Abstract
Methyl ester sulfonate (MES) is an anionic surfactant derived from palm oil through sulfonation of fatty acid methyl esters. Due to limited ecotoxicological data on MES, this study was initiated to evaluate the ecotoxicological properties of MES and its impact to the environment. The respirometric method (OECD 301F) was used to monitor the biodegradation of various homologues of MES over 28 days. The algae growth inhibition test (OECD 201) was conducted to assess the effects of palm-based MES towards green algae by exposing exponentially-growing cultures of Pseudokirchneriella subcapitata (P. subcapitata) to five concentrations of MES with maximum concentrations of 100 mg/L. Results showed all MES samples were readily biodegradable, where the biodegradability of each homologue surpassed 60% within 28 days. It was also observed that the longer the carbon chain length of MES, the solubility and the biodegradability rate decreased. The ecotoxicity of C12 and C14 MES towards P. subcapitata after 72 h of experiment showed no inhibition of algae growth in C12 MES while, the growth of algae decreased as the concentration of C14 MES increases. The EC50 value for C14 MES and C16 MES towards green algae was >100 and >10 mg/L, respectively. It can be concluded that C12 and C14 MES were practically non-toxic towards P. subcapitata and the toxicity increased with an increase in chain length of the surfactant (EC50 value decreases). Therefore homologues of palm-based MES are not expected to cause environmental concern due to their biodegradability and low toxicity in the aquatic environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are widely used as active ingredients in household and industrial applications. The world production of synthetic surfactants reaches 13 million tons annually and they are economically important products [1]. In 2014, the surfactants world market earned more than 33 billion US-dollars. The market research institute Ceresana, Germany forecast that the annual revenues for surfactant will increase by 2.5% p.a. until 2022 [2]. The global surfactant market was forecast to grow at a compound annual rate of 6.02% from 2015 to 2019 (Asia–Pacific, Europe, North and South America, and the rest of the world), with rising demand for personal care products [3].
Surfactants are potential toxins when they enter the environment in large quantities via wastewaters, so the determination of aquatic toxicity and biodegradability of surfactants is of utmost importance [1]. Because surfactants may have adverse effects on humans and animals, it is important that in the future all new surfactants that enter the market have a product safety datasheet which includes the environmental protection parameters such as biodegradability and ecotoxicity. Biodegradation is a process whereby microorganisms decompose the organic matter in the environment through utilization of carbon as food. Biodegradation can be described as the destruction of chemicals by the metabolic activity of microorganisms [4]. In primary biodegradation, microorganisms convert the organic molecule into different products, but in ultimate biodegradation, they assimilate the organic molecule completely into simple molecules (such as carbon dioxide/methane, nitrate/ammonium and water) [5]. The ability of a chemical compound to degrade is the most significant parameter to determine the adverse effects of the compound on the ecosystem. The percentage of biodegradability of a surfactant must fulfill certain regulation requirement, such as the European environmental safety legislation, Regulation (EC) No 648/2004 on detergents [6]. For reproducible and comparable results between different laboratories, standardized methods have been used globally. There are a number of standardized biodegradability test methods available, such as the International Organization for Standardization (ISO) standard methods, for example ISO/TR 15462 on water quality-selection of test for biodegradability, and the Organization for Economic Co-operation and Development (OECD) standard methods [7]. A compound that gives a positive result in one of the ‘ready biodegradability’ tests (e.g. OECD 301 series) is assumed to be able to rapidly biodegrade in the environment, releasing non-toxic by-products like carbon dioxide and water (ultimate biodegradation). Hence, a compound that is readily biodegradable should not cause any adverse effects on living organisms in the environment [8].
Ecotoxicity studies examine the toxic effects caused by chemicals on living organisms. Aquatic toxicity tests using test species such as fish, invertebrates or algae are usually single-species tests in which the toxicity of a chemical is measured through mortality, decreased growth rate or lowered reproductive capacity, either by an acute toxicity test or a chronic toxicity test [9]. These tests have been standardized by organizations such as the OECD, ISO and American Society for Testing and Materials (ASTM), and are applied to a selected group of organisms. The ecotoxicity assays are based on exposing a population of aquatic test species to a series of test substance’s concentrations. The ecotoxicity tests provide an estimation of the concentration that cause effects such as mortality, mobility inhibition, reproduction interference, reduction of respiration, etc. to 50% of the population exposed, usually expressed in terms of EC50 (effective concentration) or LC50 (lethal concentration) [10].
