Abstract
Experimental studies were conducted to evaluate the thermal stability and rheological properties of novel surfactant–polymer (SP) systems for enhanced oil recovery applications. One in-house synthesized amphoteric amidosulfobetaine surfactant 3-(N-pentadecanamidopropyl-N,N-dimethylammonium)propanesulfonate and three different polymers were evaluated. Polymer A was a terpolymer of acrylamide, acrylamido tert-butyl sulfonate, and acrylic acid, whereas polymers B and C were terpolymers of acrylamide, N-vinylpyrrolidone, and acrylamido tert-butyl sulfonate with different anionicity. Long-term thermal stability of the surfactant was assessed using FTIR, 1H NMR, and 13C NMR. The surfactant was compatible with seawater at 90 °C and no precipitation was observed. Structural analysis showed good thermal stability and no structural changes were observed after aging at 90 °C. The effects of surfactant concentration, shear rate, salinity, and polymer concentration on rheological properties of the SP systems were determined. Polymer A showed highest viscosity among the investigated polymers in deionized and seawater. The interactions between the surfactant and polymer A were assessed using rheological measurements. In the presence of salts, the viscosity of all three polymers reduced significantly as a result of charge screening. At low shear rates, the added surfactant slightly decreased the viscosity and storage modulus of polymer A. At high shear rates, the effect of the surfactant on the viscosity and storage modulus of polymer A was insignificant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand for energy has increased over the last few decades and it is expected that consumption will quadruple by 2100 [1]. Hydrocarbons are a major source of energy and at the end of last century their share of the total energy consumption was 60 %. To meet the growing demand for oil, it is important to recover maximum oil from existing reservoirs. Only one-third of the oil present in a reservoir can be recovered using conventional oil recovery methods. To recover the remaining oil, enhanced oil recovery (EOR) methods are used. Chemical, thermal, and gas injections are the most commonly used EOR methods in industry. In chemical EOR, surfactants, polymers, and alkalis are injected to increase the displacement and sweep efficiencies [2–9].
Polymers are used in chemical EOR to improve the water/oil mobility ratio by increasing the viscosity of the displacing fluid (water). A large number of polymers have been evaluated for chemical EOR applications [5, 10–13]. Partially hydrolyzed polyacrylamide (HPAM) is still the most widely used polymer in chemical EOR owing to its good water solubility, low cost, high viscosity, and availability. HPAM is the best candidate for low-temperature and low-salinity reservoir recovery. However, the viscosity of HPAM can be severely degraded at high temperature and in the presence of salts. Increasing the molecular weight results in higher viscosity retention in hostile environments, but increasing the molecular weight of HPAM makes it more sensitive to shear degradation. The performance of HPAM can be improved by introducing some more salt-tolerant and thermally stable monomers. Acrylamido tert-butyl sulfonate (ATBS) and N-vinylpyrrolidone (NVP) are examples of monomers that have been used to improve the performance of HPAM [14–17].
Surfactants are used in chemical EOR to lower the interfacial tension between water and oil [3, 18–20]. Oil recovery is directly related to a dimensionless capillary number that is the ratio of viscous forces to inertial forces. A capillary number high enough to displace the trapped oil can be obtained by lowering the interfacial tension (IFT) between water and oil to ultra-low values (10−3 m Nm−1). As a result of high temperature and high salinity, surfactants can precipitate by interacting with reservoir brine. Adsorption of surfactants on reservoir rock surfaces is another challenge related to surfactant EOR. A higher adsorption can reduce the efficiency of a surfactant in lowering the IFT and has a negative impact on the economics of the process.
Surfactants can be classified as cationic, anionic, non-ionic, and zwitterionic on the basis of their dissociation in water. Alkyl aromatic sulfonate, alcohol sulfate, internal olefin sulfonate, and branched alpha olefin sulfonate have been extensively evaluated for chemical EOR applications under different conditions [21–23]. Among the different classes of surfactants, anionic surfactants are the most widely used for EOR applications because most of the EOR research is conducted in sandstone reservoirs. Anionic surfactants are attractive for sandstone reservoirs owing to low-temperature and low-salinity conditions. In addition, anionic surfactants have lower adsorption on the sandstone rock surfaces as a result of the presence of negative charge. However, anionic surfactants are not suitable in carbonate reservoirs because of high adsorption on the carbonate rocks. In addition, most of the carbonate reservoirs involve high-temperature, high-salinity, and heterogeneous conditions which are harsh and pose additional challenges to surfactant EOR.
