Abstract
Background and aims
The cardiovascular health benefits of eicosapentaenoic acid (EPA) have been demonstrated previously; however, the exact mechanism underlying them remains unclear. Our previous study found that lipotoxicity induced cardiomyocyte apoptosis via the inhibition of autophagy. Accordingly, in this study, we investigated whether EPA attenuated lipotoxicity-induced cardiomyocyte apoptosis through autophagy regulation. The role of EPA in mitochondrial dynamics was analyzed as well.
Methods
To explore how EPA protected against lipotoxicity-induced myocardial injury, cardiomyoblast (H9C2) cells were left untreated or were treated with 400 μM palmitic acid (PAM) and/or 80 μM EPA for 24 h.
Results
Excessive PAM treatment induced apoptosis. EPA reduced this PAM-induced apoptosis; however, EPA was unable to ameliorate the effects of PAM when autophagy was blocked by 3-methyladenine and bafilomycin A1. PAM blocked the autophagic flux, thus causing the accumulation of autophagosomes and acid vacuoles, whereas EPA restored the autophagic flux. PAM caused a decrease in polyunsaturated fatty acid (PUFA) content and an increase in saturated fatty acid content in the mitochondrial membrane, while EPA was incorporated in the mitochondrial membrane and caused a significant increase in the PUFA content. PAM also decreased the mitochondrial membrane potential, whereas EPA enhanced it. Finally, PAM elevated the expressions of autophagy-related proteins (LC3I, LC3II, p62) and mitochondrial fission protein (Drp1), whereas EPA inhibited their elevation under PAM treatment.
Conclusions
EPA reduces lipotoxicity-induced cardiomyoblast apoptosis through its effects on autophagy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autophagy is a cellular quality control system that functions to degrade and recycle excess/impaired proteins and organelles via autophagosome formation and lysosome degradation (Liang and Kobayashi 2016). The cardioprotective effects of autophagy have been demonstrated recently. Cellular stresses such as oxidative stress or lipotoxicity block autophagy, thus causing cardiomyocyte apoptosis and cardiomyopathy, whereas the activation of autophagy alleviates cardiac damage and restores heart function (Hsu et al. 2014b; Hsu et al. 2016; Liang and Kobayashi 2016).
Mitochondria are the main producers of energy in cells. Through mitochondrial fission and fusion (i.e., mitochondrial dynamics), the mitochondria meet the energy demands of cells (Ikeda et al. 2015). Any imbalance in mitochondrial dynamics, however, causes a decrease in mitochondrial membrane potential, triggering autophagy in order to eliminate the damaged mitochondria. Relatedly, any failure to degrade the damaged mitochondria causes an excessive accumulation of mitochondria that, in turn, induces apoptosis. Furthermore, it is evident that mitochondrial dynamics play an important role in the function of the heart, as any imbalance can cause mitochondrial fragmentation that can eventually lead, in turn, to heart failure (Liu et al. 2014; Wai et al. 2015).
Accumulated evidence has indicated the cardiovascular health benefits of n3 polyunsaturated fatty acids (PUFAs), including decreased dyslipidemia levels, increased insulin sensitivity, attenuation of oxidative stress and inflammation, and a reduced risk of congestive heart failure (Mozaffarian and Wu 2012). The American Heart Association thus recommends a daily intake 1 g of n3 PUFAs to reduce the risk of death from coronary heart diseases (American Heart Association Nutrition et al. 2006). The biological pathways for the effects of n3 PUFAs in cardiomyocytes have been investigated via cell and animal models recently, and such investigations have found that the membrane physiochemistry, ion channels, nuclear receptors and transcription factors, and metabolites of n3 PUFAs are regulated (Mozaffarian and Wu 2012). However, the relevance of such effects demonstrated by cell culture and animal experiments to the actual clinical effects of these fatty acids remains unclear.
It is evident that a high-fat diet (HFD) induces cardiomyocyte apoptosis via the inhibition of autophagy (Hsu et al. 2016), whereas eicosapentaenoic acid (EPA) has been found to restore heart function in rats fed with a HFD (Poudyal et al. 2013). In addition, we previously demonstrated the protective effects of EPA in cardiomyocytes placed under oxidative stress (Hsu et al. 2014a; Hsu et al. 2014b), including the activation of autophagy and the incorporation of EPA itself into mitochondrial membranes. Accordingly, we utilized a cell model in this study to investigate whether EPA attenuates lipotoxicity-induced cardiomyocyte apoptosis via the regulation of autophagy. The fatty acid composition of mitochondrial membranes and the function of mitochondria were also analyzed to elucidate the effects of EPA on mitochondria.
