Abstract
Fatty acids (FAs) are essential components of cell membranes and play an integral role in membrane fluidity. The lipophilic index [LI, defined as the sum of the products between FA levels and melting points (°C), divided by the total amount of FA: \({\text{LI}} = \frac{{\mathop \sum \nolimits_{k} [{\text{fatty acid}} \times {\text{melting point}}]}}{{\mathop \sum \nolimits_{k} {\text{fatty acid}} }}\)] is thought to reflect membrane and lipoprotein fluidity and may be associated with the risk of coronary heart disease (CHD). Therefore, we examined the associations of dietary and plasma phospholipid (PL) LI with CHD risk among postmenopausal women. We determined dietary LI for the cohort with completed baseline food frequency questionnaires and free of prevalent cardiovascular diseases in the Women’s Health Initiative (WHI) observational study (N = 85,563). We additionally determined plasma PL LI in a matched case-control study (N = 2428) nested within the WHI observational cohort study. Cox proportional hazard regression and multivariable conditional logistic regression were used to calculate HRs/ORs for CHD risk between quartiles of LI after adjusting for potential sources of confounding and selection bias. Higher dietary LI in the cohort study and plasma PL LI in the case-control study were significantly associated with increased risk of CHD: HR = 1.18 (95% CI 1.07–1.31, P for trend <0.01) and OR = 1.76 (95% CI 1.33–2.33, P for trend <0.01) comparing extreme quartiles and adjusting for potential confounders. These associations still persisted after adjusting for the polyunsaturated to saturated fat ratio. Our study indicated that higher LI based on either dietary or plasma measurements, representing higher FA lipophilicity, was associated with elevated risk of CHD among postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the US, which accounts for 31.3% of total death [1]. Coronary heart disease (CHD) is the most common type of CVD and accounts for over 30% of prevalent CVD and 47.7% of CVD death [2]. Fatty acids (FAs) play important roles in cardiovascular pathophysiology. Different FAs have different properties such as binding affinity, viscosity and lipophilicity, which determine the orientation of membrane-bound proteins, further influencing lipoprotein metabolism and the activity of membrane-bound enzymes, receptors and other proteins that can affect CVD risk [3, 4]. A unique characteristic of FA is the melting point, which is determined by the length and degree of unsaturation of FA chains and has been shown to reflect the lipophilicity of FA. The lipophilic index (LI) was first developed by Ding in 2008, summarizing individual FA levels and their melting points, and was applied for predicting CHD risk using erythrocyte and plasma FA [5]. LI provides a novel method to capture overall FA lipophilicity, with a lower value indicating lower lipophilicity and higher membrane fluidity.
Two studies have examined the association between LI and CHD risk. In a nested case-control study of the Health Professionals Follow-up Study, which included US men aged 40–70 years, Wu et al. [6] examined the association of plasma and erythrocyte LI with CHD risk. They found that higher plasma LI was significantly associated with an increased risk of CHD [risk ratio (RR) = 1.61, 95% confidence internal (CI) 1.03–2.53, comparing extreme quintiles], while erythrocyte LI was not. In another matched case-control study of Hispanic Americans living in the Central Valley of Costa Rica by Toledo et al. [7], the LIs of diet, plasma, red blood cells and adipose tissue were used to evaluate the association with myocardial infarction. Higher LIs derived from diet and adipose tissue were associated with an elevated risk of myocardial infarction (RR = 1.57, 95% CI 1.22–2.02 and 1.30, 95% CI 1.00–1.69, respectively, comparing extreme quintiles). Currently, evidence regarding the relationship between LI and CHD is limited, especially the evidence from a large representative population of US women. Given these findings, we examined the association of dietary LI with CHD risk among postmenopausal women who participated in the Women’s Health Initiative (WHI) observational cohort study and performed a separate analysis of plasma phospholipid (PL) LI in a case-control study nested in the observational cohort study as plasma PL FAs were not measured for all WHI cohort participants. We hypothesized that LI will be positively associated with CHD risk.
