Abstract
Palmitic acid, a main fatty acid (FA) in human nutrition, can induce apoptosis of cardiomyocytes. However, a specific combination of palmitic, myristic and palmitoleic acid (CoFA) has been reported to promote beneficial cardiac growth. The aim of this study was to investigate the relevance of CoFA for cardiac growth and to delineate the underlying signaling pathways of CoFA and palmitic acid treatment. CoFA treatment of C57Bl/6 mice increased FA serum concentrations. However, morphologic and echocardiographic analysis did not show myocardial hypertrophy. Cell culture studies using rat ventricular cardiomyocytes revealed an increased phosphorylation of AMP activated protein kinase α (AMPKα) to 155 ± 19% and its target acetyl-CoA-carboxylase to 177 ± 23% by CoFA. Treatment with myristic acid also increased AMPKα phosphorylation to 189 ± 32%. Palmitic acid did not activate AMPKα but increased expression of the FA translocase CD36 (FAT/CD36) to 163 ± 23% and adipose-differentiation-related-protein (ADRP), a sensitive marker of lipid accumulation, to 168 ± 42%. This was associated with an increased phosphorylation of the stress-activated-protein-kinase/Jun-amino-terminal-kinase (SAPK/JNK) to 173 ± 27%. In CoFA-treated cells, phosphorylation of SAPK/JNK was unaltered. FACS analysis revealed increased apoptosis to 159 ± 5% by palmitic acid but not by CoFA. AMPK activator AICAR (5-aminoimidazole-4-carboxamide ribonucleotide) prevented up-regulation of ADRP and increased apoptosis by palmitic acid. Confirming these findings, inhibition of AMPK by compound C in CoFA-treated cardiomyocytes resulted in an increased expression of ADRP to 154 ± 27%, FAT/CD36 to 167 ± 28% and apoptosis to 183 ± 12%. These data reveal that AMPK activation plays an important role in prevention of palmitic acid-induced apoptosis and lipid accumulation in cardiomyocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac hypertrophy develops as an adaptive or a maladaptive response to increased workload. Physiological hypertrophy can occur in highly trained athletes [1]. Pathological hypertrophy can be a result of chronically increased afterload, e.g. in patients with hypertension or aortic stenosis, and predisposes for chronic heart failure [2, 3]. Under physiological conditions, fatty acids are the main energy source for cardiomyocytes. More than half of the cellular ATP is produced by beta oxidation of saturated and mono unsaturated fatty acids [4, 5]. An increased energy demand leads to a shift of substrate preference. Maladaptive myocardial hypertrophy is characterized by increased glucose uptake and utilisation and decreased fatty acid oxidation [6,7,8]. The physiological balance between carbohydrate and fatty acid utilisation is important to maintain contractile cardiac function [9].
Fatty acids are not only an important source of energy. An increase in cellular fatty acid uptake can lead to lipid accumulation and cardiac dysfunction. The failing hypertrophied heart is characterized by an impaired beta oxidation and a resulting lipotoxic cardiomyopathy [10]. The clinical importance of lipotoxicity is observed in animal models and in humans [5]. Obesity and insulin resistance lead to increased cardiac lipid content, cardiac remodelling and impaired diastolic function [11, 12]. Interestingly, there is no lipid accumulation in the exercise training-induced hypertrophic heart [13]. Furthermore, fatty acids and fatty acid derivatives are able to act as signalling molecules in several pathways [5, 14].
A central regulator of cellular energy metabolism is the AMP activated protein kinase (AMPK) which is responsive to metabolic conditions [15]. The main function of AMPK in the myocardium is to minimize energy demanding processes and to enhance energy providing processes [16, 17]. The AMP/ATP ratio increases with energy consumption and activates AMPK [17]. AMPK consists of a catalytic alpha subunit and regulatory beta and gamma subunits with several isoforms [18]. An important downstream target of AMPK is the acetyl CoA carboxylase (ACC). ACC is a regulator of fatty acid oxidation. ACC is inhibited by phosphorylation through AMPK which leads to increased fatty acid oxidation [19]. Furthermore AMPK activation is involved in translocation of the fatty acid translocase CD36 (FAT/CD36) from intracellular stores to the plasma membrane [20]. FAT/CD36 is of importance for the uptake of long chain fatty acids into cardiac myocytes. Increased expression of FAT/CD36 under obese conditions is associated with intracellular lipid accumulation and insulin resistance [21]. However, the influence of AMPK on FAT/CD36 expression is incompletely understood, as there is also evidence for an AMPK independent regulation of FAT/CD36 expression [22]. Fatty acids can activate AMPK by allosteric effects [23]. In addition, AMPK increases glucose uptake by membrane translocation of glucose transporter 4 (GLUT-4) which is associated with increased glucose uptake and glycolysis [20, 24, 25]. Many studies show the importance of AMPK in metabolic changes in the post-ischemic heart [19, 26]. However, data about its role in the hypertrophied heart are sparse.