Materials and methods
Cell culture and treatment
The H9C2 cell line, a myoblastic cell line derived from embryonic BD1X rat heart tissue, was obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Hyclone, Auckland, NZ), 2 mM l-glutamine, 0.1 mM nonessential amino acids (Gibco BRL, Grand Island, NY, USA), 100 units/ml of penicillin, and 100 μg/ml of streptomycin (Gibco BRL, Grand Island, NY, USA) at 37 °C in a humidified chamber with 5% CO2. After the subcultured cells had been incubated for 24 h and had become attached to the plate, they were left untreated or were treated with 400 μM palmitic acid (PAM) and/or 80 μM EPA (Sigma-Aldrich, St. Louis, MO, USA) for 24 h. To regulate autophagy, 3-methyladenine (3-MA, 5 mM), bafilomycin A1 (Baf A1, 50 nM), or rapamycin (0.5 μM) was added for 1 h before the addition of 400 μM PAM and incubation for 24 h. After treatment, the cells were harvested for analysis.
Subcellular fractionation
The method for subcellular fractionation was performed as described in our previous study (Hsu et al. 2014a). The cells were lysed with 250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, and 1 mM ETTA and then centrifuged at 720g for 5 min. The supernatant was removed and centrifuged at 10,000g for 20 min to obtain the mitochondrial fraction, then the supernatant was centrifuged at 100,000g for 60 min to obtain the membrane and cytosol fractions.
Determination of fatty acid composition
To determine the fatty acid composition, lipids were extracted from the total cell lysate or mitochondrial fraction. Each sample was homogenized in a 1:1 (v/v) mixture of methanol/chloroform and centrifuged at 1200g for 30 min. The lower phase was dried and resuspended in 14% borontrifluoride methanol (Merck Schuchardt OHG, Hohenbrunn,Germany) followed by heating at 90 °C for 40 min. After cooling, the free fatty acids were extracted with an 8:3 (v/v) mixture of pentane/water, and the organic phase was recovered and dried. The extract was resuspended in heptane for gas chromatography analysis on a capillary column (DB-23 60 m × 0.25 mm × 0.25 μm, Agilent J&W, USA). The gas chromatograph was an Agilent 7890 series GC equipped with a flame ionization detector (FID/EPC G3440A) and an autoinjector module. The oven temperature was held at 120 °C for 2 min, was increased at 8 °C per min to 200 °C and held at 200 °C for 1 min, then was increased by 3 °C per min to 240 °C, and held at 240 °C for 2 min. The injector and detector were both at 240 °C and the flow rates of the carrier gas, helium, and air and nitrogen were 40, 450, and 25 ml/min, respectively. Components were identified by comparison of the retention time with those of authentic standards (Supelco 37 Comp. Fame Mix™, Supelco Inc. Belletonte PA) (Sekikawa et al. 2008).
Mitochondrial membrane potential measurement
Mitochondrial membrane potential (∆ψ) is essential to mitochondria for maintaining their basic functions, including energy production. A loss of the membrane potential (depolarization) leads to the decoupling of the respiratory chain and the release of cytochrome c into the cytosol during apoptosis (Gottlieb et al. 2003). As a result, ∆ψ is widely used in apoptosis studies to monitor mitochondrial health. In this study, the mitochondrial ∆ψ was measured using a fluorescent cationic dye, JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide), which was provided in a commercial kit (MitoScreen™ Kit, BD Bioscience, San Jose, CA, USA). After treatment, the H9C2 cells were incubated with JC-1 working solution for 15 min at 37 °C, then were trypsinized and resuspended for analysis by FACScalibur flow cytometry (BD Bioscience, San Jose, CA, USA). Data were analyzed using WinMDI 2.8 software.
Autophagy determination
Autophagy was measured by the quantification of autophagosomes and acid vacuoles. Autophagosomes were quantitated by saponin extraction FACS assay according to the method of Eng et al. (Eng et al. 2010). LC3-I, the nonautophagososme-associated form of LC3, is soluble, while the lapidated autophagosome-associated LC3-II is membrane-bound and insoluble. Saponin extraction followed by intracellular staining for endogenous LC3 allows the formation of autophagosomes to be quantified (Eng et al. 2010). Acid vacuoles were quantified by flow cytometry using LysoTracker Red DND-99 (Thermo Fisher Scientific, Grand Island, NY USA) (Hsu et al. 2014b). After treatment, the cell pellet was collected and stained with the dye following the manufacturer’s instruction. The stained cells were subjected to analysis by FACS Calibur flow cytometry (Becton-Dickinson, Mountain View, CA).
The autophagic flux was determined by steady-state levels of autophagosomes in relation to the lysosomal inhibitor-mediated accumulation of autophagosomes according to our previous study (Hsu et al. 2016). Briefly, H9C2 cells were subjected to the indicated experimental conditions with and without Baf A1 to inhibit autophagosome-lysosome fusion. By analyzing the Baf A1-mediated increase in LC3-II accumulation within a cell population, a quantitative index of the flux of autophagosome formation and degradation was obtained (more LC3II accumulation mediated by BafA1 suggests an active autophagic flux).