Materials and Methods
Study Population
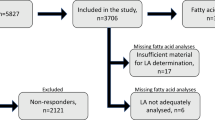
The WHI observational cohort study enrolled 93,676 postmenopausal women (age 50–79) at 40 clinical centers in the US from 1994 to 1998. A detailed description of the WHI observational cohort study design has been published elsewhere [8, 9]. All incident CHD cases, which were defined as hospitalized myocardial infarction, definite silent myocardial infarction and deaths due to definite CHD or possible CHD, were confirmed based on review of medical records and death certificates by trained physician adjudicators [10]. For the cohort analysis that evaluated the association of diet-derived LI with CHD risk, women were excluded from the 93,676 WHI participants based on the following criteria: (1) lack of completion of baseline food frequency questionnaires, (2) baseline self-reported myocardial infarction, coronary artery bypass graft, percutaneous transluminal coronary angioplasty or stroke and (3) implausible baseline dietary total energy intake (<600 or >5000 kcal/day). The final sample size in the current cohort analysis was 85,563 (Fig. 1).
As plasma PL FA profiles were not available for all WHI participants, we additionally performed a matched case-control study of 2448 participants nested in the WHI observational cohort study to evaluate the association of plasma PL LI with CHD risk. Specifically, for the matched case-control study, all adjudicated incident CHD cases from the WHI observational database in September 2005 were selected for sampling [10]. A total of 2468 potential cases were initially eligible. Potential cases were excluded according to the following criteria: (1) lack of available baseline plasma sample, (2) lack of completion of baseline food frequency questionnaires and (3) CVD reported at baseline, where CVD was defined as myocardial infarction, angina, coronary artery bypass graft surgery/percutaneous transluminal coronary angioplasty, carotid artery disease, congestive heart failure, stroke or peripheral vascular disease. Potential controls were excluded for all these criteria as well as developing CVD during follow-up (a mean of 4.5 years). Among the 1549 cases meeting the eligibility criteria, 1288 had a previously matched eligible control. Matching was done on the basis of age at screening, date of enrollment, race/ethnicity (White, Black, Hispanic, Other) and hysterectomy status at baseline. This matching process resulted in 2448 matched case-controls. Additionally, we excluded 11 participants who lacked plasma PL FA profile results and their matched pairs (N = 9). Therefore, the final sample size in the case-control analysis was 2428 (Fig. 1).
All participants signed an informed consent, which was approved by the institutional review boards at the Clinical Coordinating Center at the Fred Hutchinson Cancer Research Center and the 40 clinical centers. A separate approval to use de-identified samples and data for the plasma PL FA analysis in this study was obtained from the Tufts University/Tufts Medical Center Institutional Review Board [11].
Lipophilic Index
The primary exposure of interest was LI, which was calculated from dietary FA and plasma PL FA. Dietary FAs were measured from food frequency questionnaires at baseline and year 3, including saturated fatty acids (SFA) (4:0, 6:0, 8:0, 10:0, 12:0, 14:0, 16:0, 17:0, 18:0, 20:0, and 22:0), monounsaturated fatty acids (MUFA) (14:1, 16:1, 18:1, 20:1, and 22:1), n-3 polyunsaturated fatty acids (PUFA) (18:3, 20:5, 22:5, and 22:6), n-6 PUFA (18:2, 18:4, and 20:4) and trans fatty acids (TFA) (16:1 T, 18:1 T, and 18:2 T). Plasma PL FAs, including SFA (12:0, 14:0, 15:0, 16:0, 18:0, 20:0, 22:0, and 24:0), MUFA (14:1, 16:1N-7, 16:1N-9, 18:1N-7, 18:1N-9, 20:1N-9, and 24:1N-9), n-3 PUFA (18:3N-3, 20:5N-3, 22:5N-3, and 22:6N-3), n-6 PUFA (18:2N-6, 18:3N-6, 20:2N-6, 20:3N-6, 20:4N-6, 22:4N-6 and 22:5N-6) and TFA (all 18:1 T and 18.2 T), were measured at baseline using an established gas chromatography method and expressed as molar percentage (mol%), proportions of FA relative to the internal standard [12]. Details about internal and external quality controls can be found elsewhere [11].