Specific fatty acids have been suggested to induce adaptive myocardial hypertrophy. Riquelme et al. showed that the Burmese python, after a large meal, develops rapid and pronounced cardiac hypertrophy that is reversible [27]. The snakes did not exhibit common maladaptive characteristics of pathological hypertrophy such as fibrosis and apoptosis. The cardiomyocytes showed no lipid accumulation despite high postprandial serum concentrations of fatty acids. The authors identified a specific combination of the fatty acids, namely palmitoleic (16:1), myristic (14:0) and palmitic acid (16:0) in the molar ratio of 1:6:16, to be responsible for physiological cardiac growth in both pythons and mice. This specific combination of fatty acids (CoFA) increased fatty acid transport and oxidation as well as expression of cardioprotective enzymes. However, isolated treatment with palmitic acid, an important fatty acid occurring in human nutrition, has been observed to induce cell apoptosis [28, 29].
Heart failure is associated with profound changes in fatty acid metabolism and supply. However, the physiological and pathophysiological effects of fatty acids on the myocardium are incompletely understood. The aim of this study was therefore to investigate the effect of CoFA and palmitic acid on cardiac growth and myocardial apoptosis and lipid metabolism. As AMPK is a main regulator of cellular energy homeostasis, we tested whether the observed metabolic changes may be regulated by AMPK.
Materials and Methods
Animal Studies
Preparation of Fatty Acids Solutions
Palmitoleic, myristic and palmitic acid were conjugated separately to initially fatty acid free bovine serum albumin (BSA) and combined in the molar ratio of 1:6:16. The fatty acids were dissolved in ethanol. Na2CO3 and nitrogen gas were added at a temperature of 60 °C until the ethanol was completely evaporated. After addition of 30 µM 10% fatty acid free BSA, the fatty acids were dialysed in 0.1 M (NH4)HCO3 and lyophilised. Concentrations of the fatty acids were determined by gas chromatography as described [30], then they were put together in the mentioned molar ratio (CoFA).
Fatty Acid Treatment
C57/Bl6 mice were treated with CoFA as described by Riquelme et al. [27]. In n = 5 mice, CoFA was applied via subcutaneous mini osmotic pumps (ALZET® model 2001, 1 µl/h) for 7 days. The control group of n = 5 mice underwent sham operations. In a second experiment, C57/Bl6 mice (n = 24) were treated with either CoFA or a fatty acid free control BSA solution by daily subcutaneous injection (24 µl) over 5 weeks, then underwent either a transaortic construction or a sham operation. Each treatment was followed by echocardiographic analysis and isolation of the hearts. Fatty acid serum concentrations were quantified by gas chromatography with mass spectrometry.
Transaortic Constriction (TAC)
TAC procedures were performed as described [31]. After thoracotomy, an Ethicon thread 6–0 was put around the aorta ascendens and a knot was set with the help of a 24G needle to define the aortic diameter. Sham operated mice underwent identical anaesthesia with ketamine–xylazine and a full thoracotomy.
Histology
The hearts were put into paraffin and cut in 3 µm thick layers. These patterns underwent standard haematoxylin–eosin staining for cell size measurement.
Cell Culture Studies
Isolation of Ventricular Rat Cardiomyocytes
Animal experiments were approved by the animal ethics committee of the Universität des Saarlandes. Sprague–Dawley rats (Charles River) were kept under usual conditions. Ventricular cardiomyocytes were isolated from 3- to 5-day-old rats as described [32, 33]. Briefly, the ventricles were digested using an enzyme mixture containing collagenase type 2 (Worthington Biochemical, Cell Systems) and pancreatin (Sigma-Aldrich Chemie, München). The isolated myocytes were centrifuged for 5 min at 700 rpm and filtered to extract fibroblasts. After incubation for 1 h in F10 medium (Gibco, Invitrogen, Karlsruhe) containing 10% Horse-Serum, 5% Fetal calf serum and 1% Penicillin/Streptomycin, remaining fibroblasts adhered to the bottom of the petri dishes. The overlap with the isolated cardiomyocytes was removed and distributed in 6-well culture trays (BD Falcon 6-Well Plate 353846, BD, Franklin Lakes, NJ, USA). Cells were counted in Neubauer counting chambers after marking dead cells with trypan blue.
Fatty Acid Treatment of Isolated Cardiomyocytes
On day 2 after cardiomyocyte isolation, the medium was exchanged for a serum free medium and 50 or 100 µM of the fatty acids palmitoleic, myristic and palmitic acid, as well as the combination (CoFA), were added for 48 h. Control cells were treated with 30 µM 10% fatty acid free BSA. The AMPK activator AICAR was added at 1 mM and the AMPK inhibitor Compound C at 10 µM. Both were put into the medium 1 h before the start of fatty acid treatment. H9C2 rat cardiac myoblasts were grown in F10 medium (Gibco, Invitrogen, Karlsruhe) and treated under identical conditions.