Early apoptosis and cell death determination
Double fluorescence staining with a PE-Annexin V/7-AAD kit (BD Bioscience, San Jose, CA, USA), followed by flow cytometry analysis, was used to measure early apoptosis and cell death in the H9C2 cells. In this approach, cells that stain positive for PE-Annexin V and negative for 7-AAD are undergoing apoptosis; those that stain positive for both PE-Annexin V and 7-AAD are in the end stage of apoptosis, undergoing necrosis, or are already dead; and those that stain negative for both are alive and not undergoing measureable apoptosis. After treatment, the cells were incubated with PE-Annexin V and 7-AAD reagents at 37 °C for 2 h according to the manufacturer’s protocol and then were analyzed using FACS Calibur flow cytometry (Becton-Dickinson, Mountain View, CA).
Western blotting
The cells were harvested, and total cell lysates were prepared using RIPA lysis buffer (Sigma-Aldrich, St. Louis, MO USA). Protein concentrations were determined using Bio-Rad protein assay reagents (Bio-Rad, Hercules CA, USA). Forty to 60 μg of protein lysate was analyzed by SDS-polyacrylamide gel electrophoresis. After transfer of the proteins from the gel to a nitrocellulose membrane (Amersham Pharmacia Biotech, Freiburg, Germany), the membranes were blocked in PBS containing 0.05% Tween 20 (PBS-T) and 5% nonfat dry milk and then were incubated with monoclonal antibodies against LC3, p62, MFN2, Drp1, OPA1, PINK1, VDAC, or ß-actin (Santa Cruz Bio. Inc., MA USA), followed by horseradish peroxidase-conjugated secondary antibodies (PharMingen, San Diego CA, USA). Immunoreactive bands were visualized using an enhanced chemiluminescence kit (Perkin-Elmer Life Sciences, Boston, MA, USA) and analyzed by QUANTITY ONE (Bio-Rad Hercules CA, USA). ß-Actin or VDAC was used as the internal control.
Statistical analysis
All data are expressed as the mean ± SEM. The significance of differences was determined by one-way ANOVA followed by Fisher’s test. Statistical analyses were performed using SAS (version 6.011; SAS Institute Inc., Cary, NC. USA). A p value <0.05 was considered statistically significant.
Results
EPA reduced PAM-induced apoptosis via the regulation of autophagy
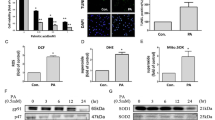
To elucidate whether EPA protects cardiomyoblasts from the PAM-induced lipotoxicity, H9C2 cells were treated with 400 μM PAM and/or 80 μM EPA for 24 h, and then the apoptosis was evaluated via flow cytometry. As shown in Fig. 1, the results indicated that PAM induced lipotoxicity in H9C2 cells and caused severe apoptosis, whereas EPA reduced the degree of this PAM-induced apoptosis. To further elucidate the role of autophagy in the protection provided by EPA against PAM-induced apoptosis, autophagy regulators (specifically, rapamycin as an autophagy activator and 3-MA and Baf A1 as autophagy inhibitors) were applied. Autophagy activation did not change the protective effects of EPA against PAM-induced apoptosis. However, EPA was unable to ameliorate the PAM-induced effect when autophagosome formation was blocked by 3MA, thus resulting in more severe apoptosis. It is notable that PAM treatment alone caused a cell death rate of 45% when the fusion of autophagosomes and lysosomes was inhibited by Baf A1 treatment, while treatment with EPA only protected the cardiomyoblasts from PAM stress to a mild degree (yielding a cell death rate of 40%). These results demonstrated the critical role of autophagy in the protective effects provided by EPA against PAM stress.
EPA attenuated lipotoxicity-induced cardiomyoblast apoptosis in an autophagy-dependent manner. H9C2 cells were (i) left untreated or (ii) treated with 400 μM PAM and/or 80 μM EPA for 24 h, then cell apoptosis (a), autophagosomes (b), and acid vacuoles (c)were measured by flow cytometry analysis. To regulate autophagy, 3-methyladenine (3-MA, 5 mM), bafilomycin A1 (Baf A1, 50 nM), or rapamycin (0.5 μM) was added for 1 h before the addition of 400 μM PAM. The results are expressed as mean ± SEM for five independent experiments. * vs Unt; + vs PAM; # vs E + P. Unt: untreated cells; PAM/ P: palmitic acid; EPA/ E: eicosapentaenoic acid
The effects of regulators on autophagy were shown in Fig. 1b, c. LC3II-positive cells and Lysotracker red-positive cells were analyzed as markers of autophagosomes and acid vacuoles, respectively. Rampamycin reduced the autophagosome accumulations in cells under PAM stress, whereas it could not decrease the rate of acid vacuoles (Fig. 1b, c). BafA1 increased the degree of LC3II-positive cells in untreated, EPA alone, EPA, and PA treatment, respectively. These results demonstrated BafA1 as a well-functioned autophagy inhibitor.