The LIs for dietary and plasma PL FAs were calculated as a summation of the products of the levels of FAs and their specific melting points (°C) using the following equations:
where i is the individual FA, and k is the number of FAs used to calculate LI. A higher LI is associated with a higher FA melting point and higher lipophilicity.
Dietary LI in the cohort study was computed for baseline and year 3 separately. We used the cumulative average diet method to evaluate the association between dietary LI and CHD risk [13, 14]. In this method, we used dietary LI derived from baseline food frequency questionnaires to capture the exposure within the first 3 years and dietary LI averaging the baseline and year 3 questionnaires to capture the exposure beyond the first 3 years. For those who failed to complete food frequency questionnaires at year 3 (N = 11,051), we used multiple imputation by chained equations to impute missing dietary measurements (more details in “Statistical analysis”) [15]. Dietary LI in the case-control study was derived based on baseline food frequency questionnaires. Since plasma PL FAs in the WHI case-control study were expressed as molar percentage, molecular weight for each FA was taken into account. Information about the melting point and molecular weight was acquired from the LipidBank Database [16]. For those FAs for which melting points were described in ranges, the midpoints were used; for FAs having isomers, the weighted averages were used, where weights were calculated based on previously published papers [7].
Covariates
Socio-demographic variables, lifestyle factors, CHD risk factors and dietary factors were assessed by interview, self-report or physical measurement at baseline using standardized questionnaires or during follow-up using the same protocol as baseline assessments [8]. Socio-demographic variables included age, region, race/ethnicity, education and income. Lifestyle factors included body mass index (BMI), physical activity and smoking. CHD risk factors included family history of myocardial infarction/diabetes/stroke, medication use (included anticoagulants, diabetes medications and lipid lowering medications), postmenopausal hormone use and self-reported baseline hypertension/diabetes/cancer/hypercholesterolemia/hysterectomy status. Dietary factors included alcohol intake, percent calories from protein and carbohydrates, and total energy intake.
For analytic purposes, education was categorized as ≤ high school, some college and post-graduate. Income was categorized as <$20,000, $20,000 to $74,999 and ≥$75,000 per year. Physical activity was measured by the total physical activity score (MET-h/week) [17]. BMI was treated as a continuous variable. Smoking was categorized as never, past or current. Postmenopausal hormone use was categorized as current estrogen + progesterone, current estrogen alone, past users and never used.
Statistical Analysis
We initially examined the distribution of baseline socio-demographic characteristics, lifestyle factors, CHD risk factors and dietary factors by quartiles of dietary LI in the cohort study and by cases status in the matched case-control study. Descriptive statistics such as medians, means, standard errors, frequencies and proportions were used to summarize the aforementioned variables. Chi-square and ANOVA tests were used for categorical and continuous variables, respectively, in the cohort study by quartiles of dietary LI. Cases and controls were compared using paired t test, Wilcoxon signed rank or McNemar tests, depending on the distribution of the data.
To examine the association between quartiles of LI and CHD risk, we used unadjusted and adjusted Cox proportional hazard regression models in the cohort study and adjusted conditional logistic regression models in the case-control study. LI was categorized into quartiles based on the distribution among participants without CHD. A test for trends was conducted by assigning the median value of each quartile to a given category and then including this categorical variable in Cox and conditional logistic regression models as a linear term. Other covariates were modeled using indicators when categorical or linear terms when continuous. The covariates that were adjusted for in the aforementioned models were based on the causal diagram of each study (Appendix 1) and selected to control for confounding bias as well as selection bias due to study exclusions and censoring because of loss to follow-up [18,19,20]. Patients were considered to be lost to follow-up 1 year after the last time they were seen at a clinic visit during the study period. Patients who were last seen within 1 year of 29 August 2014 were administratively censored at 29 August 2014. The fully adjusted model in the cohort study included age, region, race/ethnicity, education, income, BMI, physical activity, smoking, family history of myocardial infarction/diabetes/stroke, medication use, postmenopausal hormone use, self-reported baseline hypertension/diabetes/cancer/hypercholesterolemia/hysterectomy status, dietary alcohol, percent calories from protein and carbohydrates, and total energy intake. The final adjusted model examining plasma PL LI on CHD risk in the case-control study included matching factors (age, race/ethnicity, enrollment date and hysterectomy status), BMI, physical activity, smoking, family history of myocardial infarction/diabetes/stroke, medication use, postmenopausal hormone use, self-reported baseline hypertension/diabetes/cancer/hypercholesterolemia, dietary alcohol, percent calories from protein and carbohydrates, and total energy intake. No evidence of violation of the proportional hazards assumption was found on the basis of the Schoenfeld residuals or the Wald test for a product term between the exposure of interest and follow-up time (both linear and on log scale).