Western Blot Analyses
Proteins were separated by SDS polyacrylamide gel electrophoresis as described [34]. Proteins were transferred to nitrocellulose membranes using a Biorad Mini Trans Blot System (350 mA, 2 h). Membranes were incubated with primary and peroxidase conjugated secondary antibodies. Antibody bounded proteins were visualized by enhanced chemiluminescence using ECL western blotting detection reagents (GE Healthcare). Band intensities were analysed by densitometry with UVP Labworks (Version 4.6.00.0).
Flow Cytometric Analyses
The cells were cells were extracted from the 6-well plates with trypsin, centrifuged in F10 medium and analysed using the Annexin V-FITC apoptosis detection kit as described [35]; 25,000 cells of each sample were counted in the flow cytometer.
Statistical Analyses
All values are expressed as mean ± SEM. Groups were tested for normal distribution. Student’s t tests, Mann–Whitney U tests and one-way ANOVA for multiple comparisons were applied. Post hoc comparisons were performed with the Bonferroni’s multiple comparison test. Data were statistically analysed with GraphPad Prism software 6.0 (GraphPad Software Inc., CA, USA). All results are shown in percent of control. Differences were considered significant at p < 0.05.
Results
Fatty Acid Combination Exerted No Effect on Cardiac Hypertrophy in C57/Bl6 Mice
Treatment of C57/Bl6 mice for 7 days with the fatty acids palmitoleic (16:1), myristic (14:0) and palmitic acid (16:0) in the molar ratio of 1:6:16 (CoFA) [27] via osmotic pumps led to increased serum concentrations of myristic, palmitic and palmitoleic acid (Fig. 1a–c). However, cardiac dimensions measured by echocardiography and cardiomyocyte size, quantified by haematoxylin–eosin staining after 7 days, remained unaffected (Table 1a; Fig. 1d). There was no difference in heart weight between the control group and the CoFA-treated mice (cardiac weight/tibia length control vs. CoFA 7 days: 0.0783 ± 0.001 vs. 0.0786 ± 0.013). CoFA treatment over 5 weeks increased cell area to 130% (458.29–593.72 µm2, Fig. 1e), but the effect was not statistically significant. Heart weights were not affected by CoFA after 5 weeks (cardiac weight/tibia length Control Sham vs. CoFA Sham: 0.0752 ± 0.004 vs. 0.0737 ± 0.004). As expected, transaortic constriction (TAC)-induced myocardial hypertrophy after 5 weeks (Fig. 1e) [33]. CoFA did not influence this effect (cardiac weight/tibia length Control TAC vs. CoFA TAC: 0.0934 ± 0.005 vs. 0.0979 ± 0.005, Fig. 1e; Table 1b).
Effect of CoFA on cardiac hypertrophy in C57/Bl6 mice in vivo and in neonatal cardiomyocytes in vitro. Representative gas chromatography with mass spectrometry (GCMS) analysis of the serum concentration of the fatty acids a myristic, b palmitic and c palmitoleic acid in C57/Bl6 mice after 7 days treatment with CoFA. Representative analysis of cardiomyocyte area after hematoxylin–eosin staining of C57/Bl6 mice after treatment with CoFA for d 7 days (n = 10) and e 5 weeks (n = 22). f Microscopic analysis of neonatal cardiomyocyte size after 48 h of incubation with CoFA, palmitoleic, myristic and palmitic acid, n = 65–93. TAC transaortic constriction (*p < 0.05; **p < 0.01)
Myristic Acid-Induced Cellular Hypertrophy
Riquelme et al. described an increased cell size in cell culture experiments after treatment with CoFA [27]. Therefore, cultured primary rat cardiomyocytes were treated with 100 µM CoFA as well as palmitoleic, myristic and palmitic acid for 48 h. Only cells treated with myristic acid exhibited mild hypertrophy (Fig. 1f).
Palmitic Acid But Not CoFA Increased Apoptosis of Cardiomyocytes
Flow cytometry analysis revealed an increase of cardiomyocyte apoptosis to 159 ± 5% by 48 h treatment with palmitic acid at 50 µM and to 292 ± 29% by palmitic acid at 100 µM. CoFA did not influence apoptosis (Fig. 2).
CoFA and Myristic Acid, But Not Palmitic Acid, Increased Phosphorylation of AMPKα and ACC
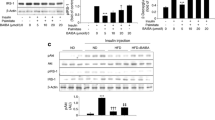
AMP activated protein kinase (AMPK) is an important regulator of cellular energy demand that is regulated by metabolic stress [15]. AMPK is activated by phosphorylation [36, 37]. Western blot analysis showed an increased phosphorylation of AMPKα at threonine 172 in CoFA-treated cardiomyocytes to 155 ± 19% (Fig. 3a). To test whether this observation also applies for adult cardiac myocytes, H9C2 cells were treated with CoFA under the same conditions as described for the neonatal cardiomyocytes. CoFA led to a similar increase of AMPKα phosphorylation in H9C2 cells (162.5 ± 25%, Fig. 4a). Palmitic acid did not increase AMPKα phosphorylation in neonatal cardiomyocytes. Pre-incubation of palmitic acid-treated cells with the AMPK activator AICAR increased phosphorylation to 170 ± 30% (Fig. 3a). In addition, treatment with myristic acid increased AMPK phosphorylation to 189 ± 32%, which was prevented by the AMPK inhibitor compound C (Fig. 5a). AMPKα expression was unaffected under all conditions (Figs. 3b, 4b, 5b). An important downstream target and main regulator of fatty acid oxidation is Acetyl-CoA-carboxylase (ACC), which inhibits fatty acid oxidation by Malonyl-CoA production. AMPK inactivates ACC by phosphorylation and increases fatty acid oxidation [19]. Consistent with the results described above, CoFA, but not palmitic acid, increased ACC phosphorylation to 177 ± 23%. Pre-incubation of palmitic acid-treated cardiomyocytes with AICAR led to an increased ACC phosphorylation to 242 ± 33% (Fig. 3c). CoFA and palmitic acid treatment had no effect on total ACC expression (Fig. 3d).