EPA restored autophagic flux in cardiomyoblasts under PAM stress
To further investigate the possible mechanisms underlying the protective effects of EPA against PAM-induced apoptosis, the roles of autophagy-related mechanisms were determined. Excess PAM caused a 16.0% of LC3II-positive cells, while cotreatment of EPA and PAM resulted in a lower rate of LC3II-positive cells (10.8%) (Fig. 1b). As shown in Fig. 1c, more acid vacuoles were observed in the cells under the condition of PAM treatment, whereas EPA reduced this PAM effect. These data demonstrated that treatment with PAM alone caused greater accumulation of autophagosomes and acid vacuoles, whereas the addition of EPA treatment to the PAM treatment substantially reduced the degree of such accumulation caused by PAM.
Autophagy is a dynamic process that plays a key role in the cellular protein quality control system. As a result, monitoring the autophagic flux would further clarify the protective role of EPA against PAM-induced lipotoxicity. Since Baf A1 treatment clearly suppressed the fusion process between autophagosomes and lysosomes, monitoring the amounts of autophagosomes produced in the cells with and without Baf A1 treatment was used to determine the autophagic flux. Treatment of cells with both Baf A1 and PAM resulted in a lower percentage of LC3 II-positive cells (10.6%) than treatment with PAM alone (16.0%) (Fig. 1b). In contrast, cotreatment with EPA, PAM, and Baf A1 caused an increase in the percentage of LC3II-positive cells (21.8%) as compared with the PAM and BafA1 cotreatment. These data demonstrated that PAM treatment blocked the autophagic flux and resulted in greater autophagosome accumulation, while EPA enhanced the autophagic flux, thus ameliorating the negative effect caused by PAM. Taken together, these results indicate that PAM caused an impairment in autophagic flux, whereas EPA restored the autophagic flux and reduced the accumulation of autophagosomes resulting from PAM stress.
Autophagy-related protein expression
The expression levels of autophagy-related proteins were monitored, and the results are shown in Fig. 2. Similar to the results of the control treatment, low expression levels of LC3I and LC3II were observed for treatment with EPA alone. PAM treatment caused a relatively high expression of LC3II, and EPA decreased this effect of PAM. PAM treatment resulted in the highest ratio of LC3II to LC3I among the four treatments, while the addition of EPA decreased the ratio of LC3II to LC3I caused by PAM treatment. The expression of p62 was upregulated by PAM treatment, while EPA reduced this upregulation of p62 expression caused by the PAM treatment. Overall, these protein expression results supported our hypothesis that EPA protected the cardiomyoblasts from PAM-induced apoptosis via the activation of functional autophagy.
EPA regulated autophagy in cardiomyoblasts under lipotoxicity. H9C2 cells were (i) left untreated or (ii) treated with 400 μM PAM and/or 80 μM EPA for 24 h, then the expression of autophagy-related protein was measured by Western blotting. After the individual treatment, cells were harvested and proteins extracted for Western blotting using antibodies against the indicated proteins, with β-actin as the internal control. For the quantitative density results, the intensity of the band of interest was divided by that of the β-actin band and this value expressed relative to that in the untreated control. The results are expressed as mean ± SEM. * vs Unt; + vs PAM; # vs E + P; $ vs P + Baf A1. Unt: untreated cells; PAM/ P: palmitic acid; EPA/ E: eicosapentaenoic acid; BafA1: bafilomycin A1
EPA modulated the fatty acid composition
Our previous study demonstrated that EPA changed the fatty acid composition in mitochondria and then regulated the mitochondrial function in doxorubicin-treated cells (Hsu et al. 2014a); accordingly, the fatty acid composition was also analyzed in this study. To analyze the fatty acid composition in the cells, PAM and/or EPA were added to H9C2 cells for 24 h, then the cells were harvested and separated into subcellular compartments. Table 1 shows that in the total cell lysate, excessive PAM resulted in a significant increase in palmitic acid (16:0) at the expense of a significant decrease of stearic acid (18:0), oleic acid (18:1 n9), and arachidonic acid (20:4 n6) as compared to the untreated cells, suggesting that a suppression of acetyl-CoA elongation by PAM occurred in the H9C2 cells. Compared to the untreated cells, EPA treatment did not cause a change in the level of saturated fatty acids (SFA), but increased the composition of PUFA, especially n3 PUFA. EPA treatment increased the biosynthesis of docosapentaenoic acid (22:5 n3, DPA) and docosahexaenoic acid (22:6, n3, DHA). Compared with the PAM alone treatment, a decrease in 16:0 and a significant increase in PUFA (especially n3 PUFA) were observed in the cotreatment of EPA and PAM.
A similar pattern of fatty acid profiles was observed in the mitochondria. Compared with the untreated cells, PAM treatment caused a higher SFA content, with 16:0 being the dominant SFA, while treatment with EPA alone caused an increase in PUFA without affecting the SFA content. An increase in PUFA content was also observed in the PAM and EPA cotreament as compared with the PAM alone treatment. It is noticeable that EPA significantly decreased the SFA content caused by PAM treatment, suggesting that EPA increased the membrane flexibility and then modulated the mitochondrial function in the cells under PAM stress.