Amphoteric surfactants have shown good tolerance to high temperature and high salinity and are potential candidates for surfactant flooding in harsh reservoir conditions [24]. Recently hydrocarbon and fluorinated carboxybetaine-based amphoteric surfactants have shown good thermal and IFT properties under harsh conditions [24–27]. However, amidosulfobetaine surfactants have not been investigated in detail for EOR applications.
In this work, the thermal stability of our in-house synthesized amidosulfobetaine surfactant was determined using a novel approach based on structural analysis. FTIR and NMR were used to determine the structural changes in the surfactant after exposing it to 90 °C. The amidosulfobetaine surfactant was thermally stable at 90 °C. HPAM, ATBS, and NVP-based polymers were evaluated using rheological measurements. These polymers were selected owing to their good thermal stability [28]. Surfactant–polymer interactions were also assessed using rheological measurements. Surfactant concentration, polymer concentration, temperature, and salinity are the main parameters covered in this work.
Experimental
Materials
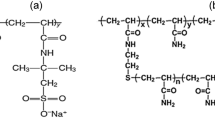
The amidosulfobetaine surfactant 3-(N-pentadecanamidopropyl-N,N-dimethylammonium)propanesulfonate was synthesized as described in our previous publication [29]. Three different commercially available polymers from SNF Floerger were evaluated. Flopaam 5220 SH (polymer A) is a terpolymer of acrylamide, ATBS, and acrylic acid and has a molecular weight of around 12 million daltons. Superpusher SAV522 (polymer B) and superpusher SAV550 (polymer C) are terpolymers of ATBS, acrylamide, and NVP that have similar molecular weight (5 million daltons) but different anionicity. Structures of the surfactant and polymers are shown in Fig. 1. The synthetic seawater (SW) of 57,643 ppm was prepared using laboratory-grade chemicals and its composition is given in Table 1.
Characterization
Thermal stability was assessed by aging the surfactant solutions in sealed tubes at 90 °C for 10 days. NMR and FTIR were used to determine the structural changes after aging at 90 °C. FTIR analysis was carried out using a Perkin-Elmer 16F model spectrometer. A JEOL 500 MHz spectrometer was used to acquire 1H and 13C NMR spectroscopic data. Tetramethylsilane was used as the reference and deuterated chloroform was used as the solvent. Rheological measurements were carried out using a Discovery hybrid rheomter (DHR-3, TA Instruments). A concentric cylinder geometry with a vapor trap was used for both steady shear and dynamic rheological testing. A shear rate ranging from 0.01 to 1000 s−1 was used in steady shear experiments. All the reported data points are within the torque limits of the machine.
Results and Discussion
Discussion of the results is divided into three parts. The first part describes the thermal stability of the synthesized amidosulfobetaine surfactant, the second part deals with rheological characterization of three polymers, and the third part explains the surfactant–polymer interactions using rheological measurements.
Thermal Stability
Compatibility of the surfactant with reservoir brine, polymers, and other injected chemicals is a primary requirement. A good surfactant should be compatible and thermally stable under reservoir conditions as it may remain inside the reservoir for several days. The harsh conditions of a reservoir can cause structural changes in the surfactant that ultimately reduce its ability to lower interfacial tension. Therefore, long-term thermal stability of the synthesized surfactant was assessed by aging the surfactant at 90 °C for 10 days in sealed vials. Short-term thermal stability of the surfactant was evaluated in our previous work [30]. Spectroscopic techniques such as NMR and FTIR were used at different periods to evaluate structural changes after aging. Only NMR and FTIR spectra of the aged sample with maximum time (10 days) are presented.
NMR and FTIR Analysis
According to 1H NMR spectra (Fig. 2) of the aged samples, the methyl [–(CH2) n –CH 3)] and methylene [–(CH 2) n –CH3)] protons that belong to the surfactant tail are still present, and methyl protons directly attached to the quaternary nitrogen [–CH2–N+(CH3)2–CH2–] also appeared. The 13C NMR spectra (Fig. 3) of the aged sample showed the presence of a terminal methyl carbon [–CH3] and the methyl carbons directly attached to the quaternary nitrogen [–CH2–N+(CH3)2–CH2–]. The two methylene carbons directly attached to the quaternary nitrogen [–CH2–N+(CH3)2–CH2–] and amide carbonyl group [–CH2–C=O–NH] were also revealed. In general, the NMR spectra obtained before and after aging exhibited similar peaks that clearly indicates that no significant structural changes occurred after aging in the presence and absence of salt.