Multiple imputation (five times) by chained equations [15] was used to impute missing values among cohort participants (N = 85,563) on dietary measurements at year 3 (dietary LI, alcohol intake, percent calories from protein and carbohydrates, and total energy, N = 11,051) and the following covariates: race/ethnicity (N = 231), education (N = 680), income (N = 3714), BMI (N = 980), physical activity (N = 958), smoking (N = 1181), family history of myocardial infarction (N = 4428)/diabetes (N = 4115)/stroke (N = 4736), self-reported baseline hypertension (N = 1502)/diabetes (N = 86)/cancer (N = 654)/hypercholesterolemia (N = 1887) and hysterectomy status (N = 80).
Sensitivity analyses were conducted to assess the robustness of findings by (1) calculating the dietary lipophilic load (LL), which is an integrated measure of both lipophilic quantity and quality [21], for the comparison with dietary LI; (2) calculating alternative dietary LI by excluding TFA or PUFA; (3) using data on participants with complete information (N = 60,079); (4) examining the associations of dietary PUFA:SFA and long-chain n-3 PUFA with CHD risk; (5) examining the associations of blood lipids [low-density lipoprotein cholesterol (LDL-C):high-density lipoprotein cholesterol (HDL-C) ratio and triglycerides] with CHD among a subgroup of participants with blood lipids; (6) comparing dietary LI/LL calculated based on different methods (cumulative average versus baseline only); (7) validating the association between LI and CHD by additionally adjusting for the PUFA-to-SFA ratio.
All data were analyzed using SAS (version 9.4; SAS Institute, Inc., Cary, NC).
Results
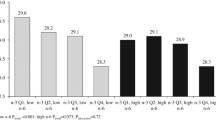
Among 85,563 women in the cohort study, we identified 4195 incident CHD events; the average (SD) follow-up person time was 13.2 (4.3) years. The median (IQR) baseline dietary LI was 27.6 (3.5), and the mean age (SD) was 63.4 (7.3). Participants in the highest dietary LI quartile compared to participants in lower dietary LI quartiles were more likely to be White/non-Hispanics, live in the Midwest and have less income (Table 1). Less physical activity and higher BMI were associated with higher dietary LI. We observed a moderate correlation between baseline and year 3 LI (Pearson’s correlation coefficient = 0.52, P < 0.01). Among all the cohort participants, 39% stayed in the same dietary LI quartile, 35% participants switched to lower LI quartiles, and 25% switched to higher LI quartiles in the second dietary measurement at year 3.
Table 2 shows the characteristics by CHD case status. The median (IQR) of plasma PL LI was 22.3 (2.5) overall, 22.1 (2.5) among controls and 22.5 (2.4) among cases. The median (IQR) of dietary LI was 27.7 (3.5) overall, 27.6 (3.5) among controls and 27.8 (3.4) among cases. The Spearman’s correlation coefficient between plasma PL LI and dietary LI was 0.12 (P < 0.01). The overall mean (SD) age was 67.8 (6.8) years, and there was no difference between cases and controls. Cases had significantly lower levels of education, income and physical activity. Cases also had higher BMI and total energy intake than controls. There was a higher proportion of smokers and family history of myocardial infarction and a lower proportion with a family history of diabetes among cases. Cases also reported a lower proportion of current hormone usage (estrogen + progesterone or estrogen alone) than controls.