EPA restored mitochondrial membrane potential
To evaluate whether EPA modulated the mitochondrial function, the membrane potential change (∆ψ) was monitored by means of JC-1 staining. As shown in Fig. 3, PAM alone treatment resulted in a significant increase in the ∆ψ, and this effect was ameliorated by cotreatment with EPA. These data support our hypothesis that EPA modulates the fatty acid composition in the mitochondria, thus restoring the mitochondrial membrane potential.
EPA restored mitochondrial membrane potential under lipotoxicity. H9C2 cells were (i) left untreated or (ii) treated with 400 μM PAM and/or 80 μM EPA for 24 h, then the mitochondrial membrane potential was measured by flow cytometry. The results are expressed as mean ± SEM. * vs Unt; + vs PAM. Unt: untreated cells; PAM/ P: palmitic acid; EPA/ E: eicosapentaenoic acid
Mitochondrial dynamics-related protein expression
To evaluate the mitochondrial dynamics, the expression levels of fusion and fission-related proteins were monitored. MFN2 and OPA1 are the markers for mitochondrial fusion, and Drp1 is the marker for mitochondrial fission. Neither PAM nor EPA regulated the expression of fusion-relation proteins in the cardiomyoblasts (Fig. 4). In contrast, EPA suppressed Drp1 expression, whereas PAM enhanced its expression as compared with the untreated cells. Moreover, EPA inhibited Drp1 in cardiomyoblasts under the condition of PAM treatment. PINK1 is the initiator for mitophagy. Compared with the untreated cells, EPA alone treatment caused a higher expression of PINK1, while PAM alone treatment did not change PINK1 expression. EPA treatment also upregulated PINK1 expression to induce mitophagy in cells under PAM stress. Taken together, these data suggested that treatment with PAM alone activated mitochondrial fission, whereas treatment with EPA suppressed the fission function. In addition, EPA regulated the mitophagy function in the cardiomyoblasts under lipotoxicity.
EPA regulated mitochondrial dynamics and mitophagy under lipotocixity. After the individual treatment, cells were harvested and proteins extracted for Western blotting using antibodies against the indicated proteins, with VDAC as the internal control. For the quantitative density results, the intensity of the band of interest was divided by that of the VDAC band and this value expressed relative to that in the untreated control. The results are expressed as mean ± SEM. * vs Unt; + vs PAM. Unt: untreated cells; PAM/ P: palmitic acid; EPA/ E: eicosapentaenoic acid
Discussion
Using a mouse model, we previously found that a HFD impaired the autophagic flux and activated maladaptive autophagy, thus causing lipotoxicity and cardiomyocyte apoptosis (Hsu et al. 2016). In contrast, the activation of adaptive autophagy by chemicals or genomic regulation reversed lipotoxicity-induced apoptosis. EPA has been demonstrated to act as an autophagy mediator (Hsu et al. 2014b), and it was applied in this study to investigate whether it would reduce lipotoxicity-induced cardiomyocyte apoptosis via an autophagy-dependent pathway. The present study showed that PAM induced lipotoxicity in cardiomyoblasts, whereas EPA lessened the rate of cell death caused by PAM stress via the restoration of adaptive autophagy. In addition, EPA modulated the mitochondrial function, with its effects including an increase in the PUFA content in the membrane, an elevated membrane potential, and regulations in the mitochondrial fission and mitophagy. Furthermore, all these changes and regulatory effects in the mitochondria might partially contribute to the protective effects of EPA in cardiomyoblasts against lipotoxicity.
Cardiomyocytes, as long-lived cells, require a well-functioning degradation system, i.e., autophagy, in order to maintain cellular homeostasis. Recent studies have focused on the modulation of autophagy to treat and prevent cardiovascular diseases, and researchers have found that tuning of the autophagic flux may provide a promising therapeutic target for the treatment of cardiovascular diseases (Schiattarella and Hill 2016). This study demonstrated the potential utility of using EPA for such tuning of the autophagic flux in cardiomyocytes under lipotoxic stress. The beneficial role of EPA in cardiovascular health has been studied extensively, and the possible mechanisms for EPA’s cardioprotective effects involve the membrane physiochemistry, ion channels, nuclear receptors and transcription factors, and metabolites of n3 PUFA (Mozaffarian and Wu 2012). The potential relevance of regulating such biological pathways with EPA to achieve clinical effects remains unclear; however, the results of the current study indicated that EPA may achieve cardioprotective effects through its tuning of autophagy.