Anionic surfactants are the most commonly used surfactants, contributing to about 60% of the world production, due to their ease and low cost of production. They include alkylbenzene sulfonates (detergents), fatty acid (soaps), lauryl and laureth sulfate (foaming agent), di-alkyl sulfosuccinate (wetting agent) and lignosulfonates (dispersants). Most of the anionic surfactants are used in detergents because of their cleaning performance and effectiveness in removing dirt [11, 12]. The three most frequently encountered anionic surfactants in the new chemical program are sulfonates, phosphonates and fatty acids. The strongest surfactants are the sulfonates and the weakest are the fatty acids. The easiest to assess are the sulfonates because of the large amount of information for linear alkyl benzene sulfonates (LAS) in terms of their acute and chronic toxicity to fish and aquatic invertebrates [13]. Due to the public concern on environmental fate of surfactants in the aquatic environment, many ecotoxicology studies have been performed on both anionic and non-ionic surfactants using different type of test species, i.e. fish, water flea and microalgae [14,15,16,17].
Methyl ester sulfonate (MES) is an anionic surfactant derived from palm oil through sulfonation of fatty acid methyl esters. It has good surface-active properties, excellent detergency and is less sensitive to water hardness compared to LAS [18,19,20]. The global fatty methyl ester sulfonates (FMES) market is currently in its growth phase due to consumer awareness on biodegradable surfactants in detergents. MES was valued at USD 0.57 billion in 2014 and is anticipated to reach USD 1.58 billion by 2020, growing at a compound annual growth rate (CAGR) of 18.6% between 2015 and 2020 [21]. This paper is intended to evaluate the biodegradability and ecotoxicity of various homologues of palm-based surfactant, MES in order to establish their impact to the environment.
Experimental
Materials
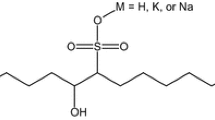
Test substances were palm-based MES of various chain lengths (C12, C14 and C16) produced from palm stearin methyl esters in the Malaysian Palm Oil Board (MPOB) MES pilot plant. The palm stearin methyl esters were purchased from Emery Oleochemical (M) Sdn Bhd, a Roundtable on Sustainable Palm Oil (RSPO) company. The purity of MES was more than 70%. Molecular structure of MES is shown in Fig. 1. C10-13 LAS was purchased from Kong Long Huat, Malaysia and neutralized prior to the biodegradation test. C10-13 LAS is used for comparison purposes.
For biodegradation test, aniline (99%) from AnalaR-BDH, UK, was used to check the validity of the test procedure. While, for ecotoxicity test, a reference compound, potassium dichromate, K2Cr2O7 from Friendemann Schmidt, Germany, was used to check the sensitivity of the test organisms and the conformity with the test procedure.
Procedure for ready biodegradability study using OECD 301F, Manometric Respirometry Test
The inoculum used in the biodegradation study was derived from activated sludge of a wastewater treatment plant treating predominantly domestic sewage. The sludge was collected from Indah Water Konsortium (IWK) Putrajaya, Malaysia and diluted in mineral medium to the concentration as stated in the OECD 301F test method [7]. Thereafter, the sludge was pre-conditioned by aerating it in a mineral medium for 5 days at the test temperature (22 ± 2 °C).
All biochemical oxygen demand (BOD) bottles were inoculated with a small volume of the inoculum to give a concentration of 30 mg/L of suspended solids as suggested in the OECD test guidelines [7]. The mineral medium for the test was prepared as described in OECD [7]. Total volume for each BOD bottle was 300 mL. The oxygen uptakes in all bottles were measured directly using the BOD EVO (Velp, Italy) system for 28 days to produce BOD curves for each test bottle. The experiment was conducted in duplicate. The six BOD bottles used in OECD 301F method were prepared as in Table 1.