The melting point, median level of each FA in the diet and plasma PL and correlations between individual FA and LI are shown in "Appendix 2". The major plasma PL FAs were 16:0 (30.5 mol%), 18:0 (13.3 mol%), 18:1n-9 (8.4 mol%), 18:2n-6 (20.7 mol%), 20:4n-6 (10.9 mol%) and 22:6n-3 (3.1 mol%), while the major dietary FAs were 16:0 (8.4 g/day), 18:0 (4.1 g/day), 18:1n-9 (16.3 g/d), 18:2n-6 (8.5 g/day), 18:3n-3 (1.0 g/day) and trans 18:1 (2.6 g/day).
Associations Between LI and CHD
Table 3 shows the relationship between LI and CHD risk for the two studies based on hazard ratios (HRs) with 95% CI for CHD associated with quartiles of LI in the cohort study and odds ratios (ORs) with 95% CI for CHD in the matched case-control study. Among the cohort study participants, higher dietary LI was significantly associated with an increased risk of CHD in both the unadjusted and adjusted models. Specifically, in model 2, participants in the highest quartile of dietary LI showed an 18% higher risk of CHD compared with participants in the lowest quartile after adjusting for confounders. Among those included in the case-control study, the OR in an adjusted model that controlled for matching factors was 1.87 (95% CI 1.45–2.40, P for trend <0.01) comparing the highest to the lowest quartile of plasma PL LI. In the model that adjusted for all proposed confounders, the OR comparing extreme quartiles was attenuated to 1.76 (95% CI 1.33–2.33, P for trend <0.01). The associations of dietary LI and plasma PL LI with CHD still persisted after the adjustment of PUFA:SFA (model 3 and model 6).
Sensitivity Analyses
In the sensitivity analysis of using cohort participants with complete information (N = 60,079), we found a stronger association between dietary LI and the risk of CHD (HR = 1.23, 95% CI 1.08–1.33) than using multiple imputation by chained equations to impute missing values (Appendix 3). In the analysis comparing different dietary lipids (LL, PUFA:SFA and n-3 PUFA) with LI, PUFA:SFA and n-3 PUFA were negatively associated with CHD. However, we did not find a significant association between dietary LL and CHD. The associations between alternative dietary LIs (without TFA or PUFA) and CHD remained statistically significant (Appendix 4). When limiting the analysis to cohort participants with blood lipids and C-reactive protein (CRP) (N = 6188), we found that blood triglycerides and the LDL:HDL ratio were positively associated with CHD risk, and the association between dietary LI and CHD risk did not change after adjusting for LDL:HDL (Appendix 5). In our analysis, CRP did not modify the associaton of dietary LI/LL on CHD risk. "Appendix 6" shows the results comparing cumulative average dietary LI/LL with baseline LI/LL. We only observed significant associations between cumulative average dietary LI and CHD risk.
Discussion
In the cohort analysis of 85,563 women in the WHI observational study (1994–2014), higher dietary LI was associated with increased risk of CHD after adjusting for potential sources of confounding and selection bias. In the adjusted case-control analysis of 2428 women selected from the WHI 2005 database, we found a significant positive association between plasma PL LI and the risk of CHD. Our results are consistent with two previous matched case-control studies [6, 7]. In these studies, dietary LI was positively associated with increased risk of CHD and myocardial infarction (RR = 1.61 and 1.57, respectively, when comparing extreme quintiles) [6, 7].
FAs regulate cellular membrane fluidity and physiological function and further influence cardio-metabolic risks. The LI was designed to summarize the quality of FA, either consumed in the diet or present in biological samples, into an index. The quality of FA with regard to lipophilicity, was defined by melting points, which reflect two main molecular characteristics of FAs: FA hydrocarbon chain length and unsaturation (number of double bonds) [22]. A higher melting point is related to a longer hydrocarbon chain, a greater degree of saturation and presence of a double bond in the trans configuration. Therefore, a higher LI is associated with higher FA lipophilicity and may indicate lower membrane fluidity.