According to FTIR spectra (Fig. 4) obtained before and after aging, two stretching bands (at 2900–3000 cm−1) were seen that correspond to the methyl (–CH3) and methylene (–CH2–) groups in the tail of the surfactant. The amide carbonyl stretching [–CH2–C=O–NH] and C–N stretching bands were observed in the spectra of the aged sample of surfactant which further confirmed the structure and survival of the surfactant in the presence of harsh conditions.
Rheological Evaluation of Polymers
Figures 5 and 6 show the comparison of three polymers at 70 °C and at fixed shear rate of 7.3 s−1 in deionized water (DW) and SW, respectively. Polymer A showed the highest viscosity compared to polymers B and C at all investigated concentrations. The higher viscosity of polymer A is associated with its higher molecular weight. In addition, polymer A is a terpolymer of acrylamide, ATBS, and AA, whereas polymers B and C are terpolymers of acrylamide, ATBS, and NVP. NVP increases the thermal stability of acrylamide polymer but it is not a good viscosifier. At least a 10-fold higher concentration of homopolymer of NVP is required to achieve the equivalent viscosity to that of HPAM [31]. Figures 5 and 6 demonstrate that all three polymers exhibit similar trends in deionized water and seawater. It is obvious that viscosity should be increased by increasing polymer concentration in both seawater and deionized water. However, as a result of charge screening, the magnitude of the viscosity in seawater is much lower compared to that in deionized water. In addition to lower molecular weight, NVP in polymers B and C is responsible for lower viscosity of the polymers. However, a significant reduction in the viscosity of all three polymers was observed in seawater. In deionized water, the higher viscosity of polymer A is associated with negative charges present on the backbone chain. Polymer chain remains stretched because of the presence of repulsive forces between negative charges which results in increasing the hydraulic radius and viscosity of the polymer. Addition of seawater brings cations into the solution and the polymer chain coiled up as a result of the decrease in repulsive forces. This decrease is associated with the interaction between the anionic polymer chain and cations present in the seawater. This charge screening decreases the hydraulic radius of the polymer that causes coiling of the polymer chain and as a result viscosity reduces.
Dynamic rheological measurements were performed to determine the storage modulus of the polymers. Initially it was believed that polymers can only improve sweep efficiency and have no effect on reducing residual oil saturation. However, recently many studies proved that the viscoelastic nature of the polymer could reduce the residual oil saturation by recovering the trapped oil [32–37]. Polymer A has the highest storage modulus compared to polymers B and C (Fig. 7). The higher storage modulus of polymer A compared to polymers B and C is associated with structural difference among these polymers. Polymers B and C contain N-vinylpyrrolidone whereas polymer A contains acrylic acid. In addition, the molecular weight of polymer A is also higher compared to polymers B and C. On the basis of its higher viscosity and storage modulus, polymer A was further evaluated and its interactions with amidosulfobetaine surfactant were determined. Figure 8 shows the steady shear viscosity of polymer A at different shear rates. At low shear rate, a Newtonian plateau was observed followed by a shear thinning region. At all shear rates, the viscosity of polymer A increased with increasing polymer concentration. However, this increase in the viscosity was more significant at low shear rates. For example, at a shear rate of 0.01 s−1, increasing the polymer concentration from 0.4 to 0.5 % causes an 80 % increase in the polymer viscosity. However, at high shear rate (10 s−1), the increase in the viscosity was only 45 %. Shear stress plots at different polymer concentrations are given in Fig. 9. At all shear rates, increasing polymer concentration shifts the stress to a higher value. However, the increase in stress is more significant at low shear rate. Zero shear viscosity (η o) and consistency index (k) of the Cross model at different polymer concentrations are given in Table 2. For all concentrations, the constant n was 0.79 which shows that shear thinning is independent of the concentrations.
Rheological Properties of Surfactant–Polymer System
Ultra-low interfacial tension is required to displace the trapped oil. The main role of surfactant addition is to lower the interfacial tension between oil and water. However, depending upon the structure it may interact with polymers in different ways and can affect the rheological properties. Therefore, the effect of surfactant on rheological properties was investigated.