The percentage of biodegradation of the sample is calculated using Eq. 1:
where BOD is given by Eq. 2.
The degree of biodegradation is expressed as a percentage of the theoretical oxygen demand (ThOD), i.e. the maximum oxygen demand required for complete biodegradation of the test substance. This maximum value is normally calculated from the molecular formula of the test substance. The ThOD for each MES and LAS samples were calculated using the formula described by Gerike [22].
Procedure for ecotoxicity study using OECD 201, Algae, Growth Inhibition Test
The ecotoxicity study of MES was performed separately from the biodegradation study. The ecotoxicity of the various homologues of MES was assessed using green algae, Pseudokirchneriella subcapitata (Korshikov) Hindak (ATCC® 22662™) (formerly Selenastrum capricornutum) obtained from the American Type Culture Collection (Maryland, USA). P. subcapitata was cultured and maintained in the mineral medium as stated in method OECD 201 [23]. The cultures were re-cultured weekly into fresh sterilized medium, composed of both micro- and macronutrients. This procedure ensured a steady supply of logarithmic exponential phase cells in 5–7 days after inoculations. The pH of algae medium was adjusted to 7.5 (±0.01) with 0.1 N NaOH or 0.1 N HCl prior to sterilization. Algae were incubated in 200-mL conical flasks covered with silicon caps in 14-h light (4000 Lux) and 10-h dark cycle incubator (EYELA FLI-2000, Japan,) at 24 °C and shaken at 100 rpm.
The algae growth inhibition test was conducted to assess the effects of palm-based MES towards P. subcapitata and to determine the 72-h EC50 value of MES according to standard method OECD 201 [23]. The exponentially-growing cultures of P. subcapitata cultured in OECD 201 media were exposed to five different concentrations of MES with maximum concentrations of 100 mg/L. The inhibition of growth in relation to a control culture was determined for 72 h. Cell concentration was estimated using a particle counter (Beckman Counter Z2, USA). The EC50 values were calculated via probit analysis using SPSS version 17 software based on the dose–response curves. The probit analysis is commonly used in toxicology to determine the relative toxicity of chemicals to living organisms.
Results and Discussion
Aerobic Biodegradability of Palm-based MES
The molecular formula and ThOD of MES and aniline are shown in Table 2. From the table, the ThOD values increase as the chain length of MES increases. The biodegradability of MES (C12, C14 and C16) determined using method OECD 301F, Manometric Respirometry are shown in Fig. 2. In principle, any substance meeting the OECD ready biodegradability pass levels (either 60% CO2 evolution, 60% BOD/ThOD or 70% dissolved organic carbon (DOC) removal within 28 days test period) in one of the biodegradability screening tests are considered as ‘‘readily biodegradable’’ [7]. The OECD 301F test method uses 60% ThOD as the pass level.
The biodegradability of C12, C14 and C16 MES was 73% within 6 days, 66% within 8 days and 63% within 16 days, respectively. From the results, it can be concluded that all MES samples were readily biodegradable since their biodegradability surpassed the 60% pass level within 28 days. MES has a linear structure which made the surfactant easily biodegraded by the microbes compared to other surfactants that are branched or having aromatic moiety. The degradation pathway of MES was well described by Masuda et al. [24] and Roberts [25], where the biodegradation of MES started with ω-oxidation to form a carboxyl group and continued with β-oxidation, removing two carbons at a time, to form monomethyl α-sulfosuccinate. This undergoes desulfonation to succinic acid, which features naturally in cell metabolism. In addition, the longer the carbon chain length of MES, the solubility and the biodegradability rate decreases. In general, biodegradability increases with increasing solubility; solubility is inversely proportional to alkyl chain length [26]. In this case, microorganisms may take longer time to degrade any substance with a longer carbon chain length. The biodegradability of MES has also been reported by Razmah and Salmiah [20]. In their report, by using method OECD 301D Closed Bottle Test, palm-based MES was found to be readily biodegradable with more than 80% degraded in only 8 days. In addition, the MES is not expected to cause environmental concern because of its high biodegradability, which will leave very little residual and therefore is not toxic to aquatic organisms. In terms of detergency performance, Zulina et al. [18] showed that C16 MES had the most excellent performance compared to other carbon chain length. The detergency performance of MES is in the order C16 > C14 > C18 > C12.