Effect of treatment with CoFA, palmitic acid and AMPK activator AICAR for 48 h on phosphorylation and expression of AMP-activated kinase α (AMPKα) and on phosphorylation and expression of acetyl CoA carboxylase (ACC). Representative western blot analysis and quantification of a phosphorylation and b expression of AMPKα and c phosphorylation and d expression of ACC in cardiomyocytes. n = 5–29 (*p < 0.05; **p < 0.01; ***p < 0.001)
AMPK Activator AICAR Prevented the Palmitic Acid-Induced Increased Expression of the Fatty Acid Translocase FAT/CD36 and Markers of Lipid Accumulation
Fatty acid translocase CD36 (FAT/CD36) is responsible for the uptake of more than 50% of fatty acids in the heart [38]. Treatment of cardiomyocytes with palmitic acid led to an increased expression of FAT/CD36 to 163 ± 23%. Neither CoFA nor AICAR increased the expression of this fatty acid translocase (Fig. 6a). These data indicated an increased fatty acid uptake by treatment with palmitic acid, while fatty acid oxidation remained unaffected (no change in ACC phosphorylation). Subsequent analysis of expression of adipose differentiation related protein (ADRP), a sensitive marker for lipid accumulation, showed an increase after treatment with palmitic acid to 168 ± 42%, but no effect of CoFA or AICAR (Fig. 6b).
Effect of treatment with CoFA, palmitic acid and AMPK activator AICAR for 48 h on expression of fatty acid translocase CD36 (FAT/CD36) and lipid accumulation marker adipose differentiation related protein (ADRP) and on phosphorylation of stress activated protein kinase (SAPK/JNK). Representative Western Blot analysis and quantification of expression of a FAT/CD36 (n = 14–21) and b ADRP (n = 8–23) and c phosphorylation of SAPK/JNK (n = 5–15) in cardiomyocytes. (*p < 0.05; **p < 0.01; ***p < 0.001)
AMPK Activator AICAR Prevented Palmitic Acid-Induced Apoptosis and Activation of the Stress Activated Protein Kinase SAPK/JNK
Flow cytometry analysis showed that apoptosis in cardiomyocytes increased by 159 ± 5 and 292 ± 29% after treatment with palmitic acid at 50 and 100 µM, respectively. AICAR completely prevented the palmitic acid-induced apoptosis (Fig. 2). Palmitic acid, but not CoFA, increased phosphorylation of SAPK/JNK at Thr 183 and Thr 185 to 172 ± 22 and 173 ± 27%, respectively. AICAR prevented the increased phosphorylation in palmitic acid-treated cells (Fig. 6c).
Inhibition of AMPK by Compound C was Associated with Increased Apoptosis, Fatty Acid Translocase CD36 and Markers of Lipid Accumulation
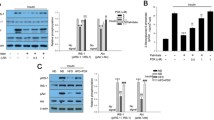
To verify the role of AMPK as an important mediator in the prevention of fatty acid-induced apoptosis, CoFA-treated cardiomyocytes were pre-incubated with the AMPK inhibitor Compound C. Inhibition of AMPK increased apoptosis to 183 ± 12% (Fig. 7c, d) and expression of FAT/CD36 to 167 ± 28% (Fig. 7a) and ADRP to 154 ± 27% (Fig. 7b).
Effect of CoFA and AMPK inhibitor Compound C for 48 h on apoptosis and the expression of the fatty acid translocase CD36 and lipid accumulation marker ADRP in cardiomyocytes. Representative western blot analysis and quantification of expression of a FAT/CD36 (n = 6) and b ADRP (n = 6–23). c Flow cytometry analysis of apoptosis of cardiomyocytes using Annexin V (n = 12–32). d Representative histograms and dot plots of flow cytometry analysis with Annexin V. (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Discussion
This study provides novel insight into the importance of fatty acids for cardiomyocyte hypertrophy. The main findings are that palmitic acid induces cardiomyocyte apoptosis, cytotoxic lipid accumulation and activation of the SAPK/JNK pathway. The experiments did not fully reproduce the previously described induction of cardiac growth in the Burmese python and in mice induced by the combination of the fatty acids myristic, palmitic and palmitoleic acid (CoFA) [24; Riquelme et al. Science 2011]. Our data show that myristic acid induces hypertrophy of rat ventricular cardiomyocytes, whereas CoFA prevents palmitic acid-induced lipid accumulation and apoptosis. Importantly, the study identifies AMPK as the central mediator of these cardio-protective effects (Fig. 8).