Mitochondria are the major energy producers in the cardiomyocytes, such that any damage to the mitochondria causes a decrease in the metabolic rate and ATP synthesis, while severe damage to the mitochondria leads to cell apoptosis and contributes to the pathogenesis of heart diseases (Ikeda et al. 2015). To maintain their own health, functional mitochondria engage in both fusion and fission to maintain their ability to produce ATP, while any damaged mitochondria are sequestered and degraded via the autophagic process. The present study showed the regulatory effects of EPA on the mitochondria; these effects included a decrease in the level of mitochondrial fission, increases in the PUFA content and membrane potential of the mitochondrial membranes, and the regulation of mitophagy, all changes that might contribute to the decrease in lipotoxicity-induced apoptosis caused by EPA.
Our study also indicated that EPA treatment increased the biosynthesis of EPA, DHA, and DPA, in addition to causing significant mitochondrial membrane enrichment in n-3 PUFAs. Many studies have indicated the cardioprotective role of EPA and DHA (Hsu et al. 2014a; Stanley et al. 2012), and DPA has received increasing attention recently due to its biological activity, such as its anti-inflammatory effect, and the improvement it caused to plasma lipid profiles (Weylandt 2016).
n3 PUFAs (especially EPA and DHA) affect the mitochondrial functions, including redox signaling, metabolic processing, ATP synthesis, and apoptosis (Hsu et al. 2014a; Stanley et al. 2012). The mitochondrial uncoupling protein 2 (UCP2) is a critical regulator of respiratory activity and ROS generation by inducing proton leak in a moderate manner (Akhmedov et al. 2015). By causing the upregulation of UCP2, EPA and DHA decrease ROS generation, thus protecting cardiomyocytes from doxorubicin-induced toxicity (Hsu et al. 2014a). Moreover, n-3 PUFAs alter the lipid content and permeability transition in mitochondria (Stanley et al. 2012). Cardiolipin, a mitochondrial protein, is responsible for the normal functions of mitochondria. Relatedly, the depletion, impaired remodeling, or oxidation of cardiolipin has been found to stimulate the release of cytochrome c and trigger apoptotic signaling molecules (Monteiro et al. 2013). EPA and DHA supplementation enhance the incorporation of themselves into the n-3 cardiolipin species in the H9C2 cells (Ting et al. 2015).
DPA, an elongated metabolite of EPA and an intermediate in the biosynthesis of DHA, is associated with a lower risk of coronary heart diseases (Del Gobbo et al. 2016). DPA itself could be an intermediate reservoir contributing to the biosynthesis of both EPA and DHA (Kaur et al. 2010), and pro-resolution mediators derived from DPA metabolites, such as D5n-3DPA(RvD5n-3DPA) and maresin (MaR)-1, possess potent anti-inflammatory and proresolving bioactivity (Weylandt 2016). The biological functions of DPA and its metabolites remain unknown; however, they might be the potential regulators of mitochondrial function.
Accordingly, we postulated that EPA and its metabolites (DPA and DHA) would affect the cardiolipin content, upregulate UCP2, and increase the unsaturation in the mitochondrial membrane, thus restoring the mitochondrial functions.
To maintain mitochondrial dynamics, the mitochondria undergo constant fusion and fission. However, excessive fission or deficient fusion causes mitochondrial fragmentation, which is linked to apoptosis in the heart (Hom and Sheu 2009). Several approaches have been applied to regulate mitochondrial dynamics, including genomic manipulation, the use of different drugs, and the use of different fat types (Hom and Sheu 2009; Lionetti et al. 2014; Sun et al. 2014). The link between n3 PUFA and mitochondria in the heart has not yet been studied; however, the effect of n3 PUFAs on mitochondrial dynamics has recently been evaluated in the mouse liver. Lionetti and his colleagues found that a HFD caused a shift towards mitochondrial fission processes in the liver, whereas fish oil increased mitochondrial fusion processes (Lionetti et al. 2014). A similar regulatory pattern of mitochondrial dynamics has been observed in the endothelial cells of HFD-fed mice (Sun et al. 2014). We also found similar results for the regulation of fission with EPA; however, neither PAM nor EPA was found to regulate the fusion process in cardiomyoblasts.
An increasing number of research studies have investigated the connection between autophagy and mitochondria. The present study suggested that the beneficial effect of EPA on the mitochondria might play an important role in the increase of autophagic flux. We found that EPA elevated the unsaturation in the mitochondrial membrane. In addition, EPA enhanced the biosynthesis of DPA and DHA, which could maintain the normal mitochondrial functions via the alteration in lipid content (cardiolipin) and gene regulation, such as UCP2. Furthermore, EPA regulated the degree of mitochondrial fission. All these changes contributed to the restoration of the health of mitochondria and the membrane potential. This was potentially because EPA decreased the number of impaired mitochondria, which further eased the loading of autophagy, thus maintaining an active autophagic flux. However, more studies are required to prove our postulation.