Figure 10 shows the effect of the amidosulfobetaine surfactant on the viscosity of polymer A. The effect of the surfactant was more prominent at low shear rates. However, at high shear rate the addition of surfactant showed no significant effect. At low shear rates, surfactant–polymer interactions were more significant and viscosity reduction was observed during addition of surfactant. However, at high shear rates, the effect of shear becomes more prominent. For example, at a shear rate of 0.01 s−1, addition of 0.1 % surfactant resulted in about 25 % reduction in the viscosity of the polymer; however, at a shear rate of 10 s−1 and higher, this viscosity reduction was not significant. For practical applications, at the typical shear rate (7.3 s−1), the effect of shear is not significant and rheological behavior approached to that of pure polymer. There is no previous report describing the effect of the amphoteric surfactant on the terpolymer of acrylamide, ATBS, and acrylic acid. However, the interactions between amphoteric surfactant and HPAM were reported previously [38, 39]. Fluorinated amphoteric surfactant led to a small decrease in the viscosity of HPAM at low shear rates. At high shear rate, the added amphoteric surfactant has no effect on the viscosity [39]. Similar behavior was also reported for the effect of a hydrocarbon betaine-based surfactant on the viscosity of HPAM [38]. The storage modulus of the surfactant at different surfactant concentration is shown in Fig. 11. A decrease in the storage modulus was observed by increasing the concentration of the surfactant. The effect of the added surfactant was more significant at the low frequency range. At 0.1 % surfactant concentration, around 35 % decrease in G′ was observed at a frequency of 0.1 rad/s. The dynamic viscoelasticity of polymer A and the surfactant–polymer system is shown in Fig. 12. The storage modulus shows elastic solid-like behavior and the loss modulus is viscous response. When the applied force is smaller than the intermolecular forces, G′ is greater than G″ and material is able to return to its original configuration. For both the polymer and polymer–surfactant system, no crossover was observed and the storage modulus exceeds the loss modulus over the entire frequency range. The surfactant–polymer system containing 0.25 % polymer A and 0.05 % surfactant was evaluated in DW and SW as shown in Fig. 13. At a shear rate of 7.3 s−1, the percentage viscosity reduction of the SP system is similar to the viscosity reduction of the polymer. The viscosity reduction of polymer A by addition of salts is due to the charge screening effect as explained earlier. In the presence of the surfactant, the added salts have similar interactions with the polymer.
Conclusions
The synthesized amidosulfobetaine surfactant showed excellent tolerance to salinity and temperature. The surfactant was compatible with the polymer and seawater at 90 °C. NMR and FTIR analyses showed that there were no structural changes in the surfactant after aging at 90 °C for 10 days. Thermal stability is an important issue in surfactant screening as only thermally stable surfactants can lower the interfacial tension for long time periods. Thermal degradation alters the ability of a surfactant to lower the interfacial tension with time. The rheological properties can simulate the field performance of a polymer. Salts present in the seawater cause a significant reduction in the viscosity of the polymer and surfactant–polymer system as a result of charge screening. The amidosulfobetaine surfactant reduced the viscosity of the terpolymer of acrylamide, ATBS, and acrylic acid at low shear rates. However, at high shear rates the decrease in the viscosity due to added surfactant was not significant. Even at low shear rate, the reduction in the viscosity of polymer due to surfactant is negligible when compared to reduction in the viscosity of the polymer due to salts. Overall, the amidosulfobetaine surfactant did not alter the rheological properties of the polymer significantly. Adsorption, IFT, and coreflooding experiments of the amidosulfobetaine surfactant are currently underway in our laboratory.