Summary. Schematic summary of the results. P-AMPK is main regulator of cellular energy homeostasis. P-AMPK inhibits ACC activity by phosphorylation. Active ACC inhibits beta oxidation by malonyl-CoA production. CoFA activates AMPK and lowers cell apoptosis. Myristic acid activates AMPK and induces hypertrophic cell growth. Palmitic acid increases expression of FAT/CD36 and of the lipid accumulation marker ADRP independent of AMPK. Furthermore, it induces apoptosis via SAP/JNK and a cytotoxic lipid accumulation. CoFA combination of the fatty acids myristic, palmitic and palmitoleic acid, SAPK/JNK stress-activated protein kinase/Jun-amino-terminal kinase, AMPK–AMP activated protein kinase, FAT/CD36 fatty acid translocase CD36, ADRP adipose differentiation related protein, ACC acetyl-CoA-carboxylase
The python has to survive long fasting periods between big meals. The result is the adaptation of the animal’s metabolism to an extreme metabolic situation. For example, the mass of the digestive tract increases up to a quarter of the body weight in the first 24–48 h post feeding [39]. Riquelme et al. observed that the Burmese python develops a significant postprandial cardiac growth [27, 40]. This adaptive cardiac hypertrophy was reversible [40]. The specific combination of the fatty acids myristic, palmitic and palmitoleic acid (CoFA) was reported to be responsible for the physiological cardiac growth in the pythons and in mice [27]. Despite achieving appropriate serum concentrations by treatment with the specific fatty acid mix, C57/Bl6 mice in this study did not exhibit increased heart weight nor cardiac hypertrophy in careful echocardiographic analyses. Afterload induced by aortic constriction increased cardiomyocyte size, however this was not altered by CoFA treatment. After 5 weeks treatment with CoFA, cardiomyocyte size showed a non-significant increase similar to the size effect in the in vivo experiments reported by Riquelme et al. In vitro, Riquelme et al. observed a small increase in cell diameter by 4.43%, which is similar to the minor 4.98% increase of cell area in this study. The data of this study, therefore, show a trend similar to that published by Riquelme et al. The lack of statistical significance is most likely explained by a biological variation. Physiologically, in animals and in humans, lipids are taken up in the gut via chylomicrons and then enter the lymphatic system. However we decided to follow the protocol described by Riquelme et al., who found elevated fatty acid levels in the python’s plasma and treated mice by subcutaneous infusion of the fatty acids. In our study, CoFA did not increase cardiac mass. While these experiments were ongoing, Jensen et al. reported no changes in ventricular mass or dimensions in the hearts of Python regius and Python molurus, despite using a meal size and animal husbandry conditions very similar to those reported by Riquelme et al. [39]. The difficulty of reproducing the data encountered by different groups using multiple approaches remains incompletely explained. Jensen et al. concluded that postprandial cardiac growth may have to be regarded as a facultative rather than an obligatory component of the postprandial response [39].
Our data show that myristic, but not palmitic or palmitoleic, acid leads to cardiomyocyte hypertrophy. These findings are in agreement with observations of Russo et al. in isolated murine cardiomyocytes [41]. Furthermore, our study shows that treatment of cardiomyocytes with palmitic acid increases apoptosis. This was associated with increased phosphorylation of stress activated protein kinase (SAPK/JNK). SAPK/JNK is activated by metabolic stress and, itself, leads to cell apoptosis [28]. We postulate that palmitic acid initiates cell apoptosis by activation of SAP/JNK pathways. These results are confirmed by the study of Li et al., who demonstrated the association between palmitic acid-induced apoptosis and activation of SAPK/JNK, and its reversibility by activating AMP activated protein kinase (AMPK)-dependent pathways [42]. JNK signalling is also associated with a reduced capacity for fatty acid oxidation and cardiomyopathy [43]. Both SAPK/JNK activation and increased apoptosis induced by palmitic acid were prevented by CoFA. To investigate the underlying mechanisms, activation of AMPK as an important regulator of cellular energy homeostasis was assessed.