The association between LI and CHD risk can be mainly explained by cell membrane fluidity and the PL fluidity of lipoproteins, which involve multiple mechanisms. Cell membrane fluidity affects membrane permeability, transport systems, receptor functions, or enzyme activities, therefore playing an important role in the pathogenesis of CVD [23]. Lower LI can increase membrane fluidity, which further improves the activity of proteins involved in ion transport, signal transduction, cell Ca2+ handling, and intracellular pH regulation [3]. In addition, lower membrane fluidity, indicated by higher LI, is associated with endothelial dysfunction through increased oxidative stress [24], impaired oxygen permeability in cell membranes [25], and impaired vascular endothelial wound closure under shear stress [26], and it is also associated with decreased insulin resistance potentially through the effect of resistin [27]. The lipoprotein fluidity, which is influenced by the PL fatty acyl composition of lipoproteins [28], can influence the structure of lipoproteins, affect the rate at which the particle or its constituent lipids are deposited in or can be removed from developing atherosclerotic plaques, and further change the risk of CHD [29]. For example, lower LI is associated with higher fluidity of HDL particles, increasing the activity of lecithin:cholesterol acyltransferase [30] and the capacity of HDL to promote cholesterol efflux [31], thus lowering the development of CHD.
Alternative explanations for the association between LI and CHD may be the differential effects of FA. In our study, dietary and plasma PL LIs were positively associated with SFA and negatively associated with PUFA. Lower LI, indicating a higher proportion of PUFA, is beneficial for CHD since PUFA has pleiotropic beneficial effects in the cardiovascular system, including anti-thrombotic, anti-atherosclerotic, anti-arrhythmic, anti-inflammatory, and anti-fibrotic properties [32,33,34]. A higher level of PUFA is also associated with diminished liver triglyceride production, an increased rate of cholesterol clearance, and increased FA oxidation by suppressing the expression of lipogenesis genes [22, 35] and improved insulin sensitivity [36].
The LI was designed to summarize the quality of overall FAs; however, it has several limitations. First, the estimated LI depends on the source of FA, and its biological function may also be source-dependent. For example, as plasma PL FAs reflect both dietary fat consumption and FA metabolism, the composition of plasma PL FA is different from that of dietary FA. Therefore, the LI derived from different sources needs to be interpreted cautiously. Second, the LI derived from any source may not be a direct measurement of membrane fluidity of various cells and plasma lipoproteins [37]. Third, melting points and molecular weights may not necessarily capture the membrane fluidity. For example, the melting points of TFA are lower than those of SFA with the same number of carbon atoms. However, TFA is a strong predictor of CHD [38, 39].
With regard to the study limitations, LIs derived from both dietary FA and plasma PL FA were prone to measurement error, especially dietary FA computed from food frequency questionnaires. Not all types of FAs with available melting points from the LipidBank database were measured by our study. However, these other FAs are present in only small amounts; hence, our measurements captured the predominant FAs. In addition, we cannot exclude the possibility of the following situations as explanations of our results: (1) residual or unmeasured selection bias due to excluding participants, (2) residual or unmeasured confounding bias due to unmeasured blood lipids, which can influence the measurement of plasma PL FA, and (3) bias due to measurement error associated with FA measurements and imputed values at year 3. Furthermore, we have a limited number of FA measurements. Specifically, dietary FAs were measured twice within the first 3 years, much shorter than the average follow-up time, and plasma PL FAs were measured only once at baseline. Since plasma PL FAs only reflect medium-term dietary fat intake, one measurement may not represent long-term average FA levels and thus are less likely to predict long-term CHD risk. Finally, the results of our study were restricted to postmenopausal women.
Our study does have some strengths compared with previous studies. Our study represents a large, multiethnic, and geographically diverse population with a long period of follow-up. In addition, we used the accumulative average diet method to calculate dietary LI based on two dietary measurements and treated dietary LI as a time-varying exposure, thus reducing measurement error in food frequency questionnaires and making dietary LI more representative of long-term dietary fats.