References
Lakatos I, Toth J, Bodi T, Lakatos-szabo J, Miskolc U, Berger PD, Christie L (2007) Application of viscoelastic surfactants as mobility-control agents in low-tension surfactant floods. In: International symposium on oilfield chemistry, 28 February–2 March, Houston, Texas, 2007. Society of Petroleum Engineers. doi:10.2118/106005-MS
Yuan F-Q, Cheng Y-Q, Wang H-Y, Xu Z-C, Zhang L, Zhang L, Zhao S (2015) Effect of organic alkali on interfacial tensions of surfactant solutions against crude oils. Colloid Surf A 470:171–178
Liyanage PJ, Lu J, Arachchilage GWP, Weerasooriya UP, Pope GA (2015) A novel class of large-hydrophobe tristyrylphenol (TSP) alkoxy sulfate surfactants for chemical enhanced oil recovery. J Petrol Sci Eng 128:73–85
Goudarzi A, Delshad M, Mohanty KK, Sepehrnoori K (2015) Surfactant oil recovery in fractured carbonates: experiments and modeling of different matrix dimensions. J Petrol Sci Eng 125:136–145. doi:10.1016/j.petrol.2014.11.008
Olajire AA (2014) Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: prospects and challenges. Energy 77:963–982. doi:10.1016/j.energy.2014.09.005
Lu J, Goudarzi A, Chen P, Kim DH, Delshad M, Mohanty KK, Sepehrnoori K, Weerasooriya UP, Pope GA (2014) Enhanced oil recovery from high-temperature, high-salinity naturally fractured carbonate reservoirs by surfactant flood. J Petrol Sci Eng 124:122–131
Khan MY, Samanta A, Ojha K, Mandal A (2009) Design of alkaline/surfactant/polymer (ASP) slug and its use in enhanced oil recovery. Pet Sci Technol 27(17):1926–1942
Samanta A, Ojha K, Sarkar A, Mandal A (2011) Surfactant and surfactant-polymer flooding for enhanced oil recovery. Adv Pet Explor Dev 2(1):13–18
Samanta A, Bera A, Ojha K, Mandal A (2012) Comparative studies on enhanced oil recovery by alkali–surfactant and polymer flooding. J Pet Explor Prod Te 2(2):67–74
Zhang XM, Guo YJ, Liu JX, Zhu YW, Hu J, Feng RS, Fu CY (2014) Adaptability of a hydrophobically associating polyacrylamide/mixed-surfactant combination flooding system to the Shengli Chengdao oilfield. J Appl Polym Sci 131:12
Kamal MS, Sultan AS, Al-Mubaiyedh UA, Hussein IA, Feng Y (2015) Rheological properties of thermoviscosifying polymers in high-temperature and high-salinity environments. Can J Chem Eng 93(7):1194–1200. doi:10.1002/cjce.22204
Saleh L, Wei M, Bai B (2104) Data analysis and novel screening criteria for polymer flooding based on a comprehensive database. In: SPE improved oil recovery symposium, 12–16 April, Tulsa, Oklahoma, 2014. Society of Petroleum Engineers. doi:10.2118/169093-MS
Kuang W, Xia Y (2014) A novel dendritic-like terpolymer as a viscosifying additive for enhanced oil recovery. Mater Lett 115:109–112
Bock J, Pace SJ, Schulz DN (1987) Enhanced oil recovery with hydrophobically associating polymers containing N-vinyl-pyrrolidone functionality. US 4709759 A
Kamal MS, Sultan AS, Al-Mubaiyedh UA, Hussien IA, Pabon M (2014) Evaluation of rheological and thermal properties of a new fluorocarbon surfactant-polymer system for eor applications in high-temperature and high-salinity oil reservoirs. J Surfactants Deterg 17(5):985–993
Kamal MS, Hussien IA, Sultan AS, Han M (2013) Rheological study on ATBS-AM copolymer-surfactant system in high-temperature and high-salinity environment. J Chem. doi:10.1155/2013/801570
Doe P, Moradi-Araghi A, Shaw J, Stahl G (1987) Development and evaluation of EOR polymers suitable for hostile environments part 1: copolymers of vinylpyrrolidone and acrylamide. SPE Reservoir Eng 2(4):461–467
Zargartalebi M, Kharrat R, Barati N (2015) Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 143:21–27. doi:10.1016/j.fuel.2014.11.040
Kamari A, Sattari M, Mohammadi AH, Ramjugernath D (2015) Reliable method for the determination of surfactant retention in porous media during chemical flooding oil recovery. Fuel 158:122–128
Kamal MS, Sultan AS, Hussein IA (2015) Screening of amphoteric and anionic surfactants for cEOR applications using a novel approach. Colloid Surf A 476:17–23
Levitt D, Jackson A, Heinson C, Britton L, Malik T, Dwarakanath V, Pope G (2009) Identification and evaluation of high-performance EOR surfactants. Society of Petroleum Engineers. doi:10.2118/100089-pa
Hirasaki G, Miller C, Puerto M (2011) Recent advances in surfactant EOR. SPE J 16(4):3–5. doi:10.2118/115386-pa
Kamal MS (2016) A review of gemini surfactants: potential application in enhanced oil recovery. J Surfactants Deterg 19(2):1–14. doi:10.1007/s11743-015-1776-5