Western blot analysis revealed an increased activation of AMPK by CoFA. As allosteric binding of fatty acids to AMPK has been described, direct activation of AMPK by the fatty acids is possible, but further investigation has to be done to clarify this mechanism [23]. Watkins et al. showed that the hypertrophic response of neonatal and adult cardiomyocytes, using H9C2 cells to established hypertrophic factors such as angiotensin II, are very similar [44]. We therefore repeated the key experiments in H9C2 cardiomyocytes to test the reproducibility of the findings and found very similar effects, suggesting that the data that the observations made on neonatal cardiomyocytes in this study are transferable to adult cardiac myocytes. Palmitic acid failed to activate AMPK, but AMPK activator AICAR increased AMPK phosphorylation in palmitic acid-treated cells. In contrast, myristic acid increased phosphorylation of AMPK to 189 ± 32%. We therefore hypothesize that myristic acid plays an important role in the mediation of the metabolic changes described for CoFA. Accordingly, CoFA and AICAR increased phosphorylation of Acetyl-CoA carboxylase (ACC), an important downstream target of AMPK and main regulator of fatty acid oxidation. Phosphorylation of ACC inhibits its carboxylase activity and consequently malonyl-CoA production, which leads to an increased fatty acid oxidation and reduced intracellular fatty acid levels [19]. On the other hand, decreasing fatty acid oxidation by deletion of Peroxisome proliferator-activated receptor delta (PPAR-delta) in mice leads to cardiac lipid accumulation and heart failure [45]. Palmitic acid had no effect on ACC but increased expression of the fatty acid translocase CD36 (FAT/CD36), which is responsible for the uptake of more than 50% of the fatty acids in the heart [20]. This transport protein is essential for the development of lipotoxic cardiomyopathy. CD36-knockout mice are protected from cardiac triglyceride accumulation and dysfunction through overexpression of PPAR-alpha [46]. AMPK is able to increase membrane expression of FAT/CD36 [20]. However, Jeppesen et al. showed that AMPK kinase dead mice were able to elevate translocation of FAT/CD36 to the membrane and consecutively increase fatty acid uptake under exercise in skeletal muscle [22]. This suggests AMPK independent pathways that may regulate membrane expression of FAT/CD36. In addition, FAT/CD36 is able to suppress AMPK. Further investigations are necessary to describe the mechanisms leading to an increased FAT/CD36 expression without AMPK activation in palmitic acid-treated cells. Taken together, these findings suggest that palmitic acid increases fatty acid uptake without influencing its oxidation. Consequently, adipose differentiation related protein (ADRP), a sensitive marker for lipid accumulation [47, 48], was elevated through palmitic acid treatment. Further studies demonstrate a cytotoxic lipid accumulation by palmitic acid in cardiomyocytes leading to increased apoptosis [49, 50]. Our data therefore contribute to the understanding of palmitic acid-induced pro-apoptotic signalling. Cytotoxic metabolites, such as ceramides and diacylglycerols, and reactive oxygen species (ROS) may contribute to the observed cell death [5, 51,52,53]. AMPK activation in palmitic acid-treated cells prevented elevated FAT/CD36, ADRP expression and, consequently, palmitic acid-induced apoptosis. CoFA caused increased fatty acid oxidation without elevating fatty acid uptake. Consequently, lipid accumulation by palmitic acid was prevented. To verify the mechanism of palmitic acid-induced cytotoxicity with AMPK as the central regulator, AMPK was inhibited by Compound C in cells treated with CoFA. In agreement with the findings described above, inhibition of AMPK prevented the beneficial effects of CoFA and led to increased apoptosis, expression of FAT/CD36 and lipid accumulation indicated by up-regulation of ADRP.
Potential limitations of the study: The experiments in mice and in isolated rat cardiomyocytes are established models for investigating cellular metabolism and signalling, however, these experimental data cannot be extrapolated directly to humans or the effects of human nutrition. Neonatal ventricular myocytes use glycolysis for energy production. Therefore, adult H9C2 cells were tested to confirm the observations in an independent cell line. This study shows that increased cardiac mass is not a mandatory effect of CoFA. However, potential additional influencing factors in the study of Riquelme et al. [27] that were absent in our extensive experiments cannot be fully excluded.
In summary, the combination of the fatty acids palmitic, myristic and palmitoleic acid (CoFA) leads to mild cardiac hypertrophy in vivo and in vitro. Myristic acid by itself induces hypertrophy of rat ventricular cardiomyocytes in vitro. Palmitic acid-induced apoptosis is mediated by cytotoxic lipid accumulation and activation of SAPK/JNK pathways. Both lipid accumulation and apoptosis are prevented by CoFA. The study identifies AMPK as the central mediator of these cardio-protective effects.