The LI, derived from diet and plasma PL, summarizing the overall FA lipophilicity, were positively associated with CHD risk. Accordingly, the LI may aid in predicting CHD risk beyond individual FA and plasma lipids, which are established CHD risk factors. Future studies with multiple measurements of FAs from different sources, including diet, plasma, erythrocytes, and adipose tissue, are necessary to strengthen the observed evidence regarding the association between LI and CHD risk. Additional research related to potential pathways between LI and CHD is also likely warranted.
Abbreviations
- BMI:
-
Body mass index
- CHD:
-
Coronary heart disease
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- FA:
-
Fatty acid
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- LI:
-
Lipophilic index
- MET:
-
Metabolic equivalent of task
- MUFA:
-
Monounsaturated fatty acid
- OR:
-
Odds ratio
- PL:
-
Phospholipid
- PUFA:
-
Polyunsaturated fatty acid
- RR:
-
Risk ratio
- SD:
-
Standard deviation
- SFA:
-
Saturated fatty acid
- TFA:
-
Trans fatty acid
- WHI:
-
Women’s Health Initiative
References
Mozaffarian D et al (2015) Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131(4):e29–e322
The Antiretroviral Therapy Cohort Collaboration (2013) Influence of geographical origin and ethnicity on mortality in patients on antiretroviral therapy in Canada, Europe, and the United States. Clin Infect Dis 56(12):1800–1809 (Epub 2013 March 1)
Cooper RA (1977) Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N Engl J Med 297(7):371–377
Hodson L, Skeaff CM, Fielding BA (2008) Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47(5):348–380
Ding EL et al (2008) Lipophilic index of fatty acid fluidity in erythrocyte and plasma and risk of coronary heart disease. Circulation 118(S_1089):10
Wu H et al (2013) A novel fatty acid lipophilic index and risk of CHD in US men: the health professionals follow-up study. Br J Nutr 110(3):466–474
Toledo E et al (2013) A novel fatty acid profile index—the lipophilic index—and risk of myocardial infarction. Am J Epidemiol 178(3):392–400
The Women’s Health Initiative Study Group (1998) Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 19(1):61–109
Hays J et al (2003) The Women’s Health Initiative recruitment methods and results. Ann Epidemiol 13(9 Suppl):S18–S77
Curb JD et al (2003) Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol 13(9 Suppl):S122–S128
Matthan NR et al. (2014) Plasma phospholipid fatty acid biomarkers of dietary fat quality and endogenous metabolism predict coronary heart disease risk: a nested case-control study within the Women’s Health Initiative Observational Study. J Am Heart Assoc 3(4). doi:10.1161/JAHA.113.000764
Lichtenstein AH et al (2006) Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am J Clin Nutr 84(3):497–504
Hu FB et al (1999) Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 149(6):531–540
Bertoia ML et al (2013) Long-term alcohol and caffeine intake and risk of sudden cardiac death in women. Am J Clin Nutr 97(6):1356–1363
Resche-Rigon M, White IR (2016) Multiple imputation by chained equations for systematically and sporadically missing multilevel data. Stat Methods Med Res. doi:10.1177/0962280216666564
Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: Update of 2014 Federal Actions to Achieve National Goals and Improve Outcomes Along the HIV Care Continuum. 2014
McTiernan A et al (2003) Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA 290(10):1331–1336
Hernán MA, Hernandez-Diaz S, Robins JM (2004) A structural approach to selection bias. Epidemiology 15(5):615–625
Howe CJ et al (2016) Selection bias due to loss to follow up in cohort studies. Epidemiology 27(1):91–97
Hernan MA et al (2002) Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 155(2):176–184
Ding EL et al (2015) Dietary lipophilic load and dietary lipophilic index with risk of coronary heart disease in middle-aged women: beyond conventional fat classifications. Circulation 131(Suppl 1):A19
van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9(2):112–124
Zicha J, Kunes J, Devynck MA (1999) Abnormalities of membrane function and lipid metabolism in hypertension: a review. Am J Hypertens 12(3):315–331
