/10.1007/s11746-011-1825-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1825-9