Abbreviations
- CoFA:
-
Combination of the FA palmitic, myristic and palmitoleic acid
- FA:
-
Fatty acid
- AMPK:
-
AMP activated protein kinase
- ACC:
-
Acetyl-CoA-carboxylase
- FAT/CD36:
-
Fatty acid translocase CD36
- ADRP:
-
Adipose differentiation related protein
- SAPK/JNK:
-
Stress-activated protein kinase/Jun-amino-terminal kinase
- AICAR:
-
5-Aminoimidazole-4-carboxamide ribonucleotide
- GLUT-4:
-
Glucose transporter 4
- BSA:
-
Bovine serum albumin
- TAC:
-
Transaortic constriction
- PPAR:
-
Peroxisome proliferator-activated receptor
- ROS:
-
Reactive oxygen species
References
Kemppainen J, Fujimoto T, Kalliokoski KK, Viljanen T, Nuutila P, Knuuti J (2002) Myocardial and skeletal muscle glucose uptake during exercise in humans. J Physiol 542(Pt 2):403–412
Calderone A, Takahashi N, Izzo NJ Jr, Thaik CM, Colucci WS (1995) Pressure- and volume-induced left ventricular hypertrophies are associated with distinct myocyte phenotypes and differential induction of peptide growth factor mRNAs. Circulation 92(9):2385–2390
Gidh-Jain M, Huang B, Jain P, Gick G, El-Sherif N (1998) Alterations in cardiac gene expression during ventricular remodeling following experimental myocardial infarction. J Mol Cell Cardiol 30(3):627–637
de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M (1997) Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res 38(7):1384–1394
Drosatos K, Schulze PC (2013) Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr Heart Fail Rep 10(2):109–121
Stuck BJ, Lenski M, Bohm M, Laufs U (2008) Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J Biol Chem 283(47):32562–32569
Kagaya Y, Kanno Y, Takeyama D, Ishide N, Maruyama Y, Takahashi T, Ido T, Takishima T (1990) Effects of long-term pressure overload on regional myocardial glucose and free fatty acid uptake in rats. A quantitative autoradiographic study. Circulation 81(4):1353–1361
Lehman JJ, Kelly DP (2002) Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Fail Rev 7(2):175–185
Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF (2002) Energy metabolism in the hypertrophied heart. Heart Fail Rev 7(2):161–173
Heggermont WA, Papageorgiou AP, Heymans S, van Bilsen M (2016) Metabolic support for the heart: complementary therapy for heart failure? Eur J Heart Fail 18(12):1420–1429
Utz W, Engeli S, Haufe S, Kast P, Hermsdorf M, Wiesner S, Pofahl M, Traber J, Luft FC, Boschmann M, Schulz-Menger J, Jordan J (2011) Myocardial steatosis, cardiac remodelling and fitness in insulin-sensitive and insulin-resistant obese women. Heart (British Cardiac Society) 97(19):1585–1589
Lopaschuk GD (2016) Fatty acid oxidation and its relation with insulin resistance and associated disorders. Ann Nutr Metab 68(Suppl 3):15–20
Dobrzyn P, Pyrkowska A, Duda MK, Bednarski T, Maczewski M, Langfort J, Dobrzyn A (2013) Expression of lipogenic genes is upregulated in the heart with exercise training-induced but not pressure overload-induced left ventricular hypertrophy. Am J Physiol Endocrinol Metab 304(12):E1348–E1358
Lopaschuk GD, Ussher JR (2016) Evolving concepts of myocardial energy metabolism: more than just fats and carbohydrates. Circ Res 119(11):1173–1176
Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE (1996) Mammalian AMP-activated protein kinase subfamily. J Biol Chem 271(2):611–614
Verhoeven AJ, Woods A, Brennan CH, Hawley SA, Hardie DG, Scott J, Beri RK, Carling D (1995) The AMP-activated protein kinase gene is highly expressed in rat skeletal muscle. Alternative splicing and tissue distribution of the mRNA. Eur J Biochem 228(2):236–243
Ponticos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D (1998) Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J 17(6):1688–1699
Liu WY, Jiang RS (2013) Advances in the research of AMPK and its subunit genes. Pak J Biol Sci PJBS 16(22):1459–1468
Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD (1996) Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochem Biophys Acta 1301(1–2):67–75
Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF (2003) Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes 52(7):1627–1634
Glatz JF, Angin Y, Steinbusch LK, Schwenk RW, Luiken JJ (2013) CD36 as a target to prevent cardiac lipotoxicity and insulin resistance. Prostaglandins Leukot Essent Fatty Acids 88(1):71–77
Jeppesen J, Albers PH, Rose AJ, Birk JB, Schjerling P, Dzamko N, Steinberg GR, Kiens B (2011) Contraction-induced skeletal muscle FAT/CD36 trafficking and FA uptake is AMPK independent. J Lipid Res 52(4):699–711
Watt MJ, Steinberg GR, Chen ZP, Kemp BE, Febbraio MA (2006) Fatty acids stimulate AMP-activated protein kinase and enhance fatty acid oxidation in L6 myotubes. J Physiol 574(Pt 1):139–147
Russell RR 3rd, Bergeron R, Shulman GI, Young LH (1999) Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol 277(2 Pt 2):H643–H649
Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L (2000) Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol CB 10(20):1247–1255
Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Vanoverschelde JL, Ashworth A, Jovanovic A, Alessi DR, Bertrand L (2006) Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol Metab 290(5):E780–E788