Mitophagy, the downstream process of mitochondrial degradation, is similar to autophagy in that it involves the same core machinery as autophagy, such as autophagosome formation and lysosome degradation; however, some differences have been observed, such as the role of PINK1/Parkin, which is a well-characterized for the initiation of mitophagy (Ikeda et al. 2015). The disruption of the mitochondrial dynamics activates PINK1 and Parkin and then activates mitophagy. The results of the present study suggested that the effect of EPA is somewhat dependent on mitophagy-related mechanisms. However, further research is needed to completely elucidate the connection between mitophagy and autophagy under lipotoxic conditions.
Interestingly, rapamycin, an autophagy activator, did not suppress PAM-induced apoptosis in the present study. Rapamycin is a pharmacological inhibitor of mTOR signaling. The effects of mTOR signaling include activating mitochondrial biogenesis, increasing protein synthesis and cell proliferation, and inhibiting autophagy (Sciarretta et al. 2014). Partially inhibiting mTOR signaling has a cardioprotective effect in obesity, metabolic syndrome, and cardiovascular diseases (Sciarretta et al. 2014). However, our data showed that rapamycin did not inhibit PAM-induced apoptosis, and a possible reason for this might have been the dosage of rapamycin used. The rapamycin concentration required for activating autophagy varies from 10 to 250 nM (Chan et al. 2016; Li et al. 2017; Sishi et al. 2013). In this study, 500 nM of rapamycin decreased PAM-induced autophagosome accumulation efficiently (16 vs. 4.8%, Fig. 1b); meanwhile, this concentration might have been too high to inhibit the physiological effects of mTOR signaling, thus causing a negative effect in the cardiomyocytes. Accordingly, this dosage of rapamycin (500 nM) was not regarded as an appropriate activator of autophagy in the present study.
In conclusion, our data support the conclusion that treatment with PAM elevated the degree of mitochondrial fission and increased the SFA content in the mitochondrial membrane, which contributed, in turn, to a decrease in mitochondrial membrane potential. PAM also inhibited the autophagosome formation and lysosome degradation (autophagy), causing apoptosis as a result. In contrast, EPA decreased the degree of fission and increased the level of PUFA in the mitochondrial membrane, thus maintaining the mitochondrial membrane potential (Fig. 5). In addition, EPA restored autophagy to a functional level, which balanced the mitochondrial dynamics and enhanced the degree of cell survival under lipotoxic conditions. That said, the links among mitochondrial fission, mitophagy, and autophagy require further study.
A proposed molecular mechanism for EPA’s protection against lipoxicity. EPA elevated the unsaturation in the mitochondrial membrane. In addition, EPA enhanced the biosynthesis of DPA and DHA, which could maintain the normal mitochondrial functions via the alteration in lipid content (cardiolipin) and gene regulation, such as UCP2. Moreover, EPA decreased the degree of mitochondrial fission. All these changes contributed to restore the health of mitochondria and the membrane potential. Furthermore, EPA restored autophagy to a functional level, which balanced the mitochondrial dynamics and enhanced the degree of cell survival under lipotoxic conditions
Abbreviations
- Baf A1:
-
Bafilomycin A1
- EPA:
-
Eicosapentaenoic acid
- HFD:
-
High-fat diet
- 3-MA:
-
Methyladenine
- ∆ψ:
-
Mitochondrial membrane potential
- PAM:
-
Palmitic acid
- PUFA:
-
Polyunsaturated fatty acid
- SFA:
-
Saturated fatty acid
References
Akhmedov AT, Rybin V, Marin-Garcia J. Mitochondrial oxidative metabolism and uncoupling proteins in the failing heart. Heart Fail Rev. 2015;20:227–49. doi:10.1007/s10741-014-9457-4.
American Heart Association Nutrition C, Lichtenstein AH, Appel LJ, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association nutrition committee. Circulation. 2006;114:82–96. doi:10.1161/CIRCULATIONAHA.106.176158.
Chan YK, Sung HK, Jahng JW, et al. Lipocalin-2 inhibits autophagy and induces insulin resistance in H9c2 cells. Mol Cell Endocrinol. 2016;430:68–76. doi:10.1016/j.mce.2016.04.006.
Del Gobbo LC, Imamura F, Aslibekyan S, et al. Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease pooling project of 19 cohort studies. JAMA Intern Med. 2016;176:1155–66. doi:10.1001/jamainternmed.2016.2925.
Eng KE, Panas MD, Hedestam GBK, et al. A novel quantitative flow cytometry-based assay for autophagy. Autophagy. 2010;6:634–41.
Gottlieb E, Armour SM, Harris MH, et al. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10:709–17. doi:10.1038/sj.cdd.4401231.
Hom J, Sheu SS. Morphological dynamics of mitochondria—a special emphasis on cardiac muscle cells. J Mol Cell Cardiol. 2009;46:811–20. doi:10.1016/j.yjmcc.2009.02.023.
Hsu HC, Chen CY, Chen MF. N-3 polyunsaturated fatty acids decrease levels of doxorubicin-induced reactive oxygen species in cardiomyocytes—involvement of uncoupling protein UCP2. J Biomed Sci. 2014a;21:101. doi:10.1186/s12929-014-0101-3.