The petroleum-based surfactant, LAS, was also readily biodegraded with 60% biodegradability achieved within 8 days (Fig. 2). The biodegradability of C10-13 LAS was lower and slower than the biodegradability of palm-based C12 and C14 MES, although all these three surfactants have about the same carbon chain lengths. According to Siti Afida et al. [27], LAS has an aromatic moiety and this made the surfactant more difficult to biodegrade. The biodegradability of LAS also had been reported by Matthew and Malcolm [28]. The mechanisms of LAS breakdown begins with ω-oxidation at the end of the alkyl chain, yielding a mono- and dicarboxylic sulfophenyl acid, which is then subjected to β-oxidation, followed by cleavage of the benzene ring with conversion of the sulfonate group to inorganic sulfate in the environment [29].
Ecotoxicity of Palm-based MES
The sensitivity test was performed using potassium dichromate (K2Cr2O7) and Fig. 3 shows the effect of K2Cr2O7 on algae growth. At the concentration 10 mg/L and 30 mg/L, the growth of algae was 100% inhibited. According to ISO 8692 [30], the acceptable EC50 value range for the sensitivity test is 1.19 ± 0.27 mg/L. The EC50 value for K2Cr2O7 obtained in the experiment was 1.13 mg/L, which was within the acceptable range and confirmed that the cultured P. subcapitata in this experiment can be used as a test species for ecotoxicity studies of MES samples.
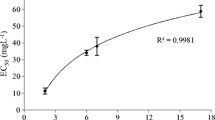
After 72 h of the experiment on C12 and C14 MES, no inhibition of algae growth was observed in C12 MES while, the growth of algae decreased as the concentration of C14 MES increases. At a concentration of 100 mg/L, the growth of algae was inhibited by about 26%. The EC50 value for C14 MES towards green algae was >100 mg/L (Fig. 4). The C16 MES sample has low water solubility, i.e. 10 mg/L at 24 °C (test temperature). For ecotoxicity test on poorly soluble substance, the toxicity test should be conducted only up to the maximum dissolved concentration under the test conditions [31]. Therefore, the highest concentration tested for C16 MES was 10 mg/L, where at this concentration about 7% of algae growth was inhibited. The EC50 value for C16 MES sample was >10 mg/L.
Table 3 shows the summary of the effect of MES homologues towards P. subcapitata. According to GESAMP [32] rating scheme for acute aquatic toxicity, C12 and C14 MES were practically non-toxic (EC50 > 100 mg/L) towards P. subcapitata and the toxicity increased with an increase in chain length of the surfactant (EC50 value decreases). This ecotoxicity trend has also been reported using fish as the test species [20]. For anionic surfactants, the aquatic toxicity depends mainly on the length of the carbon chain in the molecule. The toxicity level of a substance has a correlation with the chain length of the alkyl group [33]. This correlation has also been observed in the homologues of alcohol sulfates and alkylbenzene sulfonates where, the longer the carbon chain, the more toxic the anionic surfactant [34, 35]. The probable reason for the toxicity increase with homologue chain length was a greater interaction of the heavier homologues with cell membranes [29]. However, a systematic dependence of the toxicity on the chain length is only recognizable in fully water-soluble compounds [36].
The ecotoxicity of C10-13 LAS towards P. subcapitata was measured at the highest concentration tested (100 mg/L), where the algae growth inhibition was about 69% and the EC50 calculated was 78 mg/L. This findings show that MES is less toxic than LAS at similar carbon chain length. The finding in this study was in agreement with the study by Torben et al. [37] and Verge et al. [38] where the EC50 values of C12 LAS and C14 LAS were 48 mg/L and 18 mg/L, respectively.