Fuseni A, Han M, Al-Mobith (2013) A phase behavior and interfacial tension properties of an amphoteric surfactant for EOR application. In: SPE Saudi Arabia section technical symposium and exhibition, 2013. Society of Petroleum Engineers. doi:10.2118/168104-MS
Wu X, Han M, Zahrani BH, Guo L (2015) Effect of surfactant-polymer interaction on the interfacial properties for chemical EOR. Society of Petroleum Engineers. doi:10.2118/172706-MS
Zhou X, Han M, Fuseni A, Yousef A (2012) Adsorption of an amphoteric surfactant onto permeable carbonate rocks. Society of Petroleum Engineers. doi:10.2118/153988-MS
Lv W, Bazin B, Ma D, Liu Q, Han D, Wu K (2011) Static and dynamic adsorption of anionic and amphoteric surfactants with and without the presence of alkali. J Petrol Sci Eng 77(2):209–218. doi:10.1016/j.petrol.2011.03.006
Gaillard N, Giovannetti B, Leblanc T, Thomas A, Braun O, Favero C (2015) Selection of customized polymers to enhance oil recovery from high temperature reservoirs. In: SPE Latin American and Caribbean petroleum engineering conference, 2015. Society of Petroleum Engineers. doi:10.2118/177073-MS
Shakil Hussain SM, Muhammad Shahzad Kamal MAA, Nisar U, Ibnelwaleed AH, Abdullah SS (2016) Synthesis, characterization and surface properties of amidosulfobetaine surfactants bearing odd-number hydrophobic tail. J Surfactants Deterg 19(2):413–420
Hussain SS, Animashaun MA, Kamal MS, Ullah N, Hussein IA, Sultan AS (2016) Synthesis, characterization and surface properties of amidosulfobetaine surfactants bearing odd-number hydrophobic tail. J Surfactants Deterg 19(2):413–420
Kamal MS, Sultan AS, Al-Mubaiyedh UA, Hussein IA (2015) Review on polymer flooding: rheology, adsorption, stability, and field applications of various polymer systems. Polym Rev 55(3):491–530. doi:10.1080/15583724.2014.982821
Wang D, Cheng J, Xia H, Li Q, Shi J (2001) Ltd DOILCO Viscous-elastic fluids can mobilize oil remaining after water-flood by force parallel to the oil-water interface. In: SPE Asia Pacific improved oil recovery conference, 6–9 Oct. Kuala Lumpur, Malaysia, 2001. Society of Petroleum Engineers. doi:10.2118/72123-ms
L-j Zhang, X-a Yue (2008) Displacement of polymer solution on residual oil trapped in dead ends. J Central South Univ Technol 15:84–87
Xia H, Wang D, Wu J, Kong F (2004) Elasticity of HPAM solutions increases displacement efficiency under mixed wettability conditions. In: SPE Asia Pacific oil and gas conference and exhibition, Australia. Society of Petroleum Engineers. doi:10.2118/88456-MS
Zhang Z, Li J, Zhou J (2011) Microscopic roles of “viscoelasticity” in HPMA polymer flooding for EOR. Transport Porous Med 86(1):199–214. doi:10.1007/s11242-010-9616-6
Wang D, Xia H, Liu Z, Yang Q (2001) Study of the mechanism of polymer solution with visco-elastic behavior increasing microscopic oil displacement efficiency and the forming of steady “oil thread” flow channels. In: SPE Asia Pacific oil and gas conference and exhibition, 17–19 April, Jakarta, Indonesia. Society of Petroleum Engineers. doi:10.2118/68723-ms
Urbissinova TS, Trivedi JJ, Kuru E (2010) Effect of elasticity during viscoelastic polymer flooding: a possible mechanism of increasing the sweep efficiency. In: SPE western regional meeting, 27–29 May, Anaheim, California. 2010. Society of Petroleum Engineers. doi:10.2118/133471-MS
Bataweel MA, Nasr-El-Din HA (2012) Rheological study for surfactant-polymer and novel alkali-surfactant-polymer solutions. In: North Africa technical conference and exhibition, 20–22 February 2012, Cairo, Egypt. Society of Petroleum Engineers. doi:10.2118/150913-MS
Al-Amodi AO, Al-Mubaiyedh UA, Sultan AS, Kamal MS, Hussein IA (2016) Novel fluorinated surfactants for enhanced oil recovery in carbonate reservoirs. Can J Chem Eng 94(3):454–460. doi:10.1002/cjce.22406
Acknowledgments
This work was supported by King Abdulaziz City for Science and Technology (KACST) through the Science & Technology Unit at King Fahd University of Petroleum & Minerals (KFUPM) through project No. 10-OIL1378-04 as part of the National Science Technology and Innovation Plan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kamal, M.S., Shakil Hussain, S.M. & Sultan, A.S. Development of Novel Amidosulfobetaine Surfactant–Polymer Systems for EOR Applications. J Surfact Deterg 19, 989–997 (2016). https://doi.org/10.1007/s11743-016-1848-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1848-1