Hsu HC, Chen CY, Chiang CH, et al. Eicosapentaenoic acid attenuated oxidative stress-induced cardiomyoblast apoptosis by activating adaptive autophagy. Eur J Nutr. 2014b;53:541–7. doi:10.1007/s00394-013-0562-2.
Hsu HC, Chen CY, Lee BC, et al. High-fat diet induces cardiomyocyte apoptosis via the inhibition of autophagy. Eur J Nutr. 2016;55:2245–54. doi:10.1007/s00394-015-1034-7.
Ikeda Y, Shirakabe A, Brady C, et al. Molecular mechanisms mediating mitochondrial dynamics and mitophagy and their functional roles in the cardiovascular system. J Mol Cell Cardiol. 2015;78:116–22. doi:10.1016/j.yjmcc.2014.09.019.
Kaur G, Begg DP, Barr D, et al. Short-term docosapentaenoic acid (22:5 n-3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. Br J Nutr. 2010;103:32–7. doi:10.1017/S0007114509991334.
Li YY, Xiang Y, Zhang S, et al. Thioredoxin-2 protects against oxygen-glucose deprivation/reperfusion injury by inhibiting autophagy and apoptosis in H9c2 cardiomyocytes. Am J Transl Res. 2017;9:1471–82.
Liang Q, Kobayashi S. Mitochondrial quality control in the diabetic heart. J Mol Cell Cardiol. 2016;95:57–69. doi:10.1016/j.yjmcc.2015.12.025.
Lionetti L, Mollica MP, Donizzetti I, et al. High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS One. 2014;9:e92753. doi:10.1371/journal.pone.0092753.
Liu J, Wang P, Zou L, et al. High-fat, low-carbohydrate diet promotes arrhythmic death and increases myocardial ischemia-reperfusion injury in rats. Am J Physiol Heart Circ Physiol. 2014;307:H598–608. doi:10.1152/ajpheart.00058.2014.
Monteiro JP, Oliveira PJ, Jurado AS. Mitochondrial membrane lipid remodeling in pathophysiology: a new target for diet and therapeutic interventions. Prog Lipid Res. 2013;52:513–28. doi:10.1016/j.plipres.2013.06.002.
Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr. 2012;142:614S–25S. doi:10.3945/jn.111.149633.
Poudyal H, Panchal SK, Ward LC, et al. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J Nutr Biochem. 2013;24:1041–52. doi:10.1016/j.jnutbio.2012.07.014.
Schiattarella GG, Hill JA. Therapeutic targeting of autophagy in cardiovascular disease. J Mol Cell Cardiol. 2016;95:86–93. doi:10.1016/j.yjmcc.2015.11.019.
Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014;114:549–64. doi:10.1161/CIRCRESAHA.114.302022.
Sekikawa A, Curb JD, Ueshima H, et al. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men—a cross-sectional study. J Am Coll Cardiol. 2008;52:417–24. doi:10.1016/j.jacc.2008.03.047.
Sishi BJ, Loos B, van Rooyen J, et al. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem Pharmacol. 2013;85:124–34. doi:10.1016/j.bcp.2012.10.005.
Stanley WC, Khairallah RJ, Dabkowski ER. Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr. 2012;15:122–6. doi:10.1097/Mco.0b013e32834fdaf7.
Sun R, Wang X, Liu Y, et al. Dietary supplementation with fish oil alters the expression levels of proteins governing mitochondrial dynamics and prevents high-fat diet-induced endothelial dysfunction. Br J Nutr. 2014;112:145–53. doi:10.1017/S0007114514000701.
Ting HC, Chao YJ, Hsu YHH. Polyunsaturated fatty acids incorporation into cardiolipin in H9c2 cardiac myoblast. J Nutr Biochem. 2015;26:769–75. doi:10.1016/j.jnutbio.2015.02.005.
Wai T, Garcia-Prieto J, Baker MJ, et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science. 2015;350:aad0116. doi:10.1126/science.aad0116.
Weylandt KH. Docosapentaenoic acid derived metabolites and mediators - the new world of lipid mediator medicine in a nutshell. Eur J Pharmacol. 2016;785:108–15. doi:10.1016/j.ejphar.2015.11.002.
Acknowledgements
This work was supported by the research grants MOST 104-2313-B-002-038-MY3 (from the Ministry of Science and Technology) and NTU-CESRP-104R7615-3 (from National Taiwan University). The authors are grateful to Miss Mai-Jun Lai and Miss Cian-Jyun Kao for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hsu, HC., Li, SJ., Chen, CY. et al. Eicosapentaenoic acid protects cardiomyoblasts from lipotoxicity in an autophagy-dependent manner. Cell Biol Toxicol 34, 177–189 (2018). https://doi.org/10.1007/s10565-017-9406-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-017-9406-9