Conclusion
Palm-based MES are readily biodegradable surfactants as shown through studies carried out using the OECD 301F Manometric respirometry standard test method, where their biodegradability surpassed the 60% pass level. The results showed that as the carbon chain length of MES increases, it became less biodegradable because more time was required by the microbes to degrade this surfactant into simple molecules. On the other hand, the presence of aromatic structure in LAS may also extend the biodegradation process. C12 and C14 MES were practically non-toxic (EC50 > 100 mg/L) towards P. subcapitata and the toxicity increased with an increase in chain length of the surfactant (EC50 value decreases). Hence MES are not expected to cause environmental concern due to only 10–30% of MES is used in detergent products and it is readily biodegradable. Thus MES can be a potential alternative to LAS as the workhorse surfactant for the detergent industry.
References
Linda P. Screening methods for aquatic toxicity of surfactants. MSc Thesis. Chalmers University of Technology, Sweden; 2012.
Ceresana Research. Market study on surfactants, 2nd edn. http://www.ceresana.com/en/market-studies/chemicals/surfactants/. Accessed 3 Aug 2015.
Mathew J, Eadsforth C, Schowanek D, Delfosse T, Riddle A, Budgen N. Comprehensive review of several surfactants in marine environments: fate and ecotoxicity. Environ Toxicol Chem. 2016;35:1077–86.
Balson T, Felix MSB. The biodegradability of non-ionic surfactants. In: Karsa DR, Porter MR, editors. Biodegradability of surfactant. Glasgow: Blackie Academic and Professional; 1995. p. 204–30.
Betton CI. Lubricants and their environmental impact. In: Mortier RM, Orszulik ST, editors. Chemistry and technology of lubricants, 3rd ed. New York: Springer; 2009. p. 547.
European Commission. Regulation (EC) No 648/2004 of the European Parliament and the Council of 31 March 2004 on detergents. (DO L 104, 04/08/2004).
OECD. OECD guidelines for the testing of chemicals. Section 3: Degradation and accumulation. Test 301: Ready biodegradability. Updated guideline, adopted 17 July 1992. OECD, Paris, France;1992.
Painter HA. Detailed review paper on biodegradability testing. OECD Test Guidelines programme. Periodical review; 1992.
Van Leeuwen CJ, Hermen JLM. Risk assessment of chemicals: an introduction. Netherlands: Springer; 2010. p. 688.
Lechuga M, Fernández-Serrano M, Jurado E, Núñez-Olea J, Ríos F. Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotoxicol Environ Saf. 2016;125:1–8.
Salager JL. Surfactants Types and Uses. Booklet. Universidad De Los Andes; 2002.
Maria A. Acute toxicity to Daphnia magna in river water; Investigating mitigation and bioavailability of pure cationic surfactants and mixtures with SPME. MSc Thesis. University of Gothenburg; 2012.
Wayne GL, Jane SH, Michael AL. Environmental toxicology and risk assessment. Philadelphia: ASTM; 1916. p. 47.
Serrano MF, Jurado E, Arteaga AF, Rios F, Lechuga M. Ecotoxicological assessment of mixtures of ether carboxylic derivative and amine-oxide-based non-ionic surfactants on the aquatic environment. J Surfact Deterg. 2014. doi:10.1007/s11743-014-1621-2.
Jurado E, Serrano MF, Olea JN, Lechuga M, Jimenez JL, Rios F. Acute Toxicity of Alkylpolyglucosides to Vibrio fischeri, Daphnia magna and Microalgae: a comparative study. Bull Environ Contam Toxicol. 2011. doi:10.1007/s00128-011-0479-5.
Rios F, Lechuga M, Serrano MF, Arteaga AF. Aerobic biodegradation of amphoteric amine-oxide-based surfactants: effect of molecular structure, initial surfactant concentration and pH. Chemosphere. 2017;171:324–31.
Jurado E, Serrano MF, Lechuga M, Rios F. Environmental impact of ether carboxylic derivative surfactants. J Surfact Deterg. 2012;15:1–7.
Zulina AM, Razmah G, Parthiban S, Zahariah I, Salmiah A. Alpha-sulfonated methyl ester as an active ingredient in palm-based powder detergents. J Surfactants Deterg. 2006;9:161–7.
Salmiah A, Zahariah I, Jasmin S. Palm-based sulphonated methyl esters and soap. J Oil Palm Res. 1998;10:15–35.