Riquelme CA, Magida JA, Harrison BC, Wall CE, Marr TG, Secor SM, Leinwand LA (2011) Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science (New York, N.Y.) 334(6055):528–531
Miller TA, LeBrasseur NK, Cote GM, Trucillo MP, Pimentel DR, Ido Y, Ruderman NB, Sawyer DB (2005) Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochem Biophys Res Commun 336(1):309–315
Mancini A, Imperlini E, Nigro E, Montagnese C, Daniele A, Orru S, Buono P (2015) Biological and nutritional properties of palm oil and palmitic acid: effects on health. Molecules (Basel, Switzerland) 20(9):17339–17361
Bartelt A, Orlando P, Mele C, Ligresti A, Toedter K, Scheja L, Heeren J, Di Marzo V (2011) Altered endocannabinoid signalling after a high-fat diet in Apoe(−/−) mice: relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia 54(11):2900–2910
Adam O, Frost G, Custodis F, Sussman MA, Schafers HJ, Bohm M, Laufs U (2007) Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol 50(4):359–367
Laufs U, Kilter H, Konkol C, Wassmann S, Bohm M, Nickenig G (2002) Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc Res 53(4):911–920
Custodis F, Eberl M, Kilter H, Bohm M, Laufs U (2006) Association of RhoGDIalpha with Rac1 GTPase mediates free radical production during myocardial hypertrophy. Cardiovasc Res 71(2):342–351
Weber K, Pringle JR, Osborn M (1972) Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol 26:3–27
Gomez CCE, Carreno AA, Ishiwara DGP, San Martin Martinez E, Lopez JM, Hernandez NP, Gomez Garcia NC (2016) Decatropis bicolor (Zucc.) Radlk essential oil induces apoptosis of the MDA-MB-231 breast cancer cell line. BMC Complement Altern Med 16:266
Woods A, Vertommen D, Neumann D, Turk R, Bayliss J, Schlattner U, Wallimann T, Carling D, Rider MH (2003) Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem 278(31):28434–28442
Davies SP, Helps NR, Cohen PT, Hardie DG (1995) 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett 377(3):421–425
Kuang M, Febbraio M, Wagg C, Lopaschuk GD, Dyck JR (2004) Fatty acid translocase/CD36 deficiency does not energetically or functionally compromise hearts before or after ischemia. Circulation 109(12):1550–1557
Jensen B, Larsen CK, Nielsen JM, Simonsen LS, Wang T (2011) Change of cardiac function, but not form, in postprandial pythons, comparative biochemistry and physiology Part A. Mol Integr Physiol 160(1):35–42
Andersen JB, Rourke BC, Caiozzo VJ, Bennett AF, Hicks JW (2005) Physiology: postprandial cardiac hypertrophy in pythons. Nature 434(7029):37–38
Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, Cowart LA (2012) Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Investig 122(11):3919–3930
Li N, Zhao Y, Yue Y, Chen L, Yao Z, Niu W (2016) Liraglutide ameliorates palmitate-induced endothelial dysfunction through activating AMPK and reversing leptin resistance. Biochem Biophys Res Commun 478(1):46–52
Drosatos K, Drosatos-Tampakaki Z, Khan R, Homma S, Schulze PC, Zannis VI, Goldberg IJ (2011) Inhibition of c-Jun-N-terminal kinase increases cardiac peroxisome proliferator-activated receptor alpha expression and fatty acid oxidation and prevents lipopolysaccharide-induced heart dysfunction. J Biol Chem 286(42):36331–36339
Watkins SJ, Borthwick GM, Arthur HM (2011) The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In vitro Cell Dev Biol Anim 47(2):125–131
Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q (2004) Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med 10(11):1245–1250
Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP (2007) CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res 100(8):1208–1217
Mak KM, Ren C, Ponomarenko A, Cao Q, Lieber CS (2008) Adipose differentiation-related protein is a reliable lipid droplet marker in alcoholic fatty liver of rats. Alcohol Clin Exp Res 32(4):683–689
Muthusamy K, Halbert G, Roberts F (2006) Immunohistochemical staining for adipophilin, perilipin and TIP47. J Clin Pathol 59(11):1166–1170
Hickson-Bick DL, Buja LM, McMillin JB (2000) Palmitate-mediated alterations in the fatty acid metabolism of rat neonatal cardiac myocytes. J Mol Cell Cardiol 32(3):511–519
Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH (2000) Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97(4):1784–1789
D’Souza K, Nzirorera C, Kienesberger PC (2016) Lipid metabolism and signaling in cardiac lipotoxicity. Biochem Biophys Acta 1860(10):1513–1524
Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T (2011) MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res 92(1):75–84
Wei CD, Li Y, Zheng HY, Tong YQ, Dai W (2013) Palmitate induces H9c2 cell apoptosis by increasing reactive oxygen species generation and activation of the ERK1/2 signaling pathway. Mol Med Rep 7(3):855–861
Acknowledgements
We thank Ellen Becker and Simone Jäger for an excellent technical assistance. This study was funded by the Deutsche Forschungsgemeinschaft (DFG), the HOMFOR program and the Universität des Saarlandes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
About this article
Cite this article
Adrian, L., Lenski, M., Tödter, K. et al. AMPK Prevents Palmitic Acid-Induced Apoptosis and Lipid Accumulation in Cardiomyocytes. Lipids 52, 737–750 (2017). https://doi.org/10.1007/s11745-017-4285-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-017-4285-7