Singh U, Jialal I (2006) Oxidative stress and atherosclerosis. Pathophysiology 13(3):129–142
Dumas D et al (1999) Membrane fluidity and oxygen diffusion in cholesterol-enriched endothelial cells. Clin Hemorheol Microcirc 21(3–4):255–261
Gojova A, Barakat AI (2005) Vascular endothelial wound closure under shear stress: role of membrane fluidity and flow-sensitive ion channels. J Appl Physiol (1985) 98(6):2355–2362
Tsuda K (2015) Association of resistin with impaired membrane fluidity of red blood cells in hypertensive and normotensive men: an electron paramagnetic resonance study. Heart Vessels 31(10):1724–1730. doi:10.1007/s00380-015-0755-0
Sola R et al (1990) Effects of dietary fats on the fluidity of human high-density lipoprotein: influence of the overall composition and phospholipid fatty acids. Biochim Biophys Acta 1043(1):43–51
Soutar A (1978) Does dietary fat influence plasma lipoprotein structure? Nature 273(5657):11–12
Parks JS et al (2000) Phosphatidylcholine fluidity and structure affect lecithin:cholesterol acyltransferase activity. J Lipid Res 41(4):546–553
Berrougui H et al (2007) Age-related impairment of HDL-mediated cholesterol efflux. J Lipid Res 48(2):328–336
Harper CR, Jacobson TA (2001) The fats of life: the role of omega-3 fatty acids in the prevention of coronary heart disease. Arch Intern Med 161(18):2185–2192
Demaison L, Moreau D (2002) Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: a possible mechanism of action. Cell Mol Life Sci 59(3):463–477
Mozaffarian D, Wu JH (2011) Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58(20):2047–2067
Clarke SD (2004) The multi-dimensional regulation of gene expression by fatty acids: polyunsaturated fats as nutrient sensors. Curr Opin Lipidol 15(1):13–18
Manco M, Calvani M, Mingrone G (2004) Effects of dietary fatty acids on insulin sensitivity and secretion. Diabetes Obes Metab 6(6):402–413
Emken EA et al (1979) Incorporation of deuterium-labeled cis- and trans-9-octadecenoic acids in humans: plasma, erythrocyte, and platelet phospholipids. Lipids 14(6):547–554
Mozaffarian D et al (2006) Trans fatty acids and cardiovascular disease. N Engl J Med 354(15):1601–1613
Sun Q et al (2007) A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation 115(14):1858–1865
Acknowledgements
WHI investigators Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA). Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings. Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Yvonne Michael; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael Simon. Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no potential conflict of interest.
Appendices
Appendix 1. Causal diagrams hypothesized for the two study designs
See Fig. 2.
I Dietary LI in cohort study. II Plasma PL LI in matched case-control study. C1 includes age, region, race/ethnicity, education, income, BMI, physical activity, smoking, family history of myocardial infarction/diabetes/stroke, medication use, postmenopausal hormone use, self-reported baseline hypertension/diabetes/cancer/hypercholesterolemia/hysterectomy status, dietary alcohol, percent calories from protein and carbohydrates, and total energy intake. C2 includes age, region, race/ethnicity, education, income, self-reported baseline hypertension/diabetes/cancer/hypercholesterolemia, and hysterectomy status. C3 includes age, race/ethnicity, hysterectomy status, BMI, physical activity, smoking, family history of myocardial infarction/diabetes/stroke, medication use, postmenopausal hormone use, self-reported baseline hypertension/diabetes/cancer/hypercholesterolemia, dietary alcohol, percent calories from protein and carbohydrates, and total energy intake. A box around a node represents conditioning on that node
Appendix 2
See Table 4.
Appendix 3
See Table 5.
Appendix 4
See Table 6.
Appendix 5
See Table 7.
Appendix 6
See Table 8.
About this article
Cite this article
Liu, Q., Lichtenstein, A.H., Matthan, N.R. et al. Higher Lipophilic Index Indicates Higher Risk of Coronary Heart Disease in Postmenopausal Women. Lipids 52, 687–702 (2017). https://doi.org/10.1007/s11745-017-4276-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-017-4276-8