Razmah G, Salmiah A. Biodegradability and ecotoxicity of palm stearin-based methyl ester sulphonates. J Oil Palm Res. 2004;16:39–44.
Market Research Store. Global Fatty Methyl Ester Sulfonates (FMES) Market is expected to Reach USD 1.58 Billion in 2020. http://www.marketresearchstore.com/news/global-fatty-methyl-ester-sulfonates-fmes-market-is-45. Accessed 15 Aug 2015.
Gerike P. The biodegradability of poorly water soluble compounds. Chemosphere. 1984;13:169–90.
OECD 201. OECD guidelines for the testing of chemicals. Freshwater alga and cyanobacteria, growth inhibition test. Updated guideline, adopted 28 July 2011. OECD, Paris, France; 2011.
Masuda M, Odake H, Miura K, Ito K, Yamada K, Oba K. Biodegradation of 2-sulfonat fatty acid methyl ester (α-SFMe). II. Biodegradation pathway of α-SFMe. J Jpn Oil Chem Soc. 1993;42:21–5.
Roberts DW. Chemistry of methyl ester sulfonates. Biorenewable resources. 2008;5:2–9.
USGS. Biodegradation. Available at: http://toxics.usgs.gov/definitions/biodegradation.html. Accessed 11 Aug 2015.
Siti Afida I, Razmah G, Zulina AM. Biodegradation of various homologues of palm-based methyl ester sulphonates (MES). Sains Malaysiana. 2016;45:951–6.
Matthew JS, Malcom NJ. The biodegradation of surfactants in the environment. Biochem Biophys Acta. 2000;1508:235–51.
Ivankovic T, Hrenovic J. Surfactants in the environment. Arh Hig Rada Toksikol. 2010;61:95–110.
ISO 8692. Water quality—freshwater algae growth inhibition test with Scenedesmus subspicatus and Pseudokirchneriella subcapitata. International organization for standardization, Geneva, Switzerland; 2000.
Weyman GS, Rufli H, Weltje L, Salinas ER, Hamitou M. Aquatic toxicity tests with substances that are poorly soluble in water and consequences for environmental risk assessment. Environ Toxicol Chem. 2012;. doi:10.1002/etc.1856.
GESAMP. Revised GESAMP Hazard evaluation procedure for chemical substances carried by Ships. 2nd ed. London: International Maritime Organization; 2014. p. 32 (ISSN: 1020-4873).
Toshiharu T, Horoshi O, Kazuaki M, Yutaka T. Ecotoxicity of tetradecanoic acid, 2-sulfo-, 1-methylester, sodium salt (C14MES). J Oleo Sci. 2006;55:121–6.
Potokor MS. Acute, subacute and chronic toxicity data on anionics. Anionic surfactants, biochemistry, dermatology. In: Gloxhuber C, Kunstler K, editors. Surfactant science series, vol. 43. 2nd ed. 1992. p. 81–116.
Fendinger NJ. Environmental behaviour and fate of anionic surfactants. In: Baker LA, editor. Environmental chemistry of lakes and reservoirs. Washington: American Chemical Society; 1994. p. 528–57.
Garcia MT, Ribosa I, Guindulain T, Sanchez-Leal J, Vives-Rego J. Fate and effect of monoalkyl quaternary ammonium surfactants in the aquatic environment. Environ Pollut. 2001;111:169–75.
Torben M, Helle BB, Dorthe N, Anne RP, Gitte IP, Flemming S. Environmental and health assessment of substances in household detergents and cosmetic detergent products. Denmark: Centre for Integrated Environment and Toxicology; 2001. p. 32 (environmental project no. 615).
Verge C, Moreno A, Roque S. Toxicity of anionic surfactants to green microalgae Scenedesmus subspicatus and Selenestrium capricornutum. Tenside Surfactant Deterg. 1996;33:166–9.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ishak, S.A., Ghazali, R., Abd Maurad, Z. et al. Ecotoxicology Study of Various Homologues of Methyl Ester Sulfonates (MES) Derived from Palm Oil. J Surfact Deterg 20, 1467–1473 (2017). https://doi.org/10.1007/s11743-017-2011-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-017